Abstract
In elderly populations, diabetes is associated with reduced muscle strength, poor muscle quality, and accelerated loss of muscle mass. In addition, diabetes mellitus increases risk for accelerated aging and for the development of frailty syndrome. This disease is also associated with a polypathological condition, and its complications progressively affect quality of life and survival. Exercise interventions, including resistance training, represent the cornerstones of diabetes management, especially in patients at severe functional decline. This review manuscript aimed to describe the beneficial effects of different exercise interventions on the functional capacity of elderly diabetics, including those at polypathological condition. The SciELO, Science Citation Index, MEDLINE, Scopus, SPORTDiscus, and ScienceDirect databases were searched from 1980 to 2015 for articles published from original scientific investigations. In addition to the beneficial effects of exercise interventions on glycemic control, and on the cardiovascular risk factors associated with diabetes, physical exercise is an effective intervention to improve muscle strength, power output, and aerobic power and functional capacity in elderly diabetic patients. Thus, a combination of resistance and endurance training is the most effective exercise intervention to promote overall physical fitness in these patients. In addition, in diabetic patients with frailty and severe functional decline, a multicomponent exercise program including strength and power training, balance exercises, and gait retraining may be an effective intervention to reduce falls and improve functional capacity and quality of life in these patients.
Keywords: Diabetes in elderly, Functional capacity, Frailty, Exercise, Polypathology, Muscle power
Introduction
Diabetes mellitus is a chronic degenerative endocrine disease that affects millions of individuals. This disease is associated with a polypathological condition, and its complications progressively affect quality of life and survival (Huang et al. 2008; Eckert 2012). The many complications associated with diabetes include cardiovascular diseases, peripheral neuropathy, retinopathy, chronic renal failure, and impaired mental health (Blaum et al. 2007; Maiorana et al. 2002; Reeves et al. 2010), which put diabetic patients in a polypathological condition (i.e., when diabetes coexists with two or more other diseases, such as hypertension, chronic renal failure, depression, and ischemic heart disease) (Rodríguez-Mañas et al. 2014). In elderly populations, diabetes is also associated with reduced muscle strength, poor muscle quality, and accelerated loss of muscle mass (Morley 2008; Garg et al. 2009; Leenders et al. 2013; Park et al. 2007; Volpato et al. 2012; Morley et al. 2014). Indeed, diabetes mellitus and insulin resistance increase the risk for accelerated aging and for the development of frailty syndrome (Kahn 2007; Sinclair et al. 2012; Volpato et al. 2012). In addition, the frailty prevalence in elderly with diabetes is much greater than that in the general elderly population, and the frail diabetic individuals have a higher mortality than robust diabetic individuals (Morley 2011; Morley et al. 2014; Rodríguez-Mañas et al. 2014). Diabetes mellitus disease process may contribute to the increased risk of falls, institutionalization, and disability (Abdelhafiz and Sinclair, 2011). In view of this, a focus on improvements in functionality and quality of life may be more beneficial in frail elderly patients with diabetes than attention to metabolic control alone (Rodríguez-Mañas et al. 2014).
Together with pharmacological and dietary interventions, exercise interventions, including resistance training, represent the cornerstones of type 2 diabetes management (ADA 2011; Morley et al. 2014). In addition to the beneficial effects of exercise interventions on glycemic control (Umpierre et al. 2011) and on the cardiovascular risk factors associated with type 2 diabetes (Balducci et al. 2004; Figueira et al. 2014), physical exercise is an effective intervention to improve muscle strength, power output, cardiovascular function, and functional capacity in elderly diabetic patients (Balducci et al. 2012; Ibañez et al. 2008; Geirsdottir et al. 2012). In this regard, combined resistance and endurance training appears to serve as an effective exercise intervention to promote overall physical fitness in diabetic patients (Balducci et al. 2012).
On the other hand, there are few data on the effects of exercise interventions in elderly at a polypathological condition. In this case, because diabetes and associated comorbidities induce functional decline, poor quality of life, and increased risk of falls, physical exercise might be especially important because it improves functional status and quality of life of these individuals (Cadore et al. 2013a, 2013b). In fact, in frail elderly diabetics with severe functional decline, multicomponent exercise programs composed of resistance, endurance, balance, and gait retraining should be employed to increase functional capacity and quality of life and to avoid falls, institutionalization, and disability (Cadore et al. 2014). Indeed, it has been recently reported that multicomponent exercise training including explosive resistance training improved neuromuscular function and functional outcomes in frail institutionalized nonagenarians (Cadore et al. 2013a), as well as in frail polypathological patients (Cadore et al. 2013b). Among the patients assessed in these studies, the most prevalent comorbidity was diabetes. Therefore, exercise intervention may improve functional capacity even in polypathological aging patients that coexist with diabetes mellitus at severe functional decline.
Although descriptive and systematic reviews have been written on the effects of exercise interventions on glycemic control of diabetics patients (Snowling and Hopkins 2006; Umpierre et al. 2011; Hwang and Kim 2014; Asano et al. 2014; Figueira et al. 2014), at the best of the authors’ knowledge, no previous reviews have focused the effects of resistance, endurance, or combined resistance and endurance training in the glycemic control, neuromuscular and cardiovascular function, as well as functional capacity in elderly with type 2 diabetes. In addition, the effects of exercise interventions in the functional capacity in diabetic elderly at a polyphatological conditions and severe functional decline have not been addressed in previous reviews. Thus, to optimize the exercise prescription in elderly with type 2 diabetes, it seems reasonable to identify the most effective type of exercise to improve the functional capacity along with glycemic control in elderly populations with this disease. Therefore, this review aims to provide information regarding the exacerbated reductions in functional capacity in elderly diabetic patients compared with healthy elderly individuals and to describe the beneficial effects of different exercise interventions on the functional capacity of polypathological aging patients that coexist with diabetes mellitus and severe functional decline. In addition, the aim of this review is to describe the effects of different exercise interventions on glycemic control in elderly diabetic patients.
Literature search
Search strategy
The SciELO, Science Citation Index, MEDLINE, Scopus, SPORTDiscus, and ScienceDirect databases were searched from 1980 to 2015 for articles published from original scientific investigations. Search terms included various combinations of the keywords “exercise and diabetes in elderly,” “functional capacity and diabetes,” “concurrent strength and endurance training in elderly,” “resistance training and diabetes,” “endurance training and diabetes,” “combined resistance and endurance training and diabetes,” “frailty and diabetes,” and “polypathology.” The names of authors cited in some studies were also utilized in the search.
Criteria for study consideration: types of studies, outcome measures, and participants
The search criteria were as follows: (i) studies must be from English, Spanish, or Portuguese peer-reviewed scholarly journals; (ii) dissertations, theses, and conference proceedings were excluded; (iii) for the first purpose of study, studies must refer to the effects of diabetes on neuromuscular function and/or cardiovascular function and/or functional capacity in elderly or refer to the effects of exercise interventions in the neuromuscular function and/or cardiovascular function and/or functional capacity in elderly, frail elderly, elderly with polypathology, and elderly at severe functional decline, and for the second purpose of study, studies must refer to the effects of resistance training, endurance training, or concurrent resistance and endurance training on glycemic control in elderly with type 2 diabetes; (iv) only randomized studies using technical procedures which the validity and reliability have been shown in the literature were included (low intra-measures coefficient of variation and high intra-class correlation coefficients); and, (v) studies were included if the type of participants was older men and women with mean age ≥60 years.
