Abstract
Resistance training has been recommended for maintenance or improvement of the functional health of older adults, but its effect on acute cardiovascular responses remains unclear. Thus, the purpose of this study was to analyze the effect of 12 weeks of resistance training on post-exercise blood pressure (BP) in normotensive older women. Twenty-eight normotensive and physically inactive women (≥60 years) were randomly assigned to a training group (TG) or a control group (CG). The TG underwent a resistance training program (12 weeks, 8 exercises, 2 sets, 10–15 repetitions, 3 days/week), while the CG performed stretching exercises (12 weeks, 2 sets, 20 s each, 2 days/week). At baseline and after the intervention, participants were randomly submitted to two experimental sessions: a resistance exercise session (7 exercises, 2 sets, 10–15 repetitions) and a control session. BP was obtained pre- and post-sessions (90 min), through auscultation. Post-exercise hypotension was observed for systolic, diastolic, and mean BP in the TG (−6.1, −3.4, and −4.3 mmHg, respectively; P < 0.05) and in the CG (−4.1, −0.7, and −1.8 mmHg, respectively; P < 0.05). After the intervention period, the magnitude and pattern of this phenomenon for systolic, diastolic, and mean BP were similar between groups (TG −8.8, −4.1, and −5.7 mmHg, respectively; P < 0.05 vs CG −11.1, −5.8, and −7.6 mmHg, respectively; P < 0.05). These results indicate that a single session of resistance exercise promotes reduction in post-exercise BP and 12 weeks of resistance training program do not change the occurrence or magnitude of this hypotension. (ClinicalTrial.gov: NCT02346981)
Keywords: Resistance exercises, Female, Aging, Acute hemodynamic responses
Introduction
The aging process is characterized by physiological and functional changes that make older people more susceptible to the development of cardiovascular diseases (American College of Sports Medicine position stand 2009a). Decreased estrogen levels in older women after menopause may lead to an increase in sympathetic activity and a decrease in endothelial function, which may raise blood pressure (BP), increasing the chances for developing hypertension and other cardiovascular disease (Martins et al. 2001).
Resistance exercises have been recommended as a component of a comprehensive training program for older adults, since they attenuate the decrease in strength, power, and muscle mass that usually occur with the aging process (American College of Sports Medicine position stand 2009a; 2009b; Garber et al. 2011). In addition to these effects, a single session of resistance exercise acutely decreases BP to values lower than those observed at rest and/or values measured on a control day without exercise, a phenomenon called post-exercise hypotension (Kenney and Seals 1993). These responses have been considered clinically relevant, since the decreases in BP can be sustained for several hours after the end of an exercise session (Halliwill 2001).
The individual characteristics have played an important role in the acute post-exercise cardiovascular responses (Atkinson et al. 2005). Studies have shown that hypertensive patients and subjects with higher BP levels at rest demonstrate greater acute decreases in BP after resistance exercises when compared with normal BP levels (Atkinson et al. 2005). Recent studies have also observed that a higher training status or a chronic resistance training program attenuates acute BP decreases after a single bout of resistance exercises in adults or older adults (Costa et al. 2010; Moraes et al. 2012; Mota et al. 2013). However, these results are based in cross-sectional or noncontrolled interventional studies.
The possible attenuation of decreases in BP might restrict the benefit of post-resistance exercise BP decreases, which should be considered when prescribing resistance exercises for older subjects. Thus, the purpose of this study was to analyze the effect of 12 weeks of resistance training on post-exercise BP in normotensive older women. Although post-exercise hypotension might have higher clinical relevance in hypertensive patients, this study was carried out with normotensive participants in order to eliminate the effect of resistance training with drug interaction on BP responses. Based on previous studies (Costa et al. 2010; Moraes et al. 2012; Mota et al. 2013), our hypothesis was that a resistance training program would minimize the post-resistance exercise BP reduction, due to chronic reductions observed on rest BP after resistance training programs.
Methods
Experimental approach to the problem
Normotensive and physically inactive older women (≥60 years) were recruited and randomized into training group (TG) or control group (CG). The intervention programs were divided into three phases: initial (first 2 weeks), intermediate (from week 3 to week 10), and final (last 2 weeks). The TG performed a resistance training program (12 weeks, 8 exercises, 2 sets, 10–15 repetitions, 3 days/week), while the CG performed stretching exercises for the major muscle groups (12 weeks, 2 sets, 20 s each, 2 days/week).
All participants were randomly submitted to two acute experimental sessions (resistance exercise and control) in the initial and final phases of intervention. The resistance exercise session was relatively similar to those performed during the resistance training program (i.e., 7 exercises, 2 sets, 10–15 repetitions). In the control session, participants remained seated for 40 min. Before and after each session, the BP was obtained.
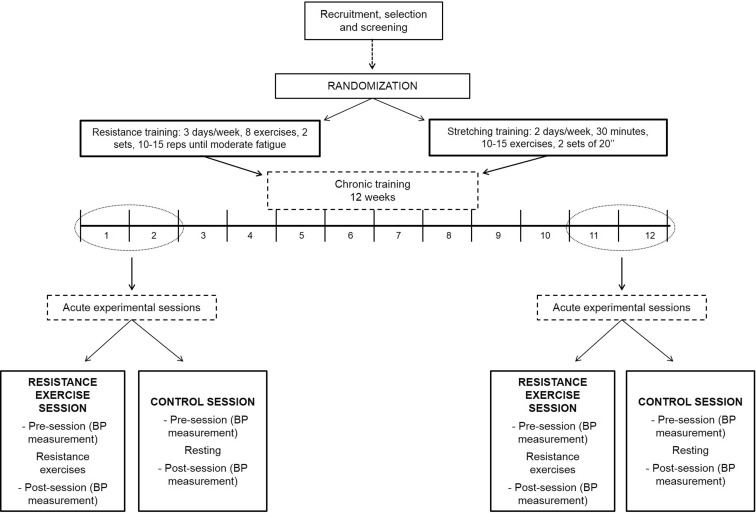
The effect of chronic resistance training on post-exercise BP was assessed by contrasting the acute BP responses to resistance exercise at the initial and final phases of intervention. Figure 1 represents a scheme of the design of the study.
