Abstract
Tourette syndrome (TS) is a common childhood-onset disorder characterized by motor and vocal tics that are typically accompanied by a multitude of comorbid symptoms. Pharmacological treatment options are limited, which has led to the exploration of deep brain stimulation (DBS) as a possible treatment for severe cases. Multiple lines of evidence have linked TS with abnormalities in the motor and limbic cortico-basal ganglia (CBG) pathways. Neurophysiological data have only recently started to slowly accumulate from multiple sources: noninvasive imaging and electrophysiological techniques, invasive electrophysiological recordings in TS patients undergoing DBS implantation surgery, and animal models of the disorder. These converging sources point to system-level physiological changes throughout the CBG pathway, including both general altered baseline neuronal activity patterns and specific tic-related activity. DBS has been applied to different regions along the motor and limbic pathways, primarily to the globus pallidus internus, thalamic nuclei, and nucleus accumbens. In line with the findings that also draw on the more abundant application of DBS to Parkinson's disease, this stimulation is assumed to result in changes in the neuronal firing patterns and the passage of information through the stimulated nuclei. We present an overview of recent experimental findings on abnormal neuronal activity associated with TS and the changes in this activity following DBS. These findings are then discussed in the context of current models of CBG function in the normal state, during TS, and finally in the wider context of DBS in CBG-related disorders.
Keywords: deep brain stimulation, motor tics, Tourette syndrome, neurophysiology, basal ganglia
Introduction
Tourette syndrome.
One hundred thirty years ago, Georges Gilles de la Tourette first described patients suffering from the disorder that would later be named after him (Gilles de la Tourette 1885). In his account of these patients he described the key symptoms of this disorder including motor and vocal tics. Tics are currently defined as sudden, rapid, recurrent, nonrhythmic motor movements or vocalizations (DSM-5; American Psychiatric Association 2013). Tics range from simple motor tics that involve only one or a few muscle groups, to complex motor or vocal tics that involve sequential activation of several muscle groups. Tourette syndrome (TS) is defined as arising in childhood (<18 yr), having at least two types of motor tics and one type of vocal tic, lasting at least 1 year, and not arising as a side effect of a medication for other disorders (DSM-5). The disorder typically appears in early childhood with a 0.8% prevalence of TS among children (Knight et al. 2012). In adults, the prevalence of TS decreases dramatically with an 80–90% reduction in the number of patients. Pharmacological interventions may reduce tics but rarely eliminate them. The most effective and studied medications for TS are the D2 dopamine receptor antagonists (antipsychotic drugs); however, their use is restricted because of their side effects (Eddy et al. 2011). α2-Adrenergic receptor agonists have smaller tic-suppressing potency but also lower risk of adverse reactions (Eddy et al. 2011). Other alternative medications are tetrabenazine, Δ9-tetrahydrocannabinol, and botulinum toxin (Botox) injections (Roessner et al. 2011). Tics are associated with a premonitory urge that has been reported by >90% of patients. These patients typically report that the tics are (at least partially) voluntary responses to the premonitory urge and are not an involuntary movement (Leckman et al. 1993). Tics are the hallmark symptom of TS; however, they seldom appear by themselves. Over 90% of TS patients suffer from additional comorbidities (Freeman et al. 2000). The most common comorbid symptoms are attention deficit hyperactivity disorder (ADHD) and obsessive-compulsive disorder and behavior (OCD and OCB, respectively), each affecting over half of all TS patients. Thus both the study of the underlying mechanism of TS and the clinical treatment of the disorder need to take into account that tic-only TS patients comprise a minority of the overall TS patient group. The underlying mechanism of tic formation in TS is still largely obscure, but multiple lines of evidence have linked tics to malfunctions in the cortico-basal ganglia (CBG) pathway (Kalanithi et al. 2005; Singer and Minzer 2003).
The cortico-basal ganglia loop and its relation to TS.
The basal ganglia (BG) comprise a group of interconnected subcortical nuclei involved in motor, associative, and limbic functions (Alexander et al. 1986). Information from multiple areas of the cerebral cortex and thalamus is integrated in the BG and conveyed via the thalamus back to frontal cortical areas, thus forming the cortico-basal ganglia (CBG) loop. The BG is composed of the striatum [subdivided into the putamen, caudate, and nucleus accumbens (NAc)], the subthalamic nucleus (STN), the globus pallidus external and internal segments (GPe and GPi, respectively), and the substantia nigra pars compacta and pars reticulata (SNc and SNr, respectively). Excitatory (glutamatergic) projections from the cerebral cortex and the centromedian and parafascicular thalamic nuclei (CM-Pf complex) converge in the striatum and STN, the BG's input structures (Parent and Hazrati 1995a, 1995b; Sadikot and Rymar 2009). Dopaminergic input to the striatum is received through its reciprocal connections with neurons in the midbrain (SNc and ventral tegmental area) (Haber et al. 2000). Efferent projections from the BG's input structures induce opposing effects via the inhibitory (GABAergic) projections of the striatum and the excitatory projections of the STN (Smith and Parent 1988; Tremblay and Filion 1989). Information from these input structures is transmitted, either directly or via the GPe, to the GPi and SNr, the BG's output structures. The output structures then send inhibitory (GABAergic) projections to the thalamus and brain stem. Multiple thalamic areas are innervated by the BG: the ventral anterior, ventral lateral, and medial dorsal (MD) nuclei and the CM-Pf complex (Haber and McFarland 2001; Sadikot and Rymar 2009). These nuclei send excitatory projections to the cortex, completing the CBG partially closed loop (Hoover and Strick 1993; Tokuno et al. 1992).
Cortical inputs are processed in the BG in multiple parallel, functionally defined pathways including the motor, associative, and limbic loops. Within each one of these pathways, partially segregated projections from functionally related cortical areas traverse discrete portions of the BG and thalamus. Thus the pathways remain mostly specific throughout the CBG loop (Alexander et al. 1986; Parent and Hazrati 1995b) despite some integrative projections (Draganski et al. 2008; Oguri et al. 2013). Subregions within the motor pathway are somatotopically organized throughout the different nuclei (Alexander and DeLong 1985; DeLong et al. 1985). Information flows through the BG circuits in three feedforward pathways, each of which has a different influence on the output nuclei (GPi/SNr) and thus affects cortical activity differentially. In the “direct pathway,” striatal neurons project to the GPi/SNr, thereby decreasing their activity and facilitating cortical activation (Alexander and Crutcher 1990). The other two pathways, the “indirect” and “hyperdirect” pathways, increase the GPi/SNr discharge and therefore suppress cortical activity. Striatal neurons in the “indirect pathway” inhibit the GPe, which then disinhibits the STN, causing an increase in GPi excitation (Alexander and Crutcher 1990). In the “hyperdirect pathway,” cortical projections reach the STN directly, bypassing the striatal afferent and efferent fibers (Nambu et al. 2000), and excite the output nuclei. Dopaminergic projections modulate the CBG pathways by activating the D1 and D2 receptors in the striatum. Dopamine (DA) has opposing effects on these pathways by facilitating the direct pathway through the D1 receptors and inhibiting the indirect pathway through the D2 receptors expressed in neurons belonging to these pathways (Gerfen et al. 1990). Hence, higher levels of DA in the striatum increase cortical activation, whereas decreased DA levels suppress cortical activation (Albin et al. 1989; DeLong 1990).
Striatal neurons can be divided into two populations: projection neurons and interneurons. GABAergic projection neurons comprise the vast majority (70–95%) of striatal neurons and are referred to as medium spiny neurons (MSNs) because of their morphological structure (Gerfen 1988). MSN activity is mediated by axon collaterals of neighboring MSNs and feedforward interneuron inhibition (Tepper and Bolam 2004). The remaining striatal neurons consist of several types of GABAergic interneurons and one type of cholinergic interneuron, classified on the basis of their morphology, neurochemistry, and physiology (Kawaguchi 1993). Parvalbumin-positive (PV+) interneurons are the best-characterized GABAergic interneurons in the striatum, exhibiting brief action potentials and a high discharge rate. They are referred to as fast-spiking interneurons (FSIs). Individual FSIs innervate large populations of MSNs and can induce potent inhibition (Bennett and Bolam 1994; Kita et al. 1990; Koós and Tepper 1999). Cholinergic interneurons are characterized by their tonic discharge and are referred to as tonically active neurons (TANs) (Kimura et al. 1984). TANs activate GABAergic interneurons and therefore indirectly inhibit MSNs (English et al. 2011).
Behaviors associated with TS are generally attributed to malfunctions of the BG in general and the striatum specifically. The activation of motor tics in TS is presumed to result from a malfunction of the motor pathway, whereas the premonitory urge is presumed to result from the limbic pathway. Functional and anatomical imaging studies of tic expression have reported aberrant activity in sensorimotor, associative, and limbic circuits, correlating tic severity with BG activation (Baym et al. 2008; Peterson et al. 1998; Stern et al. 2000; Wang et al. 2011). Structural abnormalities in the CBG loop measured by magnetic resonance imaging (MRI) present reduced caudate nucleus volumes in TS subjects regardless of age, whereas the volumes of the putamen and GP nuclei decreased mostly in adults with TS (Peterson et al. 2003). The severity of tics in late adolescence and early adulthood was reported to be inversely correlated with caudate nucleus volumes in childhood (Bloch et al. 2005). Hinting at a system-wide change, abnormalities were found in the CBG white matter pathways and in particular exhibited an enhanced structural connectivity of the striatum and thalamus with the primary motor and sensory cortices, among other cortical areas (Worbe et al. 2015), and in thalamic and sensorimotor cortical volume (Miller et al. 2010; Sowell et al. 2008).
Multiple neuromodulator/neurotransmitter systems are altered in TS patients, most notably the dopaminergic and GABAergic systems. Improvements in tic expression after dopamine D2 antagonist treatment have suggested a supersensitivity of dopaminergic receptors (Singer et al. 1982). Likewise, increased striatal expression of presynaptic dopamine carrier sites in postmortem brain tissue from TS patients has suggested enhanced dopamine innervation within the striatum (Singer et al. 1991). Consistent with these findings, neurochemical imaging studies have indicated abnormal dopaminergic innervation specifically in the ventral striatum (Albin et al. 2003; Cheon et al. 2004; Wong et al. 2008). Anomalies in the GABAergic system were found in postmortem studies of TS subjects, indicating a decreased number of the GABAergic PV+ FSIs in the striatum (Kalanithi et al. 2005; Kataoka et al. 2010). This is complemented by significant changes in the PV+ projection neuron population in both parts of the GP: a decreased number in the GPe and an increased number in the GPi (Kalanithi et al. 2005). Decreased binding of GABA receptors in the ventral parts of the striatum and GP, as observed in neurochemical imaging studies, further emphasizes the involvement of the BG GABAergic system, and its limbic pathway in particular, in TS (Lerner et al. 2012).
Animal models of TS and tic expression.