Inclusion of studies
From the preliminary search, 6959 manuscripts had their title read and 907 were selected to a second analysis, which included the reading of the abstracts. Twenty original research studies that investigated the effects of diabetes in neuromuscular function and/or cardiovascular function and/or functional capacity in elderly patients were included. Other studies that investigated the effects of exercise intervention in oldest old at severe functional decline, who diabetes was one of the most prevalent comorbidities, were included. In addition, additional studies on frailty research, diabetes research, and functional capacity research were included in order to discuss the plausible effects of exercise. Moreover, 18 original research articles that investigated the effects of exercise interventions in the glycemic control and/or neuromuscular function, cardiovascular function, and functional capacity in elderly with type 2 diabetes were included and had their results described (Table 1). From these studies, eight have investigated the effects of resistance training, four have investigated the effects of combined resistance and endurance training, and six have investigated the effects of endurance training.
Table 1.
Summary of some studies that investigated the effects of resistance, endurance, or combined resistance and endurance training in elderly with type 2 diabetes
| Authors | Subjects | Intervention, period, and weekly frequency | Training volume and intensity | Main results and adverse effects |
|---|---|---|---|---|
| Tessier et al. (2000) |
n = 39; Age 59 ± 8.7 (diabetics) and 62 ± 6.7 (control); Men and women |
ET; 16 weeks; 3 times per week |
30–40 min at intensities progressing in intensity. | No changes in HbA1c; ↓ glucose excursion during OGTT (AUC); ↑ total time on treadmill test. No adverse effects mentioned |
| Castaneda et al. (2002) |
n = 62; Age 60 ± 1; Men and women |
RT; 16 weeks; 3 times per week |
3 sets × 8 repetitions, 60–80 % of 1 RM |
↓ HbA1c (1.1 %); ↑ whole-body 1 RM (33 %); ↓ systolic blood pressure (10 mmHg). No complications or injuries were reported |
| Dustan et al. (2002) |
n = 29; Age 67.6 ± 5 and 66.9 ± 5; Men and women |
RT combined with weight loss program; 24 weeks; 3 times per week |
3 sets × 8–10 repetitions, 50–85 % of 1 RM | ↓ HbA1c (1.2 %); ↑ whole-body 1RM (33 %); ↓ systolic blood pressure (6.7 mmHg); ↓ diastolic blood pressure (4.4 mmHg). No complications or injuries were reported |
| Ibañez et al. (2005) |
n = 20, Age 66.6; men |
RT; 16 weeks; 2 times per week |
3–5 sets × 6–15 repetitions, 50–80 % of 1 RM. Slow and explosive muscle contractions | ↓ intra-abdominal fat (10.3 %); ↑ leg and arm 1 RM (17–18 %); ↑ insulin sensitivity (46.3 %); ↓ fasting glucose (7 %). No complications or injuries were reported |
| Brooks et al. (2007) |
n = 62; Age 66 ± 1; Men and women |
RT; 16 weeks; 3 times per week |
3 sets × 8 repetitions, 60–80 % of 1 RM | ↓ HbA1c (1.1 %); ↑ leg and arm 1 RM (68 and 36 %) No adverse effects mentioned |
| Ibañez et al. (2008) |
n = 20; Age 64.8 (diabetics), 66.6 (control); Men |
RT; 16 weeks; 2 times per week |
3–4 sets × 5–15 repetitions, 50–80 % of 1 RM. Slow and explosive muscle contractions | ↑ leg 1 RM: control (37 %) > diabetics (24 %); ↑ arm 1 RM: control (36 %) > diabetics (17 %); ↑ leg and power output (30 % of 1 RM) (22–33 %), no differences between groups No adverse effects mentioned |
| Cheung et al. (2009) |
n = 37; Age 59 ± 8.7 (diabetics) and 62 ± 6.7 (control); Men and women |
Home-based RT with elastic bands; 4 months; 5 times per week |
2 sets × 12 repetitions | No changes in physical functioning; No changes in hand grip strength; No changes in timed up and go test; No changes in HbA1c; No adverse effects mentioned |
| Geirsdottir et al. (2012) |
n = 213; Age 74 ± 1; Men and women |
RT; 12 weeks; 3 times per week |
3 sets × 6–8 repetitions, 75–80 % of 1 RM | ↑ leg peak torque (15 %); ↑ hand grip (19 %); ↑ 6 min walking distance (6 %); ↑ TUG performance (5 %); No changes in HbA1c No adverse effects mentioned, but some of them dropped out because they did not like the program (n = 8) |
| Nuttamonwarakul et al., 2012 |
n = 40; Age 60 ± 1; Men and women |
ET; 12 weeks; 3 times per week |
30 min at 70 % of HRmax
Water exercises |
↓ HbA1c (1.1 %); ↑ VO2máx (1 %) No adverse effects mentioned |
| Simmonds et al. (2012) |
n = 16; Age 68 ± 4; Women |
ET; 12 weeks; 4 times per week |
30 min at intensities progressing to anaerobic threshold. | ↑ VO2 at anaerobic threshold (10 %) No changes in HbA1c No adverse effects mentioned |
| Sung and Bae (2012) |
n = 40; Age 70 ± 5; Men and women |
ET; 24 weeks; 3 times per week |
30–35 min at 55–75 % of HRmax | No changes in HbA1c (−0.41 %, non-significant) No adverse effects mentioned |
| Tan et al. (2012) |
n = 16; Age 68 ± 4; Women |
CT; 12 weeks; 3 times per week |
RT 2 sets × 10–12 repetitions, 50–70 % of 1RM; ET 30 min at 55–70 % of HRmax. |
↓ HbA1c (0.55 %); No reported physical injuries related to the training or testing |
| Asa et al. (2012) |
n = 20; Age 65.8 ± 5.8 (diabetics) and 69 ± 8.2 (control) |
CT performed with hydrogymnastics exercises; 8 weeks; 3 times per week |
45 min at 40–75 % of HRR. | ↓ HbA1c (0.7 %); ↑ peak torque at 180°.s−1; ↑ VO2máx (14 %); ↑Wmax (22 %). 1 subject dropped out because of peripheral ulcer caused by new shoes and 1 had increased symptoms of CHD |
| Mavros et al. (2013) |
n = 103; Age not shown; Men and women |
RT; 48 weeks; 3 times per week |
3 sets × 8 repetitions, 80 % of 1 RM with concentric explosive contractions |
↑ Mid-thigh CSA; ↓ Mid-thigh muscle attenuation (↓ muscle fat infiltration) No changes in HbA1c No adverse effects mentioned |
| Terada et al. (2013) |
n = 15; Age 62 ± 3 (interval ET) and 63 ± 5 (continuous ET); Men and women |
ET; 12 weeks; 5 times per week |
Low-intensity ET 40 % of VO2R; High-intensity intermittent ET 100 % of VO2R (1 min) by 20 % (3 min). Time progressively increasing from 30 to 60 min |
↑ VO2 at anaerobic threshold (16 %); ↑ peak power output only in high-intensity group (11 %); No changes in HbA1c; No adverse effects were mentioned |
| Egger et al. 2013 |
n = 32; Age 65 ± 8; Men and women |
High- vs. low-intensity RT combined with ET; 8 weeks |
Low-intensity RT 2 sets × 25–30 repetitions, 40 % of 1 RM; High-intensity RT: 2 sets × 10–12 repetitions, 70 % of 1 RM; ET 60 min at 70 % of HRmax |
↓ basal glucose in both groups; ↑ arm 1 RM (high-intensity RT > low-intensity RT; No adverse effects mentioned |
| Mitranun et al. (2014) |
n = 43; Age 60.9 ± 2.4 (control); 61.7 ± 2.7 (continuous); 61.2 ± 2.8 (intermitent) Men and women |
ET; 12 weeks; 3 times per week |
Low-intensity ET 65 % of VO2peak; High-intensity intermittent ET 80 % of VO2peak (1 min) by 20 %. Time progressively increasing from 30 to 40 min |