Multiple animal models have been developed to address different properties of TS. Each one of these models focuses primarily on a single aspect of the disorder; i.e., motor tics, sensory motor gaiting deficits, comorbid symptoms, or the heredity of genetic mutations. These models can be divided into two general categories based on the method used to generate the model: 1) models that use pharmacological manipulations and 2) models that use genetic manipulations. Pharmacological animal models use systemic or localized administration of substances that affect different neurochemicals in the nervous system (for a detailed review see Bronfeld et al. 2013a). The most common targets are the neurotransmitter GABA and the neuromodulators dopamine, serotonin, and norepinephrine. The GABAergic model uses focal injections of GABAA antagonists such as bicuculline and picrotoxin into motor regions of the striatum. These injections lead to the appearance of sudden, rapid, and repetitive movements in both rodents and primates (Bronfeld et al. 2013b; Crossman et al. 1988; Marsden et al. 1975; McCairn et al. 2009) that resemble the simple motor tics of TS patients. Tics usually emerge within a few minutes following the injection and last for up to 2 h (Bronfeld et al. 2013b; Crossman et al. 1988; Marsden et al. 1975; McCairn et al. 2009; Patel and Slater 1987; Tarsy et al. 1978). In monkeys, a minority of the injections led to abnormal sequential movements that resembled complex motor tics. Similar injections that have been performed in different functional territories of the striatum and GPe have generated domain-specific behaviors similar to compulsive, hyperactive, and/or attention deficit symptoms (Bronfeld et al. 2010; François et al. 2004; Tarsy et al. 1978; Worbe et al. 2009), thus indicating the existence of a link between the generation of motor tics and their common comorbid disorders (OCD/OCB and ADHD). The presentation of an easily quantifiable motor symptom (simple motor tic) enables us to gain insight into the additional limbic/associative disorders associated with the BG. This is beneficial as the direct study of such disorders typically presents a large challenge in the quantification of behavior.
The dopaminergic models use systemic or focal administration of DA agonists such as amphetamine and apomorphine. After the injection, the animal expresses stereotypic behaviors, such as sniffing, licking, or biting (Kelley et al. 1988; Randrup et al. 1963), in addition to sensory motor gaiting deficits as assessed by the prepulse inhibition (PPI) paradigm (Lind et al. 2004; Mansbach et al. 1988; Wan and Swerdlow 1996). Administration of DA agonists and the associated behaviors are not unique to modeling TS and are frequently used to model other disorders such as schizophrenia, OCD, and ADHD (Geyer 2006; Kokkinidis and Anisman 1981; Weiss and Feldon 2001). Other models targeting the serotonergic and the norepinephrine neuromodulators use systemic administration of these drugs. The models display brief and repetitive movements (albeit not resembling tics) (Bedard and Pycock 1977) and sensory motor gaiting deficits (Carasso et al. 1998; Sipes and Geyer 1994).
Family studies of TS patients have indicated that genetic factors play a role in the manifestation of TS (Price et al. 1985). Several genes and chromosomal regions are considered likely candidates in the TS etiology, but most are rare, and currently no gene is known to have a major effect on TS etiology (Godar et al. 2014). Genetic animal models for TS can be divided into two groups: 1) models that are based on mutant genes that have been found in humans with TS and 2) models based on mutations affecting the dopaminergic system. Models based on human TS mutations mainly include the SLITRK1(Abelson et al. 2005a; Fabbrini et al. 2007; Verkerk et al. 2006), SAPAP3 (Crane et al. 2011), and HDC (histidine decarboxylase) genes (Ercan-Sencicek et al. 2010). The models related to the dopaminergic pathway mainly target the genes of D1 receptors (D1CT-7), D3 receptors, and the dopamine transporter (DAT). Currently, genetic animal models have not exhibited the main characteristic of TS; i.e., motor tics. Rather, most animal models can successfully produce a decrease in locomotion (SLITRK1 and HDC models) (Katayama et al. 2010; Kubota et al. 2002), increased twitching (D1CT-7 model) (Nordstrom and Burton, 2002), or TS comorbid symptoms such as OCB [SAPAP3, D1CT-7, DAT, and HDC (following amphetamine injection) models] (Berridge et al. 2005; Campbell et al. 1999; Castellan Baldan et al. 2014; Welch et al. 2007), anxiety (SLITRK1 and SAPAP3 models) (Katayama et al. 2010; Welch et al. 2007), and hyperactivity (D1CT-7 and nonfunctional D3 receptor models) (Accili et al. 1996; Campbell et al. 1999).
Currently, each of the pharmacological and genetic TS animal models presents only a limited subset of the complex set of properties associated with the disorder. Moreover, most of the models are transient in nature and do not display the complex neurodevelopmental properties of TS. Nonetheless, together these animal models provide unique and valuable insights into the aberrant neural activity underlying the symptoms of TS.
Physiology of Tourette Syndrome
The behavioral expression of TS symptoms has been studied for over a century; however, the underlying neuronal abnormalities have only been studied extensively in the last two decades. Physiological data, which historically have been limited to noninvasive methods, are currently being augmented by invasive electrophysiological recordings in human patients undergoing neurosurgery and novel experimental animal models.
TS physiology in human patients.
Information regarding the underlying neurophysiology of TS in human patients is slowly accumulating through two complementary channels: 1) noninvasive methods such as functional magnetic resonance imaging (fMRI), positron emitting tomography (PET), electroencephalogram (EEG), magnetoencephalogram (MEG), and transcranial magnetic stimulation (TMS), and 2) invasive methods such as electrophysiological recordings of local field potential (LFP) and spiking activity from TS patients during and immediately following neurosurgery (Priori et al. 2013).
Noninvasive neurophysiological methods point to abnormal CBG activity in TS both in the overall activity of specific brain regions and in the activity surrounding the timing of individual tics.
ABNORMAL ACTIVITY PRECEDING THE TIME OF THE TIC.
An fMRI study reported the activation of limbic areas, such as the anterior cingulate (ACC) and insular cortex, and the supplementary motor area (SMA) preceding the tic, followed by the activation of sensorimotor areas with the tic onset (Bohlhalter et al. 2006). Activation of the SMA and M1 were observed up to 2 s before the tic onset (Neuner et al. 2014). A stronger and broader correlation between the SMA and M1 surrounding the time of the tic was shown using fMRI (Hampson et al. 2009). Increased coherence was also found using EEG between the sensorimotor, prefrontal, and medial frontal areas during tic suppression (Serrien et al. 2005). Support for such findings comes from a PET study that found a correlation between the striatum, sensorimotor, associative, and limbic cortices and tic occurrences (Stern et al. 2000).
ABNORMAL BASELINE ACTIVITY.
A study of tic suppression using fMRI revealed changes in BG and thalamic activation that correlated inversely with the severity of tic symptoms (Peterson et al. 1998). In line with these findings, a recent study found abnormal functional connectivity in the CBG pathway including the sensorimotor, premotor, parietal, and cingulate cortices (Worbe et al. 2012). The study showed that the tics were associated primarily with motor pathway abnormalities and OCD with associative and limbic pathways abnormalities. Increased activation was also observed throughout the direct pathway of the BG, as well as increased compensatory activation in the prefrontal cortex and the STN (Baym et al. 2008). A complementary study using TMS found abnormal cortical excitability in terms of a reduction in the cortical silent period in TS patients (Ziemann et al. 1997). Finally, whereas most studies have focused on activation levels, an MEG study revealed abnormalities in the beta-band oscillatory activity of TS patients in the sensorimotor areas (Tinaz et al. 2014).
Invasive recordings in TS patients undergoing deep brain stimulation (DBS) implantation surgery have been performed in various nuclei of the thalamus and multiple locations within the GPi, both intraoperatively using microelectrodes and postoperatively through the stimulating electrode leads. Tic related changes in neuronal activity have primarily been studied in the period preceding the tic to avoid the large movement-related activity associated with the tic. Enhanced synchronization of cortical EEG with thalamic LFP recorded by the DBS electrode was observed up to 1.5 s before the tic onset (Bour et al. in press). Tic-related neuronal activity was also observed in the ventralis oralis thalamic nucleus in one patient (Priori et al. 2013) but was not evident in the other cases since no tics occurred during the operation. GPi single neurons displayed tic-related neuronal activity preceding the tic by up to 2 s (Zhuang et al. 2009). Changes to the baseline activity in TS patients were observed in the GPi (Zhuang et al. 2009), revealing low firing rates comparable to those in dystonia patients (Vitek et al. 1999) but far below those in human Parkinson's disease (PD) patients and normal nonhuman primates (Raz et al. 2000). Zhuang and colleagues also reported irregular GPi neuronal activity [coefficient of variation (CV) > 1] that was higher than the values observed in normal primates. Recordings in the ventralis oralis nucleus of the thalamus in TS patients under general anesthesia revealed neuronal bursting and LFP oscillations in the low-frequency and alpha bands (3–13 Hz) (Marceglia et al. 2010). These recordings also exhibited decreased beta-band oscillations, contrary to the increased power in this band classically associated with PD (Stein and Bar-Gad 2013). However, these types of oscillatory activity changes have been identified in patients under general anesthesia undergoing neurosurgery for other disorders and thus may be unrelated to TS.
TS physiology in animal models.
Research using animal models of TS has focused historically on behavioral properties and the effects of pharmacological agents on the expressed behaviors. Very few studies have attempted to explore the neurophysiological characteristics of the different models and their associated expressions of abnormal behaviors. Typically, existing animal models of TS focus on a small subset of the symptoms associated with the disorder and are based on transient deficits, in contrast to the neurodevelopmental nature of TS. However, these models provide a unique data set, because the ability of examining the underlying neuronal activity in TS patient is severely limited. The dopaminergic animal model indicates changes in the baseline activity of neurons along the CBG pathway following systemic or focal dopamine agonist, typically apomorphine, injection. The direct and most consistent effect is the inhibition of the TANs in the striatum and a reduction in their correlation (Fujita et al. 2013). Reports concerning changes in the mean firing rate of the projection neurons (MSNs) are mixed: equal populations of striatal MSNs were found, which increased or decreased their rate as predicted by current theories on the function of the known dopamine receptor subtypes (Fujita et al. 2013). Mixed results were also observed in the GPe, in line with the division into two neuronal subtypes (Bergstrom et al. 1982; Boraud et al. 2001; Kelland et al. 1995). In the STN, the firing rate increased following the injection (Kreiss et al. 1996). Most studies have observed decreased firing rates in the output nuclei of the BG, i.e., the GPi and the SNr (Boraud et al. 2001; Nevet et al. 2004), but see the increased rates in an amphetamine study (Waszczak et al. 2001). To date, physiological recordings are not common in genetic models of TS. In a study performed on the genetic model of SAPAP3 knockout mice, an increased baseline firing rate was found in striatal MSNs (Burguière et al. 2013).
Unlike most current models, the striatal disinhibition animal model of TS enables the study of the underlying neuronal abnormalities associated specifically with tics. In this model, changes in neuronal activity synchronized with the tic expression were found in both individual neurons and larger populations, as reflected by LFP and EEG activity. Large transient deflections in cortical EEG activity (EEG spikes) and LFP throughout the BG and cerebellum (LFP spikes) were recorded from the ipsilateral hemisphere of the focal striatal injection (Darbin and Wichmann 2008; McCairn et al. 2013a; Muramatsu et al. 1990; Pogorelov et al. 2015; Tarsy et al. 1978). The EEG spikes appeared shortly before the earliest observable tics. However, once initiated, the tics occurred concurrently with the EEG spikes (Muramatsu et al. 1990; Tarsy et al. 1978). The tic-related LFP spikes were stereotypic, lasted several hundred milliseconds, and appeared synchronously with the tics (McCairn et al. 2009). Tic-related changes in the activity of individual neurons appeared throughout the CBG pathway. In the motor cortex, most of the recorded neurons fired abnormally, in that they discharged in highly active bursts concurrently with the tics with little or no activity between tics (McCairn et al. 2009). In the striatum, an increase was found in the overall firing rate of MSN neurons during the tic period (Worbe et al. 2009). This results from the phasic firing rate modulations around tic onset time, because the neurons responded to tic expression with a brief burst of activity around the tic onset and nearly no activity between tics (Bronfeld et al. 2011). MSN tic-related activity usually preceded both tic onset time and tic-related activity within the motor cortex (Bronfeld et al. 2011). Phasic firing rate modulations around tic onset were also found in striatal TANs, composed of increased and decreased phases of discharge rate around tic onset (Bronfeld et al. 2011). The MSNs and TANs that were locked to the tic time were found in the somatotopic region associated with the tic region within the striatum, whereas no tic-related changes were found in other regions within the striatum. In the GPe, the low-frequency bursting (LFB) neurons displayed bursts of activity around tic onset and nearly no activity between tics (Bronfeld et al. 2011). The high-frequency pauser (HFP) GPe neurons displayed both phasic increases and decreases in firing rate in relation to the tics, with most of the recorded neurons exhibiting excitation (McCairn et al. 2009). Complex response patterns were also found in the output nuclei of the BG; i.e., the GPi and the SNr. However, in these nuclei, most of the recorded neurons displayed transient decreases in firing rate in relation to the tics (McCairn et al. 2009; Muramatsu et al. 1990). In both segments of the GP, tic-related activity was distributed throughout the motor domain, regardless of the disinhibition region within the striatum and the location of the tics (Bronfeld et al. 2011). Consistent with the inhibitory response of the BG output nuclei, phasic excitations were found in the ventromedial nucleus of the thalamus in relation to the tics (Muramatsu et al. 1990). In the striatal disinhibition animal model, tic-related neuronal activity changes were not unique to the CBG loop, in that tic-related responses were also found in the cerebellum before and during tic onset (McCairn et al. 2013a).
Relation to CBG theoretical models.
These neurophysiological findings in TS human patients and in animal models of the disease may be assessed in the framework of some of the theoretical concepts dealing with the function of the CBG pathway in health and disease. Early studies utilized the mapping of pathways between the CBG components to put forward the box-and-arrow model (Albin et al. 1989; DeLong 1990). In this model, each brain area is modeled as a single simplified unit (“box”), which can either increase or decrease its overall activity depending on the activity of afferent brain areas and on the type of connection (“arrow”). Thus the CBG loop was perceived as a feedback loop with two competing elements: a positive feedback loop mediated by the direct pathway and a negative feedback loop mediated by the indirect pathway. The relative activity of the two pathways is controlled by the dopaminergic signal, which increases the activity of the direct pathway and decreases the activity of the indirect pathway. Over the years, multiple additions have been made to the original model to incorporate new anatomical and physiological data, such as the hyperdirect pathway (Nambu et al. 2000), while maintaining the same basic concept. According to the box-and-arrow model, hyperkinetic disorders in general, and TS specifically, are expected to be associated with higher dopaminergic activity. This results in enhanced activity in the direct pathway and reduced activity in the indirect pathway, leading to the inhibition of the output structures, which in turn results in disinhibition of the thalamus and cortical targets and enhances their overall activation.
Current data support the predictions of the box-and-arrow model for TS with regard to two key features: changes in dopaminergic activity and increased cortical activity due to decreased BG output (Table 1, top panel). The supposition of hyperdopaminergic activity is supported by the success of dopamine antagonist treatment in handling the expression of tics (Jankovic 2009). Secondary support comes from the dopamine agonist models of TS, which, despite not demonstrating tics explicitly, express comorbid symptoms and secondary phenomena typical of the disorder (Kelley et al. 1988; Mansbach et al. 1988). Direct support for the rate changes predicted by the box-and-arrow model are provided by recordings in TS patients undergoing surgery, in which a lower baseline firing rate of the BG output, the GPi, was observed (Zhuang et al. 2009). This is also supported by fMRI studies that found increased activity in the direct BG pathway (Baym et al. 2008) and increased cortical activity (Worbe et al. 2012) and by the reduced silent period in the cortex following TMS (Ziemann et al. 1997).
Table 1.
Neurophysiology of tics
| Box-and-Arrow Model | Theoretical Modela | Human Patients | Dopamine Agonist Animal Model |
|---|---|---|---|
| Dopamine levels | ↑ | ↑b | ↑c |
| Striatal baseline activity | ↓↑ | ↑d | ↓↑e |
| BG output (GPi) baseline activity | ↓ | ↓f | ↓g |
| Cortical baseline activity | ↑ | ↑h | ? |
| Action Selection Model | Theoretical Modeli | Human Patients | Striatal Disinhibition Animal Model |
|---|---|---|---|
| Striatal tic-related activity | ↑ | ↑j | ↑k |
| BG output (GPi) tic-related activity | ↓ | ↑f | ↓l |
| Cortical tic-related activity | ↑ | ↑m | ↑l |
Comparison between the predictions of theoretical Tourette syndrome (TS) models and experimental results from human patients and animal models of the disorder. BG, basal ganglia; GPi, global pallidus internus.
A leading concept in CBG functionality is its central role in action selection models. These models stress the role of the BG in choosing one or more actions out of a multitude presented to the BG by the cortex (Mink 1996). The models vary in the nature of the actions selected, which range in their definition from low-level “simple” motor actions to high-level “complex” behavioral schemes. The models also differ depending on whether the selection mechanism is achieved using lateral or recurrent inhibition within the striatum (Beiser and Houk 1998; Wickens 1993) or via feedforward competition between the different pathways (Berns and Sejnowski 1998; Gurney et al. 2001). According to the action selection model, a malfunction of the selection process, such as the one occurring following abnormal inhibition within the striatum, will result in the expression of undesired actions that are not inhibited by the chosen action (Albin and Mink 2006; Mink 2001).
Indirect support for the action selection model and its malfunction in TS is provided by recordings in human TS patients undergoing surgery (Table 1, bottom panel). These patients were seen to exhibit a burst of activity in the GPi preceding the tic (however, according to the model, a transient inhibition is expected) (Zhuang et al. 2009). Multiple fMRI and PET studies have reported transient cortical excitation before the tic, although it cannot be tied directly to the CBG pathway (Bohlhalter et al. 2006; Hampson et al. 2009; Neuner et al. 2014; Stern et al. 2000). More direct support for this concept is found in the striatal disinhibition animal model, in which tic-related activity is expressed throughout the CBG pathway, including a transient inhibition (tic release) in the BG output (GPi) (Bronfeld et al. 2011; McCairn et al. 2009) (Table 1, bottom panel).
Overall, although the physiological evidence is still sparse and may be affected by the comorbidities typical to TS, the vast majority of the data collected with the use of diverse measures in human patients and animal models points to substantial changes occurring in neuronal activity throughout the CBG motor, associative, and limbic pathways. The changes are not limited to a single brain region, but rather represent a system-wide change in two main properties: the baseline firing rate and tic-related firing rate changes. These changes, which represent both temporally global changes (baseline rate) and temporally local changes (tic related), have been reported in multiple studies, whereas measures typical of other CBG disorders, such as oscillatory and coherent activity, do not appear to play a substantial role in TS physiology.
Deep Brain Stimulation
A novel approach to treating severe cases of TS that are nonresponsive to behavioral and pharmacological treatments is a neurosurgical approach termed deep brain stimulation (DBS). This treatment, which has been previously applied successfully to multiple movement and psychiatric disorders, is used in an exploratory manner in TS.
DBS in movement disorders.
The development of stereotaxic surgery enabled accurate access to deep brain structures for patients suffering from different disorders (Spiegel et al. 1947). This led to a proliferation of ablation surgeries during the 1950s and 1960s targeting multiple brain structures, including the BG, for treating a multitude of movement disorders (Schwalb and Hamani 2008). Some of these early studies used electrical stimulation as a means of identifying brain structures, thus paving the way for stimulation-based treatments that overcame the irreversibility of ablation-based treatments. In its current form, one or more DBS electrodes are chronically implanted, and following surgery, continuous stimulation consisting of high-frequency pulses is applied.
The vast majority of DBS surgeries for movement disorders are performed for treating PD. Multiple brain targets have been used for treating the symptoms of PD, including the ventral intermediate (VIM) nucleus of the thalamus (Benabid et al. 1991), the GPi (Siegfried and Lippitz 1994), and the STN (Limousin et al. 1995) (Table 2). Thalamic targets primarily provide relief to tremor but have limited effects on other motor symptoms, whereas the GPi and the STN are used for more general treatment of the motor symptoms of PD. Each of these targets has a different effect on each of the motor symptoms and is associated with different side effects. Currently, bilateral implantation of electrodes in the STN is the de facto standard, despite comparable results from GPi implantation, with both treatments providing immediate and long-lasting improvement for a wide variety of motor symptoms (Liu et al. 2014; Odekerken et al. 2013; Rodriguez-Oroz et al. 2005). In all cases, the implantation is targeted at the motor parts of the nucleus to avoid side effects arising from the stimulation of limbic pathways.
Table 2.
DBS targets
Comparison of deep brain stimulation (DBS) targets in TS and other cortico-basal ganaglia (CBG)-related disorders. PD, Parkinson's disease; OCD, obsessive-compulsive disorder.
Dystonia is an additional movement disorder treated regularly with DBS. Multiple forms of dystonia exist, which are categorized as a function of anatomical distribution, age of onset, and division into primary and secondary. The most common form of dystonia treated with DBS is generalized primary dystonia, but some other forms of dystonia respond well to DBS (Ostrem and Starr 2008). The primary target representing the vast majority of the cases is the GPi (Guridi and Lozano 1997) (Table 2). A very small minority of implantations have been performed in the STN and thalamus, despite some successful results in these surgeries. Typically, unlike the immediate relief in symptoms occurring during DBS for PD, dystonia symptom reduction increases over weeks to months and lasts for prolonged periods.
DBS in OCD.
The effectiveness of DBS in treating movement disorders has led to its application in psychiatric conditions such as OCD. OCD is an anxiety disorder characterized by intrusive thoughts (obsessions) and repetitive or ritualistic actions (compulsions) and is a common comorbidity of TS. In severe cases, DBS in various targets associated with the limbic regions of the CBG circuit is used to ameliorate the symptoms of patients experiencing treatment-refractory OCD (Table 2). Numerous clinical reports have stated that capsulotomy lesions placed in the anterior limbs of the internal capsules (ALIC) may alleviate patients' symptoms by interfering with reciprocal thalamocortical projections and over activation of the orbitofrontal cortex (Lippitz et al. 1999). Initially, DBS electrodes were implanted bilaterally in the ALIC, located on the basis of anterior capsulotomy lesions (Abelson et al. 2005b; Nuttin et al. 1999, 2003). Symptom reduction appeared in most patients within a few weeks of treatment. In an attempt to improve results, more posterior sites were investigated, refining the target to the ventral ALIC and adjacent ventral striatum, targeting limbic CBG connections (Aouizerate et al. 2004; Goodman et al. 2010; Greenberg et al. 2006, 2010). DBS in the NAc also provides a partial reduction of OCD symptoms following both bilateral (Denys et al. 2010) and unilateral stimulation (Sturm et al. 2003). Behavioral side effects reported in PD patients who underwent STN DBS procedures have emphasized the role of the STN in behavioral integration and led to the idea of stimulation in the limbic regions of the STN to treat nonmotor symptoms. Although OCD symptoms are attenuated, serious adverse events limit the usage of this nucleus as a DBS target (Chabardès et al. 2013; Mallet et al. 2008). Overall, although long-term OCD DBS stimulation studies are still lacking, clinical outcomes of published short-term studies indicate a significant symptom reduction lasting multiple years (Greenberg et al. 2006). This underscores the important therapeutic benefits of DBS in the psychopathology of severe and intractable OCD.
DBS in TS.