↓ heart rate at rest (high-intensity group) ↓ % body fat in both training groups; ↑ VO2peak in both training groups (25 vs. 17 %, higher in high-intensity group); ↑ peak power output only in high-intensity group (11 %); ↓ HbA1c only in high-intensity group(0.9 %); ↓ insulin resistance (HOMA) in both groups; No adverse effects mentioned |
| Kim et al. (2014) |
n = 52; Age 68. 5 ± 1–73.2 ± 2.0; Women |
CT; 12 weeks; 3–4 times per week |
RT (2–3 sets per exercise) performed as circuit alternation with ET at 60–80 % of HR reserve | ↓ fat mass (5 %); ↓ total cholesterol (2.2 %); No changes in insulin sensitivity No adverse effects mentioned |
HbA1c glycated hemoglobin A1c, OGTT oral glucose tolerance test, AUC area under the curve, 1 RM one maximum repetition (maximal dynamic strength), W max maximal power at cycle ergometer, RT resistance training, ET endurance training, CT combined resistance and endurance training, HR heart rate, HR max maximal heart rate, HRR heart rate reserve, VO 2max maximal oxygen uptake, VO 2 R reserve oxygen uptake, CHD chronic heart failure
Functional capacity in elderly diabetic patients
It has been shown that aging patients with type 2 diabetes exhibit greater declines in muscle strength and functional capacity and more rapid loss of muscle mass than normoglycemic controls (Park et al. 2007; Garg et al. 2009; Leenders et al. 2013; Volpato et al. 2012). Indeed, diabetes complications such as peripheral vascular disease and peripheral neuropathy are associated with poor gait ability, impaired balance, and increased risk of falls (Ko et al. 2011; Powell et al. 2006; Wray et al. 2005; Oliveira et al. 2012; Vinik et al. 2015).
In a study investigating a large cohort, Park et al. (2007) followed 1840 elderly adults (73.5 years), 16.6 % of whom were type 2 diabetics, for 3 years. These authors showed that both the diabetics (glycated hemoglobin A1c (HbA1c) = 7.9 %) and the nondiabetics (HbA1c = 6.0 %) experienced a significant loss of initial muscle strength over 3 years but that the older adults with type 2 diabetes lost their knee extensor strength, leg lean mass, and muscle quality (maximal strength per unit of muscle mass in N m/kg) more rapidly than those without diabetes. In a different study by Levinger et al. (2012), elderly men (54.2 ± 7.4 years) with type 2 diabetes (HbA1c = 6.8 %) exhibited a lower VO2peak (21.8 vs. 25.8 ml kg min−1) and maximal strength relative to body mass (chest press + leg press) (3.3 vs. 3.7 kg kg−1 body mass) and performance on physical tasks (i.e., the 15-m rapid walking, timed up-and-go, and stair-climbing and stair-descending tests) (27.2 vs. 24.2 s) than men without diabetes (HbA1c = 5.5 %). In this study, the diabetic individuals also exhibited a more depressed mood and a lower perceived general health. In addition, Leenders et al. (2013) reported that aging individuals with type 2 diabetes exhibited a greater decline in functional capacity, along with lower-body muscle mass and strength, than normoglycemic subjects.
In another study, Ijzerman et al. (2012) investigated lower extremity muscle strength in type 2 diabetics with (62 years, HbA1c = 7.1 %) or without polyneuropathy (67 years, HbA1c = 7.3 %) and compared these diabetics with healthy individuals (68 years, HbA1c = 6.0 %). These authors showed that, compared with the healthy controls, the diabetic individuals either with or without polyneuropathy exhibited reduced muscle strength (34–47 %), mobility (28 %), and quality of life. This study also showed significant associations between muscle strength and mobility and between reduced quality of life and both muscle strength and mobility in diabetics. Similarly, Ko et al. (2011) observed an association of gait pattern alterations with type 2 diabetes (HbA1c = 6.86 %) in older adults (70 years) without peripheral neuropathy. Therefore, preservation of functional capacity should be specifically addressed in aging diabetic patients because in contrast to other chronic conditions, diabetes care is dependent on the patients’ ability to perform self-care tasks (Abdelhafiz and Sinclair 2011). In addition to metabolic control, effective strategies are needed to prevent the exacerbated loss of strength and functional capacity in aging diabetic patients because these individuals exhibit an increased risk of the development of frailty syndrome, institutionalization, and disability (Abdelhafiz and Sinclair 2011; Kahn 2007; Sinclair et al. 2012).
Resistance training improves muscle strength and power and functional capacity in elderly with type 2 diabetes
In addition to its important effect on glycemic control, resistance training is a very important intervention because it counteracts the exacerbated loss of muscle strength and functional capacity observed in elderly patients (Castaneda et al. 2002; Dunstan et al. 2002; Brooks et al. 2007; Ibañez et al. 2008; Geirsdottir et al. 2012). For example, in study of Brandon et al. (2003), 24 weeks of resistance training performed at moderate intensity induced increases in muscle strength and mobility in elderly with type 2 diabetes. In general, studies have demonstrated that applying a resistance training intervention consisting of either two or three sets of 8–15 repetitions at an intensity ranging from 50 to 85 % of one maximum repetition (1 RM) performed two or three times per week for between 8 and 24 weeks markedly increases maximal muscle strength in elderly type 2 diabetic patients (Castaneda et al. 2002; Dunstan et al. 2002; Brooks et al. 2007; Ibañez et al. 2008; Geirsdottir et al. 2012).
It should be mentioned that lower resistance training volume (i.e., one set per exercise) may induce similar neuromuscular improvements as higher volumes (i.e., two to three sets per exercise) in healthy elderly subjects, especially in early phases of training (i.e., 3 months) (Radaelli et al. 2014). This reduced volume could be especially useful to improve the functional capacity in elderly type 2 diabetic patients in a polypathological condition and at severe functional decline, in which the functional capacity should be prioritized more than glycemic control (Rodriguez-Mañas et al., 2014; Rodriguez-Mañas and Fried 2015). A shorter resistance training session could be easily applied and increase the exercise adherence. Nevertheless, because the time spent exercising should be greater than 150 min per week to exert optimal metabolic effects (Umpierre et al. 2011), future studies should investigate whether low-volume resistance training (i.e., one set per exercise) may result in similar glycemic improvement as larger resistance training volumes in elderly type 2 diabetic patients, if the goal of exercise intervention is to improve glycemic control along with functional capacity gains.