The dramatic therapeutic benefits of DBS in movement disorder and early successes in psychiatric disorders paved the way for its implementation for treating severe cases of TS in which pharmacological and behavioral treatments were found to be inefficient. A growing number of TS DBS surgeries have been reported worldwide, recently exceeding 100 cases (for a detailed review see Schrock et al. 2015). Numerous surgical targets for TS DBS have been proposed, primarily within the CBG loop (Fig. 1; Table 2), in an attempt to select appropriate targets for the alleviation of both tics and comorbid symptoms. Regions of the medial thalamus are the most commonly used targets because of their involvement in both motor and limbic pathways. Preliminary studies of thalamic DBS were performed under the assumption that bilateral stimulation in the CM-Pf complex would reduce the activity level of the dorsal (motor via CM) and ventral (limbic via Pf) striatum (Kim et al. 2013; Vandewalle et al. 1999). These surgeries resulted in partial tic reduction in both motor and vocal tics starting a week after surgery, with a further decrease over longer follow-up periods (Visser-Vandewalle et al. 2003). Unilateral stimulation was found to be ineffective compared with bilateral stimulation in a double-blind study (Maciunas et al. 2007). Findings from additional studies are consistent with these results and show a substantial reduction in tic severity following CM-Pf stimulation, with significant long-term improvement (Kaido et al. 2011; Maling et al. 2012; Okun et al. 2013; Porta et al. 2009; Servello et al. 2008). The stimulation efficacy was confirmed by a prospective, randomized, double-blind study showing a significant improvement in tic severity during the on-stimulation compared with the off-stimulation state (Ackermans et al. 2011). However, the effect of thalamic stimulation on comorbidities was conflicting; thus other targets involved in both motor and limbic modulation were sought (Viswanathan et al. 2012).
Fig. 1.
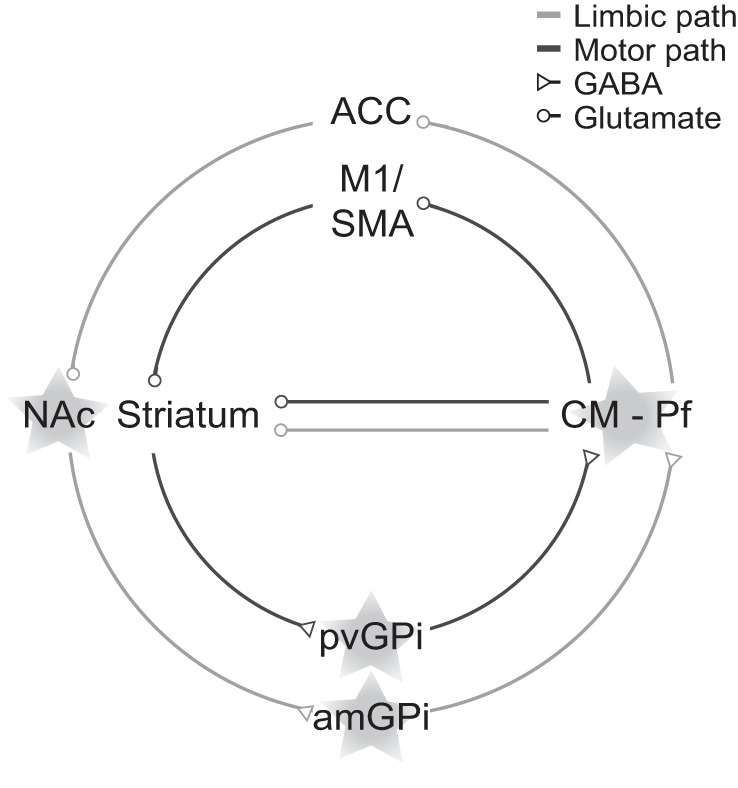
Deep brain stimulation (DBS) targets in Tourette syndrome (TS). Outline of the motor and limbic cortico-basal ganglia (CBG) pathways with the stimulation targets overlaid (stars). ACC, anterior cingulate cortex; SMA, supplementary motor area; NAc, nucleus accumbens; CM-Pf, centromedian and parafascicular thalamic nuclei complex; pvGPi, posteroventral global pallidus internus; amGPi, anteromedial global pallidus internus.
Earlier successes of GPi stimulation in treating hyperkinetic disorders such as dystonia led to the consideration of this region as a target for DBS in TS. Moreover, GPi stimulation affects both motor and limbic pathways; thus it is presumed to influence motor tics as well as psychiatric comorbidities of TS (Viswanathan et al. 2012). Some procedures have targeted the posteroventral GPi, the motor subregion, leading to improvement in motor and vocal tics several weeks after the operation, with a progressive improvement during a follow-up period, even to the point of full tic remission (Dehning et al. 2008; Diederich et al. 2005; Zhang et al. 2014). Others have found a greater improvement in stimulating the anteromedial GPi, the limbic subregion, given the involvement of the limbic loop in tic expression (Cannon et al. 2012; Martinez-Fernandez et al. 2011). In the search for an optimal surgical target, several studies have compared outcomes of stimulation in the limbic regions of the GPi and medial thalamus (Ackermans et al. 2006; Houeto et al. 2005; Motlagh et al. 2013). Although both targets produced a significant improvement in tic severity, a greater reduction of tics was obtained following the pallidal stimulation (Welter et al. 2008). A recent double-blind study of GPi DBS confirmed the significant changes in tic severity (Kefalopoulou et al. 2015). Surprisingly, successful outcomes were obtained in a case study of a patient who underwent DBS in the central GPe, an atypical target for DBS (Piedimonte et al. 2013). Finally, a case study of a patient who suffered from both TS and PD who underwent DBS in the STN, as typically targeted for treating PD, presented an improvement in both tics and parkinsonian symptoms following stimulation (Martinez-Torres et al. 2009).
The frequent association of TS and OCD/OCB is the rationale behind stimulation of the ALIC and NAc in treating TS patients' tics and comorbidities. Findings from these studies demonstrated a moderate and sustained improvement in tic severity, and a significant amelioration of OCD was observed (Kuhn et al. 2007). However, tic reduction was lower compared with that following TS DBS in other locations, as is apparent in the case report of a patient whose symptoms improved after the lead was replaced from the ALIC to the CM-Pf complex (Flaherty et al. 2005; Shields et al. 2008). Moreover, whereas double-blind studies of thalamic (Ackermans et al. 2011) and pallidal DBS (Kefalopoulou et al. 2015) have ruled out a placebo effect, such ALIC studies are still lacking.
Overall, target selections in TS DBS are multifaceted and patient specific, because ameliorating motor and vocal tics as well as comorbid symptoms needs to be considered. Currently, three main targets are used: the CM-Pf complex of the thalamus, the motor and limbic GPi, and the ALIC and NAc (Fig. 1). In sharp contrast to the immediate effects of DBS on PD symptoms, the symptomatic improvements following TS DBS are delayed and gradual. A partial improvement of tic severity occurs in all these studies within a few weeks following surgery, with further reduction occurring over longer periods of time. Despite early successes showing the efficacy of DBS in treating the symptoms of TS, additional progress in establishing the optimal target and stimulation parameters requires a better assessment of the effects of DBS on the pathophysiology of TS.
Physiological Effects of DBS
DBS surgery for different neurological disorders is a success story. However, despite its growing popularity, its underlying mechanism in general, and its specific neurophysiological effects on each of the disorders, remains obscure. A multitude of studies in human patients undergoing surgery and in animal models have greatly improved our understanding of the mechanism over the last decade. Only a very small fraction of these studies has been performed for TS; however, the larger body of evidence from other CBG-related disorders (such as PD) provides valuable information and hints at its potential mechanism in TS.
Neurophysiological effect of DBS in PD.
The mechanism of action of DBS has been studied most extensively in PD. This is a result of two main factors: the large number of PD patients who have undergone DBS implantation (currently around 150,000 worldwide) and successful animal models of the disorder, primarily 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated primates and 6-hydroxydopamine (6-OHDA)-treated rats. The mechanism hypothesized to be responsible for effect of DBS on PD symptom expression has changed dramatically over the years. Initially, the similarity in the effect of ablation and stimulation led to the assumption that the stimulation functions as a “temporary lesion” of the implanted nucleus, a concept supported by some early physiological studies (Boraud et al. 1996; Dostrovsky et al. 2000). Later studies, however, showed that the stimulation in either the STN or GPi does not inhibit the nuclei, but rather leads to a partial temporal locking of neuronal activity to the stimulation pulses (Bar-Gad et al. 2004; Hashimoto et al. 2003). These conflicting results illustrate the dissociation between the inhibition of the stimulated nucleus and the locking of downstream targets via axonal activation, which was later confirmed in theoretical and experimental studies (McIntyre et al. 2004a; Miocinovic et al. 2006; Moran et al. 2011). The changes in the firing pattern associated with the stimulation lead to reduced oscillatory activity (Moran et al. 2012) and changes in the bursting activity of individual neurons (Hahn et al. 2008). This change in regularity was also assessed in some cases by using measures associated with information theory, such as the spike train entropy (Dorval et al. 2008). Overall, the stimulation functions to regularize single-neuron activity by reducing the abnormal pattern transmission through the stimulated nucleus. In addition to changes in the activity of the neurons, the stimulation leads to changes in the interaction between neurons within the same nucleus as well as between nuclei. This is reflected in decreased coherence between neurons, both single units and multiunits, and in larger neuronal populations as evidenced by the LFP (Brown et al. 2004; Meissner et al. 2005; Moran et al. 2012). This is consistent with theoretical models showing that a stimulation-induced reduction in the oscillatory activity of the neurons in conjunction with reduced synchrony results in increased fidelity of the thalamocortical transmission, enabling information flow to the cortex (Guo et al. 2008; Rubin and Terman 2004). Finally, the effect of the stimulation is not static, but rather evolves over time (Erez et al. 2009; McCairn and Turner 2009; Moran et al. 2011), leading to decreased synaptic efficacy within the stimulated pathway. The diverse effects of the stimulation on the stimulated nucleus, its downstream targets, and additional brain areas stem directly from the complex effect of the stimulation on multiple neuronal elements around the contacts of the electrodes, including somas, efferent, and afferent axons and additional axons in passing (Lemon 1984; McIntyre et al. 2004a, 2004b). Attributing these different physiological and clinical changes to different elements has been a daunting task, which is being addressed, at least partially, by the use of optogenetic tools (Gradinaru et al. 2009).
Neurophysiological effect of DBS in TS.
The vast majority of the 100+ reported cases of TS DBS have focused on the clinical outcomes and the effect of different stimulation targets, whereas only a few have examined the effect of DBS on neuronal activity. Stimulation in the thalamus was shown to modulate abnormal LFP oscillatory activity within the thalamus in a few (but not all) patients by decreasing the power in the low (≤10 Hz) frequencies (Bour et al. in press) and increasing the power in higher (25–45 Hz) frequencies, which correlated to the improvement in the symptoms (Maling et al. 2012). These changes may be related to dopaminergic modulation, as demonstrated using PET, following thalamic stimulation (Kuhn et al. 2012). Stimulation in the GPi homolog of anesthetized normal rats revealed similar results, namely, an increase in low gamma-band activity and a decrease in low beta-band activity in both the M1 and the striatum (McCracken and Kiss 2014). However, stimulation in an upstream nucleus, the NAc, of anesthetized normal rats resulted in enhanced beta- and gamma-band oscillations in the lateral orbitofrontal cortex, the medial prefrontal cortex, and the MD thalamus (McCracken and Grace 2009). It remains unclear whether persistent changes occur following DBS. A follow-up study on one patient found a long-lasting effect of DBS in that the clinical improvement remained present for 4 years after the stimulation was turned off (Dong et al. 2014). However, another study did not find changes in thalamic LFP oscillatory activity once the stimulator stopped working (Priori et al. 2013). A similar result was reported for PD patients undergoing STN DBS surgery (Giannicola et al. 2012), indicating that DBS may not necessarily induce persistent changes within the CBG loop.
Currently, primarily due to technical difficulties, there have been no studies of the effect of DBS on single-neuron activity in human patients. A small number of DBS studies have been conducted on animal models of TS. The mechanism of DBS that they revealed was generally consistent with the one observed in PD. Stimulation in the GPi results in a partial temporal locking of the neuronal responses to the stimulation pulses both in the stimulated nucleus and in the upstream nucleus, the GPe (McCairn et al. 2013b). This temporal locking abolishes the tic-related activity of the neurons and for a short time period increases the overall firing rate of the nucleus (McCairn et al. 2012, 2013b). Thus, by influencing the firing patterns, the stimulation reduces the propagation of abnormal activity from the BG downstream to the thalamus and cortical areas (Fig. 2). In addition, the neuronal activity response to the stimulation pulses was shown to be dynamic and evolved over time (McCairn et al. 2013b), indicating that short-term plasticity plays a role in DBS function. Similar results were found in a GPi stimulation study in one normal primate (Bar-Gad and Turner 2009), supporting the notion that the DBS mechanism is similar across clinical states. In addition, in a normal primate study it was found that the stimulation did not change task-encoding neuronal activity. Thus it enabled the flow of acute behavioral information throughout the BG, despite the changes that occurred in the neuronal activity patterns. In TS animal models, the stimulation induced significant neurophysiological changes but did not cause major behavioral changes. Stimulation in the GPi did not reduce the sniffing (in the dopaminergic model) or the tic frequency (in the striatal disinhibition model) but decreased the magnitude of the tics and improved sensorimotor gating deficits (McCairn et al. 2012, 2013b; Posch et al. 2012). Behavioral effects were found immediately following STN stimulation in animals expressing stereotypic behaviors but not tics (Baup et al. 2008). The lack of immediate influence on tic frequency in animal models may be due to the short stimulation periods (seconds to minutes) that were used in these studies. This is in line with the reports that DBS stimulation in TS patients significantly reduced tic frequency with a latency of several months (Zhang et al. 2014). Studies of long-term neurophysiological changes underlying the progressive effects of DBS in TS are still missing. Translational studies of long-term DBS in animal models displaying chronic symptoms may provide new insights into this evolving therapeutic process.
Fig. 2.
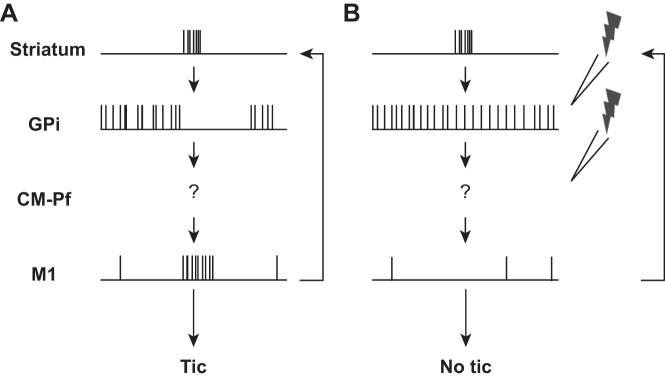
Tic encoding in the CBG pathway. Schematic illustration of neuronal activity during tic expression (A) and during DBS (B).
Overall, studies have only started recently to address the mechanism of DBS on neuronal activity in TS. Although extensive studies are still needed, results from both human and animal models are starting to accumulate. These results suggest that DBS alters the firing pattern, rather than the firing rate, of neurons in that neuronal activity has been found to be partially temporally locked to the stimulation pulses (Fig. 2). Thus DBS exerts its effect by causing functional ablation, which reduces information flow through the stimulated nucleus. The similarity between findings on TS, PD, and stimulation in normal animals support the notion that the mechanism of DBS is comparable regardless of the pathological state or region of stimulation.
Summary and Conclusions
The growing popularity of DBS treatment as a therapeutic option for controlling the symptoms of different motor and psychiatric disorders, combined with the severe limitations of pharmaceutical therapies for TS, has led to an increasing number of exploratory DBS implantation surgeries. Targets for the DBS leads vary but are primarily situated in different targets along the CBG pathway: the GPi, thalamic nuclei, and the NAc. These targets are part of either the motor pathway, presumably directly involved in tic generation, or the limbic pathway, presumably involved in the comorbidities and indirectly involved in tic generation via the premonitory urge. Early stimulation results show significant improvement in TS symptom expression following these surgeries. The improvements increase over the timeframe of weeks and months following the surgery and persist for years. Given this initial success, future progress will require further insights into the abnormal neuronal activity during TS and the ways in which this activity is modulated using DBS.
Physiological studies have reported system-wide changes throughout the CBG pathway, rather than focal neuronal abnormalities. These changes consist of an altered baseline level activity of individual neurons and whole nuclei, including changes to both firing rate and pattern. This is accompanied by abnormal interactions between cortical and thalamic areas with the BG. Finally, tic-related activity is expressed throughout the pathway, both preceding and following the expression of tics. Although the converging experimental data are mostly coherent, it is crucial to bear in mind the limitations of the current data set, which is primarily based on indirect noninvasive measures with only very few invasive recordings in human DBS patients and in only a small subset of the available animal models. Moreover, although a data set is gradually building up on the neuronal abnormalities along the motor pathways related to tic expression, little is currently known about the neuronal activity along the limbic pathways related to the premonitory urge. The modulation of this abnormal neuronal activity throughout the CBG pathway during DBS is complex and arises from the modulation of diverse neuronal populations. Most current thinking about this modulation comes indirectly from the more abundant recordings in PD patients as well as from studies in normal and disorder model animals. These studies emphasize that stimulation affects the firing pattern of individual neurons by partially entraining the neuronal firing to the stimulation pulses and a reduction in the transmission of the abnormal activity patterns, which is at least partially due to a depression of synaptic efficacy along the pathway. Moreover, the stimulation-based modulation presumably functions on the correlated neuronal activity, hence reducing the loss of specificity induced by the striatal disinhibition.
The recent proliferation of new data stemming from both human patients and novel animal models have made the last decade a watershed in our understanding of TS pathophysiology. These novel physiological data constitute a crucial platform to advance stimulation-based treatments from their current exploratory nature to standard clinical practice by supplying new measures of the neuronal modulation provided by DBS in TS.
GRANTS
This study was supported in part by Israel Science Foundation (ISF) Grant 743/13 and a Tourette Syndrome Association (TSA) grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.I., Y.L., and I.B.-G. conception and design of research; M.I. and I.B.-G. prepared figures; M.I., Y.L., and I.B.-G. drafted manuscript; M.I., Y.L., and I.B.-G. edited and revised manuscript; M.I., Y.L., and I.B.-G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. N. Ben-Aroya for helpful discussions.
REFERENCES
- Abelson JF, Kwan KY, O'Roak BJ, Baek DY, Stillman AA, Morgan TM, Mathews CA, Pauls DL, Rasin MR, Gunel M, Davis NR, Ercan-Sencicek AG, Guez DH, Spertus JA, Leckman JF, Dure LS, Kurlan R, Singer HS, Gilbert DL, Farhi A, Louvi A, Lifton RP, Sestan N, State MW. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science 310: 317–320, 2005a. [DOI] [PubMed] [Google Scholar]
- Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, Martis B, Giordani B. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry 57: 510–516, 2005b. [DOI] [PubMed] [Google Scholar]
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA 93: 1945–1949, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermans L, Duits A, van der Linden C, Tijssen M, Schruers K, Temel Y, Kleijer M, Nederveen P, Bruggeman R, Tromp S, van Kranen-Mastenbroek V, Kingma H, Cath D, Visser-Vandewalle V. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain 134: 832–844, 2011. [DOI] [PubMed] [Google Scholar]
- Ackermans L, Temel Y, Cath D, van der Linden C, Bruggeman R, Kleijer M, Nederveen P, Schruers K, Colle H, Tijssen MAJ, Visser-Vandewalle V. Deep brain stimulation in Tourette's syndrome: two targets? Mov Disord 21: 709–713, 2006. [DOI] [PubMed] [Google Scholar]
- Albin RL, Koeppe RA, Bohnen NI, Nichols TE, Meyer P, Wernette K, Minoshima S, Kilbourn MR, Frey KA. Increased ventral striatal monoaminergic innervation in Tourette syndrome. Neurology 61: 310–315, 2003. [DOI] [PubMed] [Google Scholar]
- Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends Neurosci 29: 175–182, 2006. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13: 266–271, 1990. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381, 1986. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR. Microstimulation of the primate neostriatum. II. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response properties. J Neurophysiol 53: 1417–1430, 1985. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (5th ed). Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
- Aouizerate B, Cuny E, Martin-Guehl C, Guehl D, Amieva H, Benazzouz A, Fabrigoule C, Allard M, Rougier A, Bioulac B, Tignol J, Burbaud P. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg 101: 682–686, 2004. [DOI] [PubMed] [Google Scholar]
- Bar-Gad I, Elias S, Vaadia E, Bergman H. Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation. J Neurosci 24: 7410–7419, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Gad I, Turner RS. Stimulation effect on neuronal activity in the globus pallidus of the behaving macaque. In: The Basal Ganglia IX, Advances in Behavioral Biology, edited by Groenewegen HJ, Voorn P, Berendse HW, Mulder AB, Cools AR. New York: Springer, 2009, p. 73–83. [Google Scholar]
- Baup N, Grabli D, Karachi C, Mounayar S, François C, Yelnik J, Féger J, Tremblay L. High-frequency stimulation of the anterior subthalamic nucleus reduces stereotyped behaviors in primates. J Neurosci 28: 8785–8788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baym CL, Corbett BA, Wright SB, Bunge SA. Neural correlates of tic severity and cognitive control in children with Tourette syndrome. Brain 131: 165–179, 2008. [DOI] [PubMed] [Google Scholar]
- Bedard P, Pycock CJ. “Wet-dog” shake behaviour in the rat: a possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology 16: 663–670, 1977. [DOI] [PubMed] [Google Scholar]
- Beiser DG, Houk JC. Model of cortical-basal ganglionic processing: encoding the serial order of sensory events. J Neurophysiol 79: 3168–3188, 1998. [DOI] [PubMed] [Google Scholar]
- Benabid A, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, De Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337: 403–406, 1991. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience 62: 707–719, 1994. [DOI] [PubMed] [Google Scholar]
- Bergstrom DA, Bromley SD, Walters JR. Apomorphine increases the activity of rat globus pallidus neurons. Brain Res 238: 266–271, 1982. [DOI] [PubMed] [Google Scholar]