It has been shown that resistance training programs including high-velocity muscle actions during the concentric phase are effective interventions to improve muscle strength, power output, rate of force development, and functional capacity in elderly subjects (Correa et al. 2012; Henwood et al. 2008; Pereira et al. 2012; Ramirez-Campillo et al. 2014). In fact, studies have shown that muscle power appears to serve as a more important predictor of functional performance in healthy and frail elderly than muscle strength alone (Casas-Herrero et al. 2013; Reid and Fielding 2012). In a study by Ibañez et al. (2008), elderly diabetic patients who performed a twice weekly progressive resistance training program that included high-velocity muscle actions exhibited significantly improved muscle strength and muscle power output after 16 weeks of training. Therefore, because muscle power may be a better predictor of functionality than muscle strength alone, high-speed resistance training should be considered as an alternative exercise intervention in elderly diabetic patients, because this population is at risk of functional decline. Notably, to improve functional capacity in the elderly, the volume and intensity of exercise interventions must be carefully designed because insufficient training stimuli may result in a lack of benefits to glycemic control and functional capacity in elderly type 2 diabetics. In this sense, although home-based exercise programs may facilitate exercise adherence, this type of intervention may not result in metabolic and functional improvements (Cheung et al. 2009). Table 1 presents a summary of some studies that investigated the effects of exercise in functional capacity in elderly with type 2 diabetes mellitus.
Endurance training and cardiovascular function in elderly diabetic patients
Although resistance training is an effective intervention to improve functional capacity in elderly with type 2 diabetes, its combination with endurance training is the most indicated exercise program because endurance training promotes greater increases in cardiovascular function when compared with resistance training alone (Cadore and Izquierdo 2013). Indeed, studies investigating the effects of endurance training and combined resistance and endurance training have shown marked increases in cardiorespiratory outcomes (Nuttamonwarakul et al. 2012; Simmonds et al. 2012). In addition, it has been shown that high-intensity endurance training (HIT) may be feasible in patients with type 2 diabetes (Terada et al. 2013). Terada et al. (2013) have compared the feasibility and efficacy of 12 weeks of two different intensities of endurance training in elderly patients with type 2 diabetes: high-intensity intermittent endurance training with intensity ranging from 100 % of workload corresponding to oxygen consumption reserve (VO2R) (1 min) and 20 % of VO2R (intervals 3 min); and low intensity continuous endurance training at intensity of 40 % of workload corresponding to VO2R. Both types of training were performed 5 days per week, progressing from 30 to 60 min of total exercise per session. From 126 participants showing interest to join the study, 15 individuals completed the program. On the other hand, along with similar body composition and cardiorespiratory adaptations, feeling states, self-efficacy, and adherence rates were high and did not differ between groups (over 97 %). Therefore, it seems that high-intensity interval training is an alternative to improve the cardiovascular function in elderly with type 2 diabetes. However, Terada et al. (2013) investigated patients with mean ± SD age of 62 ± 3 years (HIT group) and 63 ± 5 years (low-intensity group), and therefore, the feasibility and safety of this kind of training in oldest old individuals with type 2 diabetes as well as elderly that coexist with other comorbidities remain to be investigated in future studies.
Therefore, endurance training comprising two to five times a week at intensities around 70 % of maximal heart rate should be prescribed in combination with resistance training in order to promote benefits in cardiovascular fitness. In addition, higher intensities (around 80–90 of VO2max) during intermittent endurance training can be an alternative but should be more investigated in elderly populations in order to verify its safety, feasibility, and cost/benefits ratio.
Effects of exercise intervention on diabetic patients at severe functional decline
Frailty is an age-associated biological syndrome characterized by decreased biological reserves, which puts individuals at risk when facing minor stressors, and is associated with disability, death, and hospitalization (Rodriguez-Mañas and Fried 2015). Among several comorbidities that may coexist in frailty syndrome, diabetes is one of the most prevalent (Rodríguez-Mañas et al. 2014). In diabetic frail patients, enhancement in functional capacity is crucial and may be more beneficial than attention to metabolic control alone (Rodríguez-Mañas et al. 2014). To counteract this functional decline, exercise interventions including resistance training are quite effective in improving muscle strength and power, balance control, and gait ability and reducing incidence of falls in frail elderly (Cadore et al., 2014).
In a recent study, a 12-week multicomponent exercise program including explosive resistance training significantly increased muscle cross-sectional area, maximal strength, muscle power output, balance, gait, and sit-to-stand ability and reduced the incidence of falls of institutionalized frail nonagenarians (Cadore et al. 2013a). Among these subjects, more than 70 % suffered from type 2 diabetes among different comorbidities (i.e., polypathological condition) (Cadore et al. 2013a). In addition, it has recently been shown that 4 weeks of high-speed resistance training combined with walking, cognitive, and balance exercises improved gait ability, balance, and muscle strength (15–30 %) and reduced the incidence of falls in frail patients with dementia after long-term physical restraint during nursing care. In this study, among the several comorbidities of these patients, the most typical comorbidity was type 2 diabetes (Cadore et al. 2013b). Taken together, these results suggest that exercise intervention which includes resistance training may help to improve muscle functional capacity in elderly at a polypathological condition and severe functional decline, including those with diabetes complications. In addition, explosive resistance training can serve as an interesting alternative in these patients because of its effectiveness in improving muscle power output, muscle mass, and functional capacity in frail elderly.
The effects of exercise interventions on glycemic control in the elderly
A large body of evidence indicates that physical exercise exerts beneficial effects on glycemic control in pre-diabetic and diabetic individuals (Ibañez et al. 2005; Umpierre et al. 2011; Balducci et al. 2012). The mechanisms related to this improvement in glucose metabolism include the following: increased insulin sensitivity; upregulated GLUT4 translocation to the muscle cell membrane independently of the insulin pathway (Ebeling et al. 1993); enhanced available glucose storage capacity, thereby facilitating the clearance of glucose from the circulation; reduced levels of visceral fat (Ibañez et al. 2005), which is the primary cause of insulin resistance; and increased muscle mass, which is the primary tissue involved in glucose metabolism (Ebeling et al. 1993).
Although endurance exercise had traditionally been advocated as the most suitable mode of exercise for the treatment of cardiometabolic diseases (Borghouts and Keizer 2000), resistance training has also consistently been shown to effectively reduce the glycemic levels in pre-diabetic and diabetic individuals (Ibañez et al. 2005; Umpierre et al. 2011; Geirsdottir et al. 2012). In addition, the combination of resistance and endurance training is a more effective exercise intervention to improve neuromuscular and cardiovascular functions, than either resistance or endurance training alone. In diabetic patients, this combined training program has the advantage of increasing the total time spent undergoing physical activity, which is also beneficial to these patients. Studies on the effects of different exercise interventions in glycemic control in elderly with type 2 diabetes mellitus are also described in Table 1.