- Berns GS, Sejnowski TJ. A computational model of how the basal ganglia produce sequences. J Cogn Neurosci 10: 108–121, 1998. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW, Houchard KR, Zhuang X. Sequential super-stereotypy of an instinctive fixed action pattern in hyper-dopaminergic mutant mice: a model of obsessive compulsive disorder and Tourette's. BMC Biol 3: 4, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology 65: 1253–1258, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, Wurzman R, Hallett M. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain 129: 2029–2037, 2006. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross C. High frequency stimulation of the internal globus pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci Lett 215: 17–20, 1996. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross CE. Dopamine agonist-induced dyskinesias are correlated to both firing pattern and frequency alterations of pallidal neurones in the MPTP-treated monkey. Brain 124: 546–557, 2001. [DOI] [PubMed] [Google Scholar]
- Bour LJ, Ackermans L, Foncke EM, Cath D, van der Linden C, Visser Vandewalle V, Tijssen MA. Tic related local field potentials in the thalamus and the effect of deep brain stimulation in Tourette syndrome: report of three cases. Clin Neurophysiol. In press. [DOI] [PubMed] [Google Scholar]
- Bronfeld M, Belelovsky K, Bar-Gad I. Spatial and temporal properties of tic-related neuronal activity in the cortico-basal ganglia loop. J Neurosci 31: 8713–8721, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfeld M, Belelovsky K, Erez Y, Bugaysen J, Korngreen A, Bar-Gad I. Bicuculline-induced chorea manifests in focal rather than globalized abnormalities in the activation of the external and internal globus pallidus. J Neurophysiol 104: 3261–3275, 2010. [DOI] [PubMed] [Google Scholar]
- Bronfeld M, Israelashvili M, Bar-Gad I. Pharmacological animal models of Tourette syndrome. Neurosci Biobehav Rev 37: 1101–1119, 2013a. [DOI] [PubMed] [Google Scholar]
- Bronfeld M, Yael D, Belelovsky K, Bar-Gad I. Motor tics evoked by striatal disinhibition in the rat. Front Syst Neurosci 7: 50, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, Di Lazzaro V. Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson's disease. Exp Neurol 188: 480–490, 2004. [DOI] [PubMed] [Google Scholar]
- Burguière E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science 340: 1–15, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KM, de Lecea L, Severynse DM, Caron MG, McGrath MJ, Sparber SB, Sun LY, Burton FH. OCD-Like behaviors caused by a neuropotentiating transgene targeted to cortical and limbic D1+ neurons. J Neurosci 19: 5044–5053, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon E, Silburn P, Coyne T, O'Maley K, Crawford JD, Sachdev PS. Deep brain stimulation of anteromedial globus pallidus interna for severe Tourette's syndrome. Am J Psychiatry 169: 860–866, 2012. [DOI] [PubMed] [Google Scholar]
- Carasso BS, Bakshi VP, Geyer MA. Disruption in prepulse inhibition after alpha-1 adrenoceptor stimulation in rats. Neuropharmacology 37: 401–404, 1998. [DOI] [PubMed] [Google Scholar]
- Castellan Baldan L, Williams KA, Gallezot JD, Pogorelov V, Rapanelli M, Crowley M, Anderson GM, Loring E, Gorczyca R, Billingslea E, Wasylink S, Panza KE, Ercan-Sencicek AG, Krusong K, Leventhal BL, Ohtsu H, Bloch MH, Hughes ZA, Krystal JH, Mayes L, de Araujo I, Ding YS, State MW, Pittenger C. Histidine decarboxylase deficiency causes Tourette syndrome: parallel findings in humans and mice. Neuron 81: 77–90, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabardès S, Polosan M, Krack P, Bastin J, Krainik A, David O, Bougerol T, Benabid AL. Deep brain stimulation for obsessive-compulsive disorder: subthalamic nucleus target. World Neurosurg 80: S31.e1–S31.e8, 2013. [DOI] [PubMed] [Google Scholar]
- Cheon KA, Ryu YH, Namkoong K, Kim CH, Kim JJ, Lee JD. Dopamine transporter density of the basal ganglia assessed with [123I]IPT SPECT in drug-naive children with Tourette's disorder. Psychiatry Res 130: 85–95, 2004. [DOI] [PubMed] [Google Scholar]
- Crane J, Fagerness J, Osiecki L, Gunnell B, Stewart SE, Pauls DL, Scharf JM, the Tourette Syndrome International Consortium for Genetics. Family-based genetic association study of DLGAP3 in Tourette syndrome. Am J Med Genet Part B Neuropsychiatr Genet 156: 108–114, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman AR, Mitchell IJ, Sambrook MA, Jackson A. Chorea and myoclonus in the monkey induced by gamma-aminobutyric acid antagonism in the lentiform complex. The site of drug action and a hypothesis for the neural mechanisms of chorea. Brain 111: 1211–1233, 1988. [DOI] [PubMed] [Google Scholar]
- Darbin O, Wichmann T. Effects of striatal GABA A-receptor blockade on striatal and cortical activity in monkeys. J Neurophysiol 99: 1294–1305, 2008. [DOI] [PubMed] [Google Scholar]
- Dehning S, Mehrkens JH, Müller N, Bötzel K. Therapy-refractory Tourette syndrome: beneficial outcome with globus pallidus internus deep brain stimulation. Mov Disord 23: 1300–1302, 2008. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281–285, 1990. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Crutcher MD, Georgopoulos AP. Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol 53: 530–543, 1985. [DOI] [PubMed] [Google Scholar]
- Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, Bosch A, Schuurman R. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 67: 1061–1068, 2010. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Kalteis K, Stamenkovic M, Pieri V, Alesch F. Efficient internal pallidal stimulation in Gilles de la Tourette syndrome: a case report. Mov Disord 20: 1496–1499, 2005. [DOI] [PubMed] [Google Scholar]
- Dong S, Zhang X, Li J, Li Y. Unexpected outcome of pallidal deep brain stimulation in a patient with Tourette syndrome. Acta Neurochir (Wien) 156: 1527–1528, 2014. [DOI] [PubMed] [Google Scholar]
- Dorval AD, Russo GS, Hashimoto T, Xu W, Grill WM, Vitek JL. Deep brain stimulation reduces neuronal entropy in the MPTP-primate model of Parkinson's disease. J Neurophysiol 100: 2807–2818, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostrovsky JO, Levy R, Wu JP, Hutchison WD, Tasker RR, Lozano AM. Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol 84: 570–574, 2000. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci 28: 7143–7152, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy CM, Rickards HE, Cavanna AE. Treatment strategies for tics in Tourette syndrome. Ther Adv Neurol Disord 4: 25–45, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsáki G, Deisseroth K, Tepper JM, Koos T. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci 15: 123–130, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O'Roak BJ, Mason CE, Abbott T, Gupta A, King RA, Pauls DL, Tischfield JA, Heiman GA, Singer HS, Gilbert DL, Hoekstra PJ, Morgan TM, Loring E, Yasuno K, Fernandez T, Sanders S, Louvi A, Cho JH, Mane S, Colangelo CM, Biederer T, Lifton RP, Gunel M, State MW. l-Histidine decarboxylase and Tourette's syndrome. N Engl J Med 362: 1901–1908, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez Y, Czitron H, McCairn K, Belelovsky K, Bar-Gad I. Short-term depression of synaptic transmission during stimulation in the globus pallidus of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates. J Neurosci 29: 7797–7802, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini G, Pasquini M, Aurilia C, Berardelli I, Breedveld G, Oostra BA, Bonifati V, Berardelli A. A large Italian family with Gilles de la Tourette syndrome: clinical study and analysis of the SLITRK1 gene. Mov Disord 22: 2229–2234, 2007. [DOI] [PubMed] [Google Scholar]
- Flaherty AW, Williams ZM, Amirnovin R, Kasper E, Rauch SL, Cosgrove GR, Eskandar EN. Deep brain stimulation of the anterior internal capsule for the treatment of Tourette syndrome: technical case report. Neurosurgery 57, Suppl 4: E403, 2005. [DOI] [PubMed] [Google Scholar]
- François C, Grabli D, McCairn K, Jan C, Karachi C, Hirsch EC, Féger J, Tremblay L. Behavioural disorders induced by external globus pallidus dysfunction in primates. II. Anatomical study. Brain 127: 2055–2070, 2004. [DOI] [PubMed] [Google Scholar]
- Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countries. Dev Med Child Neurol 42: 436–447, 2000. [DOI] [PubMed] [Google Scholar]
- Fujita S, Kato R, Cui Y, Terakado M, Suga K, Koshikawa N, Kobayashi M. Apomorphine-induced modulation of neural activities in the ventrolateral striatum of rats. Synapse 67: 363–373, 2013. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Synaptic organization of the striatum. J Electron Microsc Tech 10: 265–281, 1988. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250: 1429–1432, 1990. [DOI] [PubMed] [Google Scholar]
- Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotox Res 10: 211–220, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannicola G, Rosa M, Servello D, Menghetti C, Carrabba G, Pacchetti C, Zangaglia R, Cogiamanian F, Scelzo E, Marceglia S, Rossi L, Priori A. Subthalamic local field potentials after seven-year deep brain stimulation in Parkinson's disease. Exp Neurol 237: 312–317, 2012. [DOI] [PubMed] [Google Scholar]
- Gilles de la Tourette G. Etude sur une affection nerveuse caracterisee par de l'incoordination motrice accompagnee d'echolalie et de copralalie. Arch Neurol 9: 19–42, 1885. [Google Scholar]
- Godar SC, Mosher LJ, Di Giovanni G, Bortolato M. Animal models of tic disorders: a translational perspective. J Neurosci Methods 238: 54–69, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Foote KD, Greenberg BD, Ricciuti N, Bauer R, Ward H, Shapira NA, Wu SS, Hill CL, Rasmussen SA, Okun MS. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry 67: 535–542, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science 324: 354–359, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Gabriels LA, Malone DA, Rezai AR, Friehs GM, Okun MS, Shapira NA, Foote KD, Cosyns PR, Kubu CS, Malloy PF, Salloway SP, Giftakis JE, Rise MT, Machado AG, Baker KB, Stypulkowski PH, Goodman WK, Rasmussen SA, Nuttin BJ. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry 15: 64–79, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, Salloway SP, Okun MS, Goodman WK, Rasmussen SA. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology 31: 2384–2393, 2006. [DOI] [PubMed] [Google Scholar]
- Guo Y, Rubin JE, McIntyre CC, Vitek JL, Terman D. Thalamocortical relay fidelity varies across subthalamic nucleus deep brain stimulation protocols in a data-driven computational model. J Neurophysiol 99: 1477–1492, 2008. [DOI] [PubMed] [Google Scholar]
- Guridi J, Lozano AM. A brief history of pallidotomy. Neurosurgery 41: 1169–1180, 1997. [DOI] [PubMed] [Google Scholar]
- Gurney K, Prescott TJ, Redgrave P. A computational model of action selection in the basal ganglia. I. A new functional anatomy. Biol Cybern 84: 401–410, 2001. [DOI] [PubMed] [Google Scholar]
- Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist 7: 315–324, 2001. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 20: 2369–2382, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn PJ, Russo GS, Hashimoto T, Miocinovic S, Xu W, McIntyre CC, Vitek JL. Pallidal burst activity during therapeutic deep brain stimulation. Exp Neurol 211: 243–251, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, King RA, Constable RT, Leckman JF. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry 65: 594–599, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci 23: 1916–1923, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science 259: 819–821, 1993. [DOI] [PubMed] [Google Scholar]
- Houeto JL, Karachi C, Mallet L, Pillon B, Yelnik J, Mesnage V, Welter ML, Navarro S, Pelissolo A, Damier P, Pidoux B, Dormont D, Cornu P, Agid Y. Tourette's syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry 76: 992–995, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol 8: 844–856, 2009. [DOI] [PubMed] [Google Scholar]
- Kaido T, Otsuki T, Kaneko Y, Takahashi A, Omori M, Okamoto T. Deep brain stimulation for Tourette syndrome: a prospective pilot study in Japan. Neuromodulation 14: 123–129, 2011. [DOI] [PubMed] [Google Scholar]