The effects of endurance training
Studies of endurance training in the elderly have demonstrated the beneficial effects of chronic exercise on glycemic control. In a study by Sung and Bae (2012), an interval endurance training program performed three times per week for 24 weeks at an intensity ranging from 55 to 75 % of the maximal heart rate (HRmax) resulted in a 0.41 % decrease in the HbA1c levels in elderly men and women (n = 40, age 70 years). Using a different training approach, in a study by Nuttamonwarakul et al. (2012), the cardiometabolic effects of endurance training performed in an aquatic environment at an intensity of 70 % of HRmax for a duration of 30 min per session for three sessions per week for 12 weeks were investigated. These authors demonstrated that this training protocol resulted in decreased HbA1c levels (by 1.1 %). Recently, high intensity Terada et al. (2013) have shown no changes in fasting glucose and HbA1c following 12 weeks of two high-intensity intermittent endurance or low-intensity continuous endurance training (see training details in the preceding text). Opposite results were found by Mitranun et al. (2014) investigating elderly patients with type 2 diabetes performing endurance training 12 weeks, three times per week, 30–40 min per session. This authors compared the effects of low-intensity, continuous endurance training (65 % of VO2peak) and high-intensity, interval training (higher intensity at 80 % of VO2peak), and found significant reduction in the HbA1c only after the high-intensity training protocol (60 ± 2 vs. 54 ± 2, P < 0.05). Therefore, continuous (low to moderate intensity) as well as intermittent (high intensity) endurance training performed three to five times per week may reduce the HbA1c levels in the elderly even within a short training period.
Along with the beneficial effects of endurance training on diabetic patients, endurance training has been shown to improve glycemic control in non-diabetic subjects (Seals et al. 1984; Kirwan et al. 1993). This finding is especially important because it suggests that physical training can prevent or slow the progression of diabetes in the elderly.
The effects of resistance training
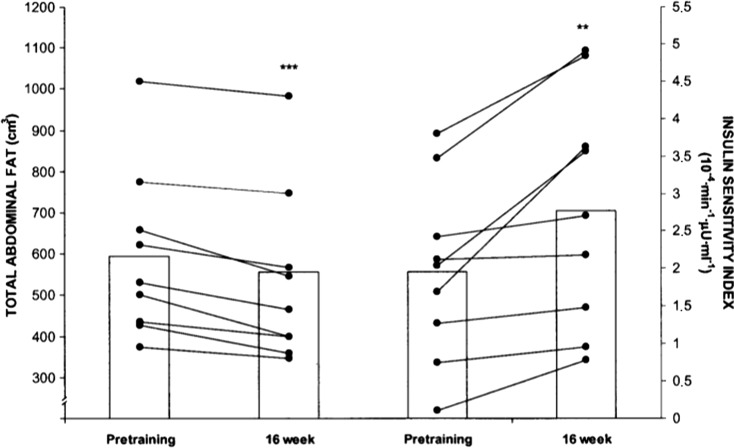
Resistance training is also an effective exercise intervention to reduce the glycemic and HbA1c levels in elderly diabetic patients. In a study by Ibañez et al. (2005), the effects of a 16-week resistance training program combining heavy and explosive loads were assessed in elderly type 2 diabetic patients. The resistance training program was performed twice weekly and included two exercises for the leg extensor muscles, one exercise for the arm extensor muscle, and from four to five exercises for the main muscle groups of the body. Heavy resistance training was performed using either three or four sets of 10–15 repetitions per set at 50–70 % of 1 RM during the first 8 weeks followed by between three and five sets of either five or six repetitions per set at 70–80 % of 1 RM. During the final 8 weeks, 20 % of the training volume of the leg extension and bench press exercises was performed as three or four sets of between six and eight repetitions at 50 % of 1 RM in an explosive manner (i.e., as rapidly as possible). The results showed that this training protocol resulted in a marked decrease in the fasting blood glucose levels (7 %, P < 0.05) and a significant improvement in insulin sensitivity by 46 % (P < 0.01) (Fig. 1). A trend toward a significant decrease in the HbA1c levels (P = 0.06) was observed in this study. Another relevant finding in this study was a significant decrease in the amount of intra-abdominal adipose tissue (10.3 %, P < 0.01). Thus, along with its beneficial impact on glycemic control, resistance training that includes heavy and explosive loads can improve the levels of intra-abdominal fat, which is a primary cause of type 2 diabetes.
Fig. 1.
Total abdominal fat and insulin sensitivity at pretraining and after a 16-week strength training period for each subject and mean values. **P < 0.01 and ***P < 0.001 vs. the corresponding pretraining value. Adapted from Ibañez et al. (2005)
Other studies investigating the effects of resistance training on glycemic control have reported positive results. In a study by Castaneda et al. (2002), a resistance training program conducted three times per week for 16 weeks induced a significant reduction in the HbA1c levels (by 1.1 %, P < 0.05) in men and women with type 2 diabetes (aged 66 ± 2 years). These authors used a progressive resistance training protocol that began at 60 % of 1 RM and progressed to 80 % of 1 RM (three sets of eight repetitions). A similar resistance training protocol was used in the study by Dustan et al. (2002), who also investigated men and women with type 2 diabetes (aged 67 ± 5 years). These authors showed that progressive resistance training (three sets of eight to ten repetitions beginning at 50–60 % of 1 RM and progressing to 75–85 % of 1 RM) performed three times per week for 24 weeks induced a significant reduction of 1.2 % in the HbA1c levels (P < 0.05). Similar results were observed by Brooks et al. (2007), who observed a reduction of 1.2 % in the HbA1c levels (P < 0.05) after 16 weeks of resistance training performed three times per week consisting of three sets of eight repetitions at an intensity ranging from 60 to 80 % of 1 RM. In all of these studies, no significant alteration in glycemic control was observed in the control group that did not perform any exercise intervention.
Importantly, not all studies investigating the effects of resistance training reported decreases in the glycemic or HbA1c levels (Honkola et al. 1997; Geirsdottir et al. 2012; Mavros et al. 2013). Some exercise interventions may not elicit sufficient stimulus to induce metabolic changes in diabetic patients. Other potential causes of the lack of a change in glycemic control after resistance training include uncontrolled diet, an insufficient sample size, and low statistical power to detect significant differences. Therefore, caution must be taken when prescribing resistance exercise interventions to improve the glycemic levels in type 2 diabetic patients.
The effects of combined resistance and endurance training
Few studies have investigated the effects of combined resistance and endurance training on glycemic control in elderly type 2 diabetic patients. In a study by Tan et al. (2012), a significant reduction in the HbA1c levels (by 0.55 %) was observed after 24 weeks of combined resistance (three times per week, two sets of 10–12 repetitions at 50–70 % of 1 RM) and endurance training (30 min at 55–70 % of HRmax). Conflicting results were reported by Egger et al. (2013) and Tessier et al. (2000), who did not observe any change in the HbA1c levels after combined resistance and endurance training for 8 or 16 weeks, respectively. In a study conducted in an aquatic environment, Asa et al. (2012) observed a significant reduction in the HbA1c levels (by 0.7 %) after 8 weeks of combined training performed using hydrogymnastic exercises.
Based on these studies, the prescription of combined resistance and endurance training at a sufficient volume and intensity may promote a reduction in the glycemic levels in elderly patients. Importantly, according to a meta-analysis conducted on a large age range of type 2 diabetic patients (Umpierre et al. 2011), the time spent exercising should be greater than 150 min per week to exert optimal beneficial effects. In this sense, the combination of resistance and endurance training should be recommended because along with enhancing neuromuscular and cardiovascular function, this combined training program increases the total time spent undergoing physical activity, which is also beneficial to type 2 diabetic patients.
Special considerations to prescribe exercise in polypathological aging patients that coexist with diabetes mellitus
In summary, along with pharmacological and dietary interventions, physical training including resistance and endurance training represents the cornerstone of diabetes management. In addition to the beneficial effects of exercise interventions on glycemic control, and on the cardiovascular risk factors associated with diabetes, physical exercise is an effective intervention to improve neuromuscular and cardiorespiratory function, as well as functional capacity and quality of life in elderly diabetic patients. Furthermore, physical exercise administration were relatively free of potential unwanted side effects caused by common medications prescribe exercised in elderly at a polypathological condition. Therefore, the combination of resistance and endurance training appears to be the most effective exercise intervention to promote overall physical fitness in elderly diabetic patients.
Based on exercise interventions used in the studies which investigated the metabolic and functional effects of exercise in elderly with type 2 diabetes, exercise interventions in this population should include the following:
Exercise interventions should be composed by, at least, 150 min of exercise per week, shared into two or three non-consecutive days. However, it has been recently shown that exercise interventions with more time than 150 min per week result in greater effects on glycemic control (Umpierre et al. 2011).
As a part of exercise intervention, resistance training should be performed at least twice weekly, including exercises for all muscle groups. These exercises should be performed using two to three sets per exercise, and repetitions ranging from 8 to 15, with workloads progressing from 50 to 80 % of 1 RM. The intensity and volume should be increased progressively. As mentioned above, even lower resistance training volume (i.e., one set per exercise) may result in improvements in the neuromuscular function in elderly, although its effects on glycemic control remains to be elucidated.
Part of resistance training exercises (especially lower limbs) should be performed as fast as possible (muscle power training) in order to optimize skeletal power output and, consequently, functional capacity.
Endurance training should be performed three times per week, with each session lasting at least 30 min. The intensity should start between 40 and 50 % of HRmax and progress to 70–80 % of HRmax. Endurance training could be performed both in aquatic environment or on dry land (i.e., walking or cycling). The use of higher intensities (higher than 80 % of VO2peak) during intermittent endurance training may also be feasible and induce positive changes on cardiovascular function and glycemic control. However, the feasibility and safety of using higher intensities in polypathological aging patients at functional decline should be further investigated.
To optimize the functional capacity of individuals, resistance training programs could include exercises in which daily activities are simulated, such as the sit-to-stand exercise. However, functional improvements induced by resistance training, especially those using explosive muscle actions, have been observed even with no daily activity exercises (Ibañez et al. 2008; Mavros et al. 2013).
Multicomponent training programs should include gradual increases in the volume, intensity, and complexity of the exercises, along with the simultaneous performance of resistance, endurance, and balance exercises.
Acknowledgments
This work was supported in part by the Spanish Department of Health and Institute Carlos III of the Government of Spain [Spanish Net on Aging and frailty; (RETICEF)(RD12/043/0002], the European Commission Seventh Framework Program (Midfrail study), as well as by the Erasmus + European Commission program [556988-EPP-1–2014–1-ES-SPO-SCP (www.vivifrail.com)].
References
- Abdelhafiz AH, Sinclair AJ (2011) Management of type 2 diabetes in older people. Diabetes Therapy: Re, Treat Educ Diabetes Relat Disord 2013; 4: 13-26. [DOI] [PMC free article] [PubMed]
- ADA Standards of medical care in diabetes. Diabetes Care. 2011;34:11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asa C, Maria S, Katharina SS, Bert A. Aquatic exercise is effective in improving exercise performance in patients with heart failure and type 2 diabetes mellitus. Evid Based Complement Alternat Med. 2012;2012:349209. doi: 10.1155/2012/349209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano RY, Sales MM, Browne RA, Moraes JF, Coelho Júnior HJ, Moraes MR, Simões HG (2014) Acute effects of physical exercise in type 2 diabetes: A review. World J Diabetes 5(5):659–665. doi:10.4239/wjd.v5.i5.659 [DOI] [PMC free article] [PubMed]
- Balducci S, Zanuso S, Cardelli P, Salvi L, Bazuro A, Pugliese L, Maccora C, Iacobini C, Conti FG, Nicolucci A, Pugliese G; Italian Diabetes Exercise Study (IDES) Investigators (2012) Effect of high- versus low-intensity supervised aerobic and resistance training on modifiable cardiovascular risk factors in type 2 diabetes; the Italian Diabetes and Exercise Study (IDES). PLoS One 7: e49297. doi:10.1371/journal.pone.0049297 [DOI] [PMC free article] [PubMed]
- Balducci S, Leonetti F, Di Mario U, Fallucca F. Is a long-term aerobic plus resistance training program feasible for and effective on metabolic profiles in type 2 diabetic patients? Diabetes Care. 2004;27:841–842. doi: 10.2337/diacare.27.3.841. [DOI] [PubMed] [Google Scholar]
- Blaum CS, West NA, Haan MN. Is the metabolic syndrome with or without diabetes, associated with progressive disability in older Mexican Americans? J Gerontol Ser A. Biol Sci Med Sci. 2007;62:766–773. doi: 10.1093/gerona/62.7.766. [DOI] [PubMed] [Google Scholar]
- Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. Int J Sports Med. 2000;21:1–12. doi: 10.1055/s-2000-8847. [DOI] [PubMed] [Google Scholar]
- Brandon LJ, Gaasch DA, Boyette LW, Lloyd AM. Effects of long-term resistive training on mobility and strength in older adults with diabetes. J Gerontol A Biol Sci Med Sci. 2003;58:740–745. doi: 10.1093/gerona/58.8.M740. [DOI] [PubMed] [Google Scholar]