- Kalanithi PSA, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci USA 102: 13307–13312, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PSA, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol 518: 277–291, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Yamada K, Ornthanalai VG, Inoue T, Ota M, Murphy NP, Aruga J. Slitrk1-deficient mice display elevated anxiety-like behavior and noradrenergic abnormalities. Mol Psychiatry 15: 177–184, 2010. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of 3 classes of interneurons in rat neostriatum. J Neurosci 13: 4908–4923, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalopoulou Z, Zrinzo L, Jahanshahi M, Candelario J, Milabo C, Beigi M, Akram H, Hyam J, Clayton J, Kass-Iliyya L, Silverdale M, Evans J, Limousin P, Hariz M, Joyce E, Foltynie T. Bilateral globus pallidus stimulation for severe Tourette's syndrome: a double-blind, randomised crossover trial. Lancet Neurol 4422: 1–11, 2015. [DOI] [PubMed] [Google Scholar]
- Kelland MD, Soltis RP, Anderson LA, Bergstrom DA, Walters JR. In vivo characterization of two cell types in the rat globus pallidus which have opposite responses to dopamine receptor stimulation: comparison of electrophysiological properties and responses to apomorphine, dizocilpine, and ketamine anesthesia. Synapse 20: 338–350, 1995. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Lang CG, Gauthier AM. Induction of oral stereotypy following amphetamine microinjection into a discrete subregion of the striatum. Psychopharmacology (Berl) 95: 556–559, 1988. [DOI] [PubMed] [Google Scholar]
- Kim JP, Min HK, Knight EJ, Duffy PS, Abulseoud OA, Marsh MP, Kelsey K, Blaha CD, Bennet KE, Frye MA, Lee KH. Centromedian-parafascicular deep brain stimulation induces differential functional inhibition of the motor, associative, and limbic circuits in large animals. Biol Psychiatry 74: 917–926, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Rajkowski J, Evarts E. Tonically discharging putamen neurons exhibit set-dependent responses. Proc Natl Acad Sci USA 81: 4998–5001, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kosaka T, Heizmann CW. Parvalbumin-immunoreactive neurons in the rat neostriatum: a light and electron microscopic study. Brain Res 536: 1–15, 1990. [DOI] [PubMed] [Google Scholar]
- Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol 47: 77–90, 2012. [DOI] [PubMed] [Google Scholar]
- Kokkinidis L, Anisman H. Amphetamine psychosis and schizophrenia: a dual model. Neurosci Biobehav Rev 5: 449–461, 1981. [DOI] [PubMed] [Google Scholar]
- Koós T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 2: 467–472, 1999. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Anderson LA, Walters JR. Apomorphine and dopamine D1 receptor agonists increase the firing rates of subthalamic nucleus neurons. Neuroscience 72: 863–876, 1996. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Ito C, Sakurai E, Sakurai E, Watanabe T, Ohtsu H. Increased methamphetamine-induced locomotor activity and behavioral sensitization in histamine-deficient mice. J Neurochem 83: 837–845, 2002. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Janouschek H, Raptis M, Rex S, Lenartz D, Neuner I, Mottaghy FM, Schneider F, Schaefer WM, Sturm V, Gründer G, Vernaleken I. In vivo evidence of deep brain stimulation-induced dopaminergic modulation in Tourette's syndrome. Biol Psychiatry 71: 11–13, 2012. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Lenartz D, Mai JK, Huff W, Lee SH, Koulousakis A, Klosterkoetter J, Sturm V. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome. J Neurol 254: 963–965, 2007. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette's syndrome. Am J Psychiatry 150: 98–102, 1993. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Methods for Neuronal Recording in Conscious Animals. New York: Wiley, 1984. [Google Scholar]
- Lerner A, Bagic A, Simmons JM, Mari Z, Bonne O, Xu B, Kazuba D, Herscovitch P, Carson RE, Murphy DL, Drevets WC, Hallett M. Widespread abnormality of the γ-aminobutyric acid-ergic system in Tourette syndrome. Brain 135: 1926–1936, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 345: 91–95, 1995. [DOI] [PubMed] [Google Scholar]
- Lind NM, Arnfred SM, Hemmingsen RP, Hansen AK. Prepulse inhibition of the acoustic startle reflex in pigs and its disruption by d-amphetamine. Behav Brain Res 155: 217–22, 2004. [DOI] [PubMed] [Google Scholar]
- Lippitz BE, Mindus P, Meyerson BA, Kihlström L, Lindquist C. Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive-compulsive disorder: relevance of the right hemisphere. Neurosurgery 44: 452–460, 1999. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li W, Tan C, Liu X, Wang X, Gui Y, Qin L, Deng F, Hu C, Chen L. Meta-analysis comparing deep brain stimulation of the globus pallidus and subthalamic nucleus to treat advanced Parkinson disease. J Neurosurg 121: 709–718, 2014. [DOI] [PubMed] [Google Scholar]
- Maciunas RJ, Maddux BN, Riley DE, Whitney CM, Schoenberg MR, Ogrocki PJ, Albert JM, Gould DJ. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg 107: 1004–1014, 2007. [DOI] [PubMed] [Google Scholar]
- Maling N, Hashemiyoon R, Foote KD, Okun MS, Sanchez JC. Increased thalamic gamma band activity correlates with symptom relief following deep brain stimulation in humans with Tourette's syndrome. PLoS One 7: e44215, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, du Montcel ST, Yelnik J, Chéreau I, Arbus C, Raoul S, Aouizerate B, Damier P, Chabardès S, Czernecki V, Ardouin C, Krebs MO, Bardinet E, Chaynes P, Burbaud P, Cornu P, Derost P, Bougerol T, Bataille B, Mattei V, Dormont D, Devaux B, Vérin M, Houeto JL, Pollak P, Benabid AL, Agid Y, Krack P, Millet B, Pelissolo A. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med 359: 2121–2134, 2008. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology (Berl) 94: 507–514, 1988. [DOI] [PubMed] [Google Scholar]
- Marceglia S, Servello D, Foffani G, Porta M, Sassi M, Mrakic-Sposta S, Rosa M, Barbieri S, Priori A. Thalamic single-unit and local field potential activity in Tourette syndrome. Mov Disord 25: 300–308, 2010. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Meldrum BS, Pycock C, Tarsy D. Focal myoclonus produced by injection of picrotoxin into the caudate nucleus of the rat. J Physiol 246: 96P, 1975. [PubMed] [Google Scholar]
- Martinez-Fernandez R, Zrinzo L, Aviles-Olmos I, Hariz M, Martinez-Torres I, Joyce E, Jahanshahi M, Limousin P, Foltynie T. Deep brain stimulation for Gilles de la Tourette syndrome: a case series targeting subregions of the globus pallidus internus. Mov Disord 26: 1922–1930, 2011. [DOI] [PubMed] [Google Scholar]
- Martinez-Torres I, Hariz MI, Zrinzo L, Foltynie T, Limousin P. Improvement of tics after subthalamic nucleus deep brain stimulation. Neurology 72: 1787–9, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn KW, Bronfeld M, Belelovsky K, Bar-Gad I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain 132: 2125–2138, 2009. [DOI] [PubMed] [Google Scholar]
- McCairn KW, Iriki A, Isoda M. High-frequency pallidal stimulation eliminates tic-related neuronal activity in a nonhuman primate model of Tourette syndrome. Neuroreport 23: 206–210, 2012. [DOI] [PubMed] [Google Scholar]
- McCairn KW, Iriki A, Isoda M. Global dysrhythmia of cerebro-basal ganglia-cerebellar networks underlies motor tics following striatal disinhibition. J Neurosci 33: 697–708, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn KW, Iriki A, Isoda M. Deep brain stimulation reduces tic-related neural activity via temporal locking with stimulus pulses. J Neurosci 33: 6581–6593, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn KW, Turner RS. Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. J Neurophysiol 101: 1941–1960, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci 29: 5354–5363, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken CB, Kiss ZH. Time and frequency-dependent modulation of local field potential synchronization by deep brain stimulation. PLoS One 9: e102576, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol 91: 1457–1469, 2004a. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol 115: 1239–1248, 2004b. [DOI] [PubMed] [Google Scholar]
- Meissner W, Leblois A, Hansel D, Bioulac B, Gross CE, Benazzouz A, Boraud T. Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain 128: 2372–2382, 2005. [DOI] [PubMed] [Google Scholar]
- Miller AM, Bansal R, Hao X, Sanchez-Pena JP, Sobel LJ, Liu J, Xu D, Zhu H, Chakravarty MM, Durkin K, Ivanov I, Plessen KJ, Kellendonk CB, Peterson BS. Enlargement of thalamic nuclei in Tourette syndrome. Arch Gen Psychiatry 67: 955–964, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425, 1996. [DOI] [PubMed] [Google Scholar]
- Mink JW. Basal ganglia dysfunction in Tourette's syndrome: a new hypothesis. Pediatr Neurol 25: 190–198, 2001. [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Parent M, Butson CR, Hahn PJ, Russo GS, Vitek JL, McIntyre CC. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. J Neurophysiol 96: 1569–1580, 2006. [DOI] [PubMed] [Google Scholar]
- Moran A, Stein E, Tischler H, Bar-Gad I. Decoupling neuronal oscillations during subthalamic nucleus stimulation in the parkinsonian primate. Neurobiol Dis 45: 583–590, 2012. [DOI] [PubMed] [Google Scholar]
- Moran A, Stein E, Tischler H, Belelovsky K, Bar-Gad I. Dynamic stereotypic responses of basal ganglia neurons to subthalamic nucleus high-frequency stimulation in the parkinsonian primate. Front Syst Neurosci 5: 21, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motlagh MG, Smith ME, Landeros-Weisenberger A, Kobets AJ, King RA, Miravite J, de Lotbiniere AC, Alterman RL, Mogilner AY, Pourfar MH, Okun MS, Leckman JF. Lessons learned from open-label deep brain stimulation for Tourette syndrome: eight cases over 7 years. Tremor Other Hyperkinet Mov (NY), 3, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S, Yoshida M, Nakamura S. Electrophysiological study of dyskinesia produced by microinjection of picrotoxin into the striatum of the rat. Neurosci Res 7: 369–380, 1990. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Ikeuchi Y, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol 84: 289–300, 2000. [DOI] [PubMed] [Google Scholar]
- Neuner I, Werner CJ, Arrubla J, Stöcker T, Ehlen C, Wegener HP, Schneider F, Shah NJ. Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Front Hum Neurosci 8: 362, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevet A, Morris G, Saban G, Fainstein N, Bergman H. Discharge rate of substantia nigra pars reticulata neurons is reduced in non-parkinsonian monkeys with apomorphine-induced orofacial dyskinesia. J Neurophysiol 92: 1973–1981, 2004. [DOI] [PubMed] [Google Scholar]
- Nordstrom EJ, Burton FH. A transgenic model of comorbid Tourette's syndrome and obsessive-compulsive disorder circuitry. Mol Psychiatry 7: 617–625, 2002. [DOI] [PubMed] [Google Scholar]
- Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet 354: 1526, 1999. [DOI] [PubMed] [Google Scholar]
- Nuttin BJ, Gabriëls LA, Cosyns PR, Meyerson BA, Andréewitch S, Sunaert SG, Maes AF, Dupont PJ, Gybels JM, Gielen F, Demeulemeester HG. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery 52: 1263–1274, 2003. [DOI] [PubMed] [Google Scholar]
- Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, Beute GN, van Vugt JP, Lenders MW, Contarino MF, Mink MS, Bour LJ, van den Munckhof P, Schmand BA, de Haan RJ, Schuurman PR, de Bie RM. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 12: 37–44, 2013. [DOI] [PubMed] [Google Scholar]
- Oguri T, Sawamoto N, Tabu H, Urayama S, Matsuhashi M, Matsukawa N, Ojika K, Fukuyama H. Overlapping connections within the motor cortico-basal ganglia circuit: fMRI-tractography analysis. Neuroimage 78: 353–362, 2013. [DOI] [PubMed] [Google Scholar]
- Okun MS, Foote KD, Wu SS, Ward HE, Bowers D, Rodriguez RL, Malaty IA, Goodman WK, Gilbert DM, Walker HC, Mink JW, Merritt S, Morishita T, Sanchez JC. A trial of scheduled deep brain stimulation for Tourette syndrome: moving away from continuous deep brain stimulation paradigms. JAMA Neurol 70: 85–94, 2013. [DOI] [PubMed] [Google Scholar]
- Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics 5: 320–330, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Rev 20: 128–154, 1995a. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev 20: 91–127, 1995b. [DOI] [PubMed] [Google Scholar]
- Patel S, Slater P. Analysis of the brain regions involved in myoclonus produced by intracerebral picrotoxin. Neuroscience 20: 687–693, 1987. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry 55: 326–333, 1998. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, King RA, Leckman JF, Staib L. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry 60: 415–424, 2003. [DOI] [PubMed] [Google Scholar]
- Piedimonte F, Andreani JC, Piedimonte L, Graff P, Bacaro V, Micheli F, Vilela Filho O. Behavioral and motor improvement after deep brain stimulation of the globus pallidus externus in a case of Tourette's syndrome. Neuromodulation 16: 55–58, 2013. [DOI] [PubMed] [Google Scholar]
- Pogorelov V, Xu M, Smith HR, Buchanan GF, Pittenger C. Corticostriatal interactions in the generation of tic-like behaviors after local striatal disinhibition. Exp Neurol 265: 122–128, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta M, Brambilla A, Cavanna AE, Servello D, Sassi M, Rickards H, Robertson MM. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology 73: 1375–1380, 2009. [DOI] [PubMed] [Google Scholar]
- Posch DK, Schwabe K, Krauss JK, Lütjens G. Deep brain stimulation of the entopeduncular nucleus in rats prevents apomorphine-induced deficient sensorimotor gating. Behav Brain Res 232: 130–136, 2012. [DOI] [PubMed] [Google Scholar]
- Price RA, Kidd KK, Cohen DJ, Pauls DL, Leckman JF. A twin study of Tourette syndrome. Arch Gen Psychiatry 42: 815–820, 1985. [DOI] [PubMed] [Google Scholar]
- Priori A, Giannicola G, Rosa M, Marceglia S, Servello D, Sassi M, Porta M. Deep brain electrophysiological recordings provide clues to the pathophysiology of Tourette syndrome. Neurosci Biobehav Rev 37: 1063–1068, 2013. [DOI] [PubMed] [Google Scholar]
- Randrup A, Munkvad I, Udsen P. Adrenergic mechanisms and amphetamine induced abnormal behaviour. Acta Pharmacol Toxicol (Copenh) 20: 145–157, 1963. [DOI] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci 20: 8559–8571, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, Kulisevsky J, Albanese A, Volkmann J, Hariz MI, Quinn NP, Speelman JD, Guridi J, Zamarbide I, Gironell A, Molet J, Pascual-Sedano B, Pidoux B, Bonnet AM, Agid Y, Xie J, Benabid AL, Lozano AM, Saint-Cyr J, Romito L, Contarino MF, Scerrati M, Fraix V, Van Blercom N. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain 128: 2240–2249, 2005. [DOI] [PubMed] [Google Scholar]
- Roessner V, Plessen KJ, Rothenberger A, Ludolph AG, Rizzo R, Skov L, Strand G, Stern JS, Termine C, Hoekstra PJ, Androutsos C, Aschauer H, Baird G, Bos-Veneman N, Brambilla A, Cardona F, Cath DC, Cavanna AE, Czernecki V, Dehning S, Eapter A, Farkas L, Gadaros J, Hartmann A, Hauser E, Heyman I, Hedderly T, Korsgaard A, Jackson GM, Larsson L, Martino D, Menghetti C, Debes NM, Muller N, Muller-Vahl K, Munchau A, Murphy T, Musil R, Nagy P, Nurnberger J, Oostra B, Paschou P, Pasquini M, Porta M, Rickards H, Robertson MM, Servello D, Tarnok Z, Van Der Griendt J, Verdellen C, Visser-Vandewalle V, Wannag E, Wolanczyck T. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: Pharmacological treatment. Eur Child Adolesc Psychiatry 20: 173–196, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JE, Terman D. High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J Comput Neurosci 16: 211–235, 2004. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Rymar VV. The primate centromedian-parafascicular complex: anatomical organization with a note on neuromodulation. Brain Res Bull 78: 122–130, 2009. [DOI] [PubMed] [Google Scholar]
- Schrock LE, Mink JW, Woods DW, Porta M, Servello D, Visser-Vandewalle V, Silburn PA, Foltynie T, Walker HC, Shahed-Jimenez J, Savica R, Klassen BT, Machado AG, Foote KD, Zhang JG, Hu W, Ackermans L, Temel Y, Mari Z, Changizi BK, Lozano A, Auyeung M, Kaido T, Agid Y, Welter ML, Khandhar SM, Mogilner AY, Pourfar MH, Walter BL, Juncos JL, Gross RE, Kuhn J, Leckman JF, Neimat JA, Okun MS. Tourette syndrome deep brain stimulation: a review and updated recommendations. Mov Disord 30: 448–471, 2015. [DOI] [PubMed] [Google Scholar]
- Schwalb JM, Hamani C. The history and future of deep brain stimulation. Neurotherapeutics 5: 3–13, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien DJ, Orth M, Evans AH, Lees AJ, Brown P. Motor inhibition in patients with Gilles de la Tourette syndrome: functional activation patterns as revealed by EEG coherence. Brain 128: 116–125, 2005. [DOI] [PubMed] [Google Scholar]
- Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry 79: 136–142, 2008. [DOI] [PubMed] [Google Scholar]
- Shields DC, Cheng ML, Flaherty AW, Gale JT, Eskandar EN. Microelectrode-guided deep brain stimulation for Tourette syndrome: within-subject comparison of different stimulation sites. Stereotact Funct Neurosurg 86: 87–91, 2008. [DOI] [PubMed] [Google Scholar]
- Siegfried J, Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery 35: 1126–1129, 1994. [DOI] [PubMed] [Google Scholar]
- Singer HS, Butler IJ, Tune LE, Seifert WE, Coyle JT. Dopaminergic dsyfunction in Tourette syndrome. Ann Neurol 12: 361–366, 1982. [DOI] [PubMed] [Google Scholar]
- Singer HS, Hahn IH, Moran TH. Abnormal dopamine uptake sites in postmortem striatum from patients with Tourette's syndrome. Ann Neurol 30: 558–562, 1991. [DOI] [PubMed] [Google Scholar]
- Singer HS, Minzer K. Neurobiology of Tourette's syndrome: concepts of neuroanatomic localization and neurochemical abnormalities. Brain Dev 25: S70–S84, 2003. [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology 33: 441–448, 1994. [DOI] [PubMed] [Google Scholar]
- Smith Y, Parent A. Neurons of the subthalamic nucleus in primates display glutamate but not GABA immunoreactivity. Brain Res 453: 353–356, 1988. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Kan E, Yoshii J, Thompson PM, Bansal R, Xu D, Toga AW, Peterson BS. Thinning of sensorimotor cortices in children with Tourette syndrome. Nat Neurosci 11: 637–639, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel EA, Wycis HT, Marks M, Lee AJ. Stereotaxic apparatus for operations on the human brain. Science 106: 349–350, 1947. [DOI] [PubMed] [Google Scholar]
- Stein E, Bar-Gad I. Beta oscillations in the cortico-basal ganglia loop during parkinsonism. Exp Neurol 245: 52–59, 2013. [DOI] [PubMed] [Google Scholar]
- Stern E, Silbersweig DA, Chee KY, Holmes A, Robertson MM, Trimble M, Frith CD, Frackowiak RS, Dolan RJ. A functional neuroanatomy of tics in Tourette syndrome. Arch Gen Psychiatry 57: 741–748, 2000. [DOI] [PubMed] [Google Scholar]
- Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, Klosterkötter J. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat 26: 293–299, 2003. [DOI] [PubMed] [Google Scholar]
- Tarsy D, Pycock CJ, Meldrum BS, Marsden CD. Focal contralateral myoclonus produced by inhibition of GABA action in the caudate nucleus of rats. Brain 101: 143–162, 1978. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol 14: 685–692, 2004. [DOI] [PubMed] [Google Scholar]
- Tinaz S, Belluscio BA, Malone P, van der Veen JW, Hallett M, Horovitz SG. Role of the sensorimotor cortex in Tourette syndrome using multimodal imaging. Hum Brain Mapp 35: 5834–5846, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuno H, Kimura M, Tanji J. Pallidal inputs to thalamocortical neurons projecting to the supplementary motor area: an anterograde and retrograde double labeling study in the macaque monkey. Exp Brain Res 90: 635–638, 1992. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Filion M. Responses of pallidal neurons to striatal stimulation in intact waking monkeys. Brain Res 498: 1–16, 1989. [DOI] [PubMed] [Google Scholar]
- Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet 353: 724, 1999. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Cath DC, van der Linde HC, Both J, Heutink P, Breedveld G, Aulchenko YS, Oostra BA. Genetic and clinical analysis of a large Dutch Gilles de la Tourette family. Mol Psychiatry 11: 954–964, 2006. [DOI] [PubMed] [Google Scholar]
- Visser-Vandewalle V, Temel Y, Boon P, Vreeling F, Colle H, Hoogland G, Groenewegen HJ, van der Linden C. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three cases. J Neurosurg 99: 1094–1100, 2003. [DOI] [PubMed] [Google Scholar]
- Viswanathan A, Jimenez-Shahed J, Baizabal Carvallo JF, Jankovic J. Deep brain stimulation for Tourette syndrome: target selection. Stereotact Funct Neurosurg 90: 213–224, 2012. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, Triche S, Mewes K, Hashimoto T, Bakay RA. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol 46: 22–35, 1999. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Swerdlow NR. Sensorimotor gating in rats is regulated by different dopamine-glutamate interactions in the nucleus accumbens core and shell subregions. Brain Res 722: 168–176, 1996. [DOI] [PubMed] [Google Scholar]
- Wang Z, Maia TV, Marsh R, Colibazzi T, Gerber A, Peterson BS. The neural circuits that generate tics in Tourette's syndrome. Am J Psychiatry 168: 1326–1337, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczak BL, Martin L, Boucher N, Zahr N, Sikes RW, Stellar JR. Electrophysiological and behavioral output of the rat basal ganglia after intrastriatal infusion of d-amphetamine: lack of support for the basal ganglia model. Brain Res 920: 170–182, 2001. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Feldon J. Environmental animal models for sensorimotor gating deficiencies in schizophrenia: a review. Psychopharmacology (Berl) 156: 305–326, 2001. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature 448: 894–900, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter ML, Mallet L, Houeto JL, Karachi C, Czernecki V, Cornu P, Navarro S, Pidoux B, Dormont D, Bardinet E, Yelnik J, Damier P, Agid Y. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol 65: 952–957, 2008. [DOI] [PubMed] [Google Scholar]
- Wickens J. A Theory of the Striatum. Oxford: Pergamon, 1993. [Google Scholar]
- Wong DF, Brasić JR, Singer HS, Schretlen DJ, Kuwabara H, Zhou Y, Nandi A, Maris MA, Alexander M, Ye W, Rousset O, Kumar A, Szabo Z, Gjedde A, Grace AA. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology 33: 1239–1251, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y, Baup N, Grabli D, Chaigneau M, Mounayar S, McCairn K, Féger J, Tremblay L. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb Cortex 19: 1844–1856, 2009. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Malherbe C, Hartmann A, Pélégrini-Issac M, Messé A, Vidailhet M, Lehéricy S, Benali H. Functional immaturity of cortico-basal ganglia networks in Gilles de la Tourette syndrome. Brain 135: 1937–1946, 2012. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Marrakchi-Kacem L, Lecomte S, Valabregue R, Poupon F, Guevara P, Tucholka A, Mangin JF, Vidailhet M, Lehericy S, Hartmann A, Poupon C. Altered structural connectivity of cortico-striato-pallido-thalamic networks in Gilles de la Tourette syndrome. Brain 138: 472–482, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JG, Ge Y, Stead M, Zhang K, Yan S, Hu W, Meng FG. Long-term outcome of globus pallidus internus deep brain stimulation in patients with Tourette syndrome. Mayo Clin Proc 89: 1506–1514, 2014. [DOI] [PubMed] [Google Scholar]
- Zhuang P, Hallett M, Zhang X, Li J, Zhang Y, Li Y. Neuronal activity in the globus pallidus internus in patients with tics. J Neurol Neurosurg Psychiatry 80: 1075–1081, 2009. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette's disorder: evidence from transcranial magnetic stimulation. Am J Psychiatry 154: 1277–1284, 1997. [DOI] [PubMed] [Google Scholar]