- Brooks N, Layne E, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceps C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci. 2007;4:19–27. doi: 10.7150/ijms.4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadore EL, Izquierdo M. How to simultaneously optimize muscle strength, power, functional capacity, and cardiovascular gains in elderly: an update. Age (Dordr) 2013;35:2329–2344. doi: 10.1007/s11357-012-9405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadore EL, Casas-Herrero A, Zambom-Ferraresi F, Idoate F, Millor N, Gómez M, Rodriguez-Mañas L, Izquierdo M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age (Dordr) 2013;36:773–785. doi: 10.1007/s11357-013-9586-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadore EL, Rodríguez-Mañas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability and balance in physically frail older adults. A systematic review. Rejuvenation Res. 2013;16:105–114. doi: 10.1089/rej.2012.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadore EL, Moneo ABB, Mensat MM, Muñoz AR, Casas-Herrero A, Rodriguez-Mañas L, Izquierdo M. Positive effects of resistance training in frail elderly patients with dementia after long-term physical restraint. Age (Dordr) 2014;36:801–811. doi: 10.1007/s11357-013-9599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas-Herrero A, Cadore EL, Zambom-Ferraresi F, Idoate F, Millor N, Martínez-Ramírez A, Gómez M, Rodríguez-Mañas L, Marcellan T, Ruiz de Gordoa A, Marques MC, Izquierdo M. Functional capacity, muscle fat infiltration, power output and cognitive impairment in institutionalized frail oldest-old. Rejuvenation Res. 2013;16:396–403. doi: 10.1089/rej.2013.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, Roubenoff R, Tucker KL, Nelson ME. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25:2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- Cheung NW, Cinnadaio N, Russo M, Marek S. A pilot randomised controlled trial of resistance exercise bands in the management of sedentary subjects with type 2 diabetes. Diabetes Res Clin Pract. 2009;83:e68–e71. doi: 10.1016/j.diabres.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Correa CS, LaRoche DP, Cadore EL, Reischak-Oliveira A, Bottaro M, Kruel LF, Tartaruga MP, Radaelli R, Wilhelm EN, Lacerda FC, Gaya AR, Pinto RS. 3 Different types of strength training in older women. Int J Sports Med. 2012;33:962–969. doi: 10.1055/s-0032-1312648. [DOI] [PubMed] [Google Scholar]
- Dustan DW, Daly RM, Owen N, Jolley D. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25:1729–1736. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- Ebeling P, Bourey R, Koranyi L, Tuominen JA, Groo LC, Henrikson J, Mueckler M, Sovijarvi A, Koivisto VA. Mechanism of enhanced insulin sensitivity in athletes, increased blood flow, muscle glucose transport protein (GLUT−4) concentration, and glycogen synthase activity. J Clin Invest. 1993;92:1623–1631. doi: 10.1172/JCI116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert K. Impact of physical activity and bodyweight on health-related quality of life in people with type 2 diabetes. Diabetes, Metab Syndr Obesity: Targets Therapy. 2012;5:303–311. doi: 10.2147/DMSO.S34835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger A, Niederseer D, Diem G, Finkenzeller T, Ledl-Kurkowski E, Forstner R, Pirich C, Patsch W, Weitgasser R, Niebauer J. Different types of resistance training in type 2 diabetes mellitus: effects on glycaemic control, muscle mass and strength. Eur J Prev Cardiol. 2013;20:1051–1060. doi: 10.1177/2047487312450132. [DOI] [PubMed] [Google Scholar]
- Figueira FR, Umpierre D, Cureau FV, Zucatti AT, Dalzochio MB, Leitão CB, Schaan BD. Association between physical activity advice only or structured exercise training with blood pressure levels in patients with type 2 diabetes: a systematic review and meta-analysis. Sports Med. 2014;44:1557–1572. doi: 10.1007/s40279-014-0226-2. [DOI] [PubMed] [Google Scholar]
- Garg PK, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, Tan J, McDermott MM. Physical activity during daily life and functional decline in peripheral arterial disease. Circulation. 2009;119:251–260. doi: 10.1161/CIRCULATIONAHA.108.791491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geirsdottir OG, Arnarson A, Briem K, Ramel A, Jonsson PV, Thorsdottir I. Effect of 12-week resistance exercise program on body composition, muscle strength, physical function, and glucose metabolism in healthy, insulin-resistant, and diabetic elderly Icelanders. J Gerontol A Biol Sci Med Sci. 2012;67:1259–1265. doi: 10.1093/gerona/gls096. [DOI] [PubMed] [Google Scholar]
- Heden TD, Winn NC, Mari A, Booth FW, Rector RS, Thyfault JP, Kanaley JA. Post-dinner resistance exercise improves postprandial risk factors more effectively than pre-dinner resistance exercise in patients with type 2 diabetes. J Appl Physiol. 2014;118:624–634. doi: 10.1152/japplphysiol.00917.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henwood TR, Riek S, Taaffe DR. Strength versus muscle power-specific resistance training in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2008;63:83–91. doi: 10.1093/gerona/63.1.83. [DOI] [PubMed] [Google Scholar]
- Honkola A, Forsén T, Eriksson J. Resistance training improves the metabolic profile in individuals with type 2 diabetes. Acta Diabetol. 1997;34:245–248. doi: 10.1007/s005920050082. [DOI] [PubMed] [Google Scholar]
- Huang ES, Zhang Q, Gandra N, Chin MH, Meltzer DO. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: a decision analysis. Ann Intern Med. 2008;149:11–19. doi: 10.7326/0003-4819-149-1-200807010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang MH, Kim S (2014) Type 2 Diabetes: Endothelial dysfunction and Exercise. J Exerc Nutrition Biochem (3):239–247. doi:10.5717/jenb.2014.18.3.239 [DOI] [PMC free article] [PubMed]
- Ibañez J, Izquierdo M, Argüelles I, Forga L. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care. 2005;28:662–667. doi: 10.2337/diacare.28.3.662. [DOI] [PubMed] [Google Scholar]
- Ibañez J, Gorostiaga EM, Alonso AM, Forga L, Arguelles I, Larrion JL, Izquierdo M. Lower muscle strength gains in older men with type 2 diabetes after resistance training. J Diabetes Complicat. 2008;22:112–118. doi: 10.1016/j.jdiacomp.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Ijzerman TH, Schaper NC, Melai T, Meijer K, Willems PJB, Savelberg HHCM. Lower extremity muscle strength is reduced in people with type 2 diabetes, with and without polyneuropathy, and is associated with impaired mobility and reduced quality of life. Diabetes Res Clin Pract. 2012;95:345–351. doi: 10.1016/j.diabres.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Kahn AJ. Central and peripheral mechanisms of aging and frailty: a report on the 8th Longevity Consortium Symposium, Santa Fe, New Mexico, May 16–18. J Gerontol A Biol Sci Med Sci. 2007;62:1357–1360. doi: 10.1093/gerona/62.12.1357. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kang CK, Park H, Lee MG (2014) Effects of vitamin D supplementation and circuit training on indices of obesity and insulin resistance in T2D and vitamin D deficient elderly women. J Exerc Nutrition Biochem 18(3):249–257. doi:10.5717/jenb.2014.18.3.249 [DOI] [PMC free article] [PubMed]
- Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, Holloszy JO. Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year-old men and women. J Gerontol Med Sci. 1993;48:M84–M90. doi: 10.1093/geronj/48.3.M84. [DOI] [PubMed] [Google Scholar]
- Ko S, Stenholm S, Chia CW, Simonsick EM, Ferrucci L. Gait pattern alterations in older adults associated with type 2 diabetes in the absence of peripheral neuropathy—results from the Baltimore longitudinal study of aging. Gait Posture. 2011;34:548–552. doi: 10.1016/j.gaitpost.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders M, Verdijk LB, van der Hoeven L, Adam JJ, van Kranenburg J, Nilwik R, van Loon LJ. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013;14:585–592. doi: 10.1016/j.jamda.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Levinger I, Selig S, Jerums G, Stewart A, Gaskin CJ, Hare DL. Depressed mood, glycaemic control and functional capacity in overweight/obese men with and without type 2 diabetes. Diabetol Metab Syndr. 2012;4:46–53. doi: 10.1186/1758-5996-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorana A, Driscoll GO, Goodman C, Taylor R, Gree D. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract. 2002;56:115–123. doi: 10.1016/S0168-8227(01)00368-0. [DOI] [PubMed] [Google Scholar]
- Mavros Y, Kay S, Anderber KA, Baker MK, Wang Y, Zhao R, Meiklejohn J, Climstein M, O’Sullivan A, Vos N, Baune BT, Blair SN, Simar D, Rooney K, Singh N, Fiatarone Singh MA. Changes in insulin resistance and HbA1c are related to exercise-mediated changes in body composition in older adults with type 2 diabetes. Diabetes Care. 2013;33:2372–2379. doi: 10.2337/dc12-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitranun W, Deerochanawong C, Tanaka H, Suksom D (2014) Continuous vs interval training on glycemic control and macro- and microvascular reactivity in type 2 diabetic patients. Scand J Med Sci Sports 24: e69-e76 [DOI] [PubMed]
- Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008;24:455–469. doi: 10.1016/j.cger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Morley JE. Frailty: diagnosis and management. J Nutr Health Aging. 2011;15:667–670. doi: 10.1007/s12603-011-0338-4. [DOI] [PubMed] [Google Scholar]
- Morley JE, Malmstrom TK, Rodriguez-Mañas L, Sinclair AJ. Frailty, sarcopenia and diabetes. J Am Med Dir Assoc. 2014;15:853–859. doi: 10.1016/j.jamda.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Nuttamonwarakul A, Amatyakul S, Suksom D. Twelve weeks of agua-aerobic exercise improve physiological adaptations and glycemic control in elderly patients with type 2 diabetes. J Exerc Physiol. 2012;15:64–70. [Google Scholar]
- Oliveira PP, Fachin SM, Tozatti J, Ferreira MC, Marinheiro LPF (2012) Comparative analysis of falls risk between patients with and without type 2 diabetes mellitus. Rev Assoc Med Bras 58: 234–239 Portuguese [PubMed]
- Park SW, Goodpaster BH, Strotmeyer ES, Kuller LH, Broudeau R, Kammerer C, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Cho YW, Newman AB. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30:1507–1512. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- Pereira A, Izquierdo M, Silva AJ, Costa AM, Bastos E, Gonzalez-Badillo JJ, Marques MC. Effects of high-speed power training on functional capacity and muscle performance in older women. Exp Gerontol. 2012;47:250–255. doi: 10.1016/j.exger.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Powell MW, Carnegie DH, Burke TJ. Reversal of diabetic peripheral neuropathy with phototherapy (MIRE) decreases falls and the fear of falling and improves activities of daily living in seniors. Age Ageing. 2006;35:11–16. doi: 10.1093/ageing/afi215. [DOI] [PubMed] [Google Scholar]
- Radaelli R, Botton CE, Wilhelm EN, Bottaro M, Brown LE, Lacerda F, Gaya A, Moraes K, Peruzzolo A, Pinto RS. Time course of low- and high-volume strength training on neuromuscular adaptations and muscle quality in elderly women. Age. 2014;36:881–892. doi: 10.1007/s11357-013-9611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Campillo R, Castillo A, de la Fuente CI, Campos-Jara C, Andrade DC, Álvarez C, Martínez C, Castro-Sepúlveda M, Pereira A, Marques MC, Izquierdo M. High-speed resistance training is more effective than low-speed resistance training to increase functional capacity and muscle performance in older women. Exp Gerontol. 2014;58:51–57. doi: 10.1016/j.exger.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Reeves MJ, Vaidya RS, Fonarow GC, Liang L, Smith EE, Matulonis R, Olson DM, Schwamm LH. Quality of care and outcomes in patients with diabetes hospitalized with ischemic stroke: findings from Get With the Guidelines-Stroke. Stroke. 2010;41:e409–e417. doi: 10.1161/STROKEAHA.109.572693. [DOI] [PubMed] [Google Scholar]
- Reid KF, Fielding RA. Skeletal muscle power: a critical determination of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40:1–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Mañas L, Fried LP (2015) Frailty in the clinical scenario. Lancet 385(9968):e7-9. doi: 10.1016/S0140-6736(14)61595-6. [DOI] [PubMed]
- Rodriguez-Mañas L, Bayer AJ, Kelly M, Zeyfang A, Izquierdo M, Laosa O, Hardman TC, Sinclair AJ, on behalf of the MID-Frail Consortium An evaluation of the effectiveness of a multi-modal intervention in frail and pre-frail older people with type 2 diabetes—the MID-Frail study: study protocol for a randomised controlled trial. Trials. 2014;15:34. doi: 10.1186/1745-6215-15-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals D, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Effects of endurance training on glucose tolerance and plasma lipids levels in older men and women. JAMA. 1984;252:645–649. doi: 10.1001/jama.1984.03350050033022. [DOI] [PubMed] [Google Scholar]
- Simmonds MJ, Minahan CL, Serre KR, Gass GC, Marshall Gradisnik SM, Haseler LJ, Sabapayhy S. Preliminary findings in the heart rate variability and haemorheology response to varied frequency and duration of walking in women 65–74 yr with type 2 diabetes. Clin Hemorheol Microcirc. 2012;51:87–99. doi: 10.3233/CH-2011-1514. [DOI] [PubMed] [Google Scholar]
- Sinclair A, Morley JE, Rodriguez-Manas L, Paolisso G, Bayer T, Zeyfang A, Bourdel-Marchasson I, Vischer U, Woo J, Chapman I, Dunning T, Meneilly G, Rodriguez-Saldana J, Gutierrez Robledo LM, Cukierman-Yaffe T, Gadsby R, Schernthaner G, Lorig K. Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc. 2012;13:497–502. doi: 10.1016/j.jamda.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Snowling NJ, Hopkins WG (2006) Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care 29(11):2518–2527 [DOI] [PubMed]
- Sung K, Bae S. Effect of a regular walking exercise program on behavior and biochemical aspects in elderly people with type II diabetes. Nurs Health Sci. 2012;11:438–495. doi: 10.1111/j.1442-2018.2012.00690.x. [DOI] [PubMed] [Google Scholar]
- Tan S, Li W, Wang J. Effects of six months of combined aerobic and resistance training for elderly patients with a long history of type 2 diabetes. J Sports Sci Med. 2012;11:495–501. [PMC free article] [PubMed] [Google Scholar]
- Terada T, Fresen A, Chahal BS, Bell GJ, McCargar LJ, Boulé NG. Feasibility and preliminary efficacy of high intensity interval training in type 2 diabetes. Diabetes Res Clin Pract. 2013;99:120–129. doi: 10.1016/j.diabres.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Tessier D, Ménard J, Fulop T, Ardilouze JL, Roy MA, Dubuc N, Dubois MF, Gauthier P. Effects of aerobic physical exercise in the elderly with type 2 diabetes mellitus. Arch Gerontol Geriatr. 2000;31:121–132. doi: 10.1016/S0167-4943(00)00076-5. [DOI] [PubMed] [Google Scholar]
- Umpierre D, Ribeiro PA, Kramer CK, Leitao CB, Zucatti AT, Azevedo MJ, Gross JL, Ribeiro JP, Schaan BD. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- Vinik AI, Vinik EJ, Colberg SR, Morrison S. Falls risk in older adults with type 2 diabetes. Clin Geriatr Med. 2015;31:89–99. doi: 10.1016/j.cger.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Volpato S, Bianchi L, Lauretani F, Bandinelli S, Guralnik JM, Zuliani G, Ferrucci L. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35:1672–1679. doi: 10.2337/dc11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray LA, Ofstedal MB, Langa KM, Blaum CS. The effect of diabetes on disability in middle-aged and older adults. J Gerontol A Biol Sci Med Sci. 2005;60:1206–1211. doi: 10.1093/gerona/60.9.1206. [DOI] [PubMed] [Google Scholar]