Fig. 1.
Design of the study
Sample and recruitment
Recruitment was carried out through advertisements in newspapers and radio and through distribution of leaflets in the metropolitan area of the city, directed specifically to older adults (≥60 years). Two hundred older women responded to the advertisements and then completed health history and physical activity questionnaires. Participants were selected according to the following inclusion criteria: normotensive female; aged 60 to 80 years old, nonsmokers, without diabetes, cardiac disease, or renal dysfunction; not practicing regular physical exercise over the 6 months preceding the beginning of the investigation; being free of any musculoskeletal or other disorders that might affect their ability to participate in the study; not taking drugs with inotropic or chronotropic actions; and not using hormonal replacement therapy. All participants passed a diagnostic, graded exercise stress test with 12-lead electrocardiogram reviewed by a cardiologist and were released with no restrictions for participation in this study. Written informed consent was obtained from each participant after a detailed description of all procedures was provided. This study was approved by the local Research Ethics Committee (Protocol 120/08) and was registered in ClinicalTrials.gov with the ID number NCT02346981.
Thirty-one older women that met the inclusion criteria were randomly assigned to perform resistance or stretching exercises for 12 weeks. Twenty-eight participants finished the study and were included in the analysis. The reasons for the three dropouts were insufficient attendance to the training sessions (<85 % of the total sessions), a car accident, and lack of time to perform the evaluations. Considering a power of 90 %, an alpha error of 5 %, and a standard deviation of 3 mmHg for systolic BP, the minimal sample size necessary to detect a difference of 4 mmHg (Queiroz et al. 2014) was 10 participants per group.
Anthropometry
Body mass was measured to the nearest 0.1 kg using a calibrated electronic scale (Filizola, model ID 110, São Paulo, Brazil), with the participants wearing light workout clothing and no shoes. Height was measured with a stadiometer to the nearest 0.1 cm, while the participants were standing without shoes. Body mass index (BMI) was calculated as body mass in kilograms divided by the square of height in meters. Anthropometric measurements were performed at baseline and after 12 weeks of intervention according to procedures described in the literature (Gordon et al. 1988).
Dietary intake
Participants were instructed by a well-trained dietitian to complete food records on three nonconsecutive days (2 weekdays and 1 weekend day) in the first and last week of the intervention period. Participants were given specific instructions regarding the recording of portion sizes and quantities to identify all food and fluid intake, in addition to viewing food models in order to improve the accuracy. Total daily energy, protein, carbohydrate, and fat intake were calculated by nutrition analysis software (Avanutri Processor Nutrition Software, Rio de Janeiro, Brazil; Version 3.1.4). All participants were asked to maintain their normal diet during the entire study period. The water ingestion was ad libitum.
Acute experimental sessions
During the first two and the last 2 weeks of intervention, all participants performed two acute experimental sessions: resistance exercise and control sessions. The order of the sessions was randomized. The sessions were performed between 7 and 10 am, and an interval of at least 48 h was kept between sessions. In both experimental sessions, after arrival at the laboratory, participants rested for 10 min in a seated position, and after which BP was measured in triplicate (auscultation method).
Afterwards, during the resistance exercise session, participants moved to the exercise facility and performed resistance exercises. The exercise session consisted of seven exercises performed on specific resistance training machines in the following order: chest press, knee extension, wide-grip front lat pulldown, leg curl, preacher curl, seated calf raise, and triceps pushdown. Participants completed two sets of 10–15 submaximal repetitions (until moderate fatigue or stopped when it began to be difficult) (Nelson et al. 2007). The rest intervals between sets and exercises were 60 to 90 s and 2 to 3 min, respectively. A personal trainer supervised all the sessions. During the control session, participants remained seated for 40 min in the exercise facility without performing any exercise.
After both experimental sessions, subjects returned to the laboratory, where they rested in a sitting position for 90 min (post-session period). During this period, BP was measured at 15, 30, 60, and 90 min. Water ingestion was ad libitum throughout the entire time.
Blood pressure measurements
Acute BP was obtained using the auscultation method with a mercury sphygmomanometer (Missouri, São Paulo, Brazil) and a stethoscope (Littmann Classic II, St. Paul, MN, USA). The right arm was positioned on a table and elevated to a height corresponding to the midpoint of the sternum. The cuff was placed securely around the arm with the indicator immediately above the antecubital area. All measurements were performed by the same experienced individual who had excellent reliability of measurement (systolic BP (SBP): ICC = 0.99, 95 % CI = 0.98–0.99; diastolic BP (DBP): ICC = 0.97, 95 % CI = 0.95–0.98).
For data analysis, the post-session BP obtained in the four moments (15, 30, 60, and 90 min) were compared were averaged and compared to rest. In addition, the net changes (Δ resistance exercise session − Δ control session) for post-session BP average at baseline and after 12 weeks were also calculated. A post-session BP difference from resting values or a net change below zero was considered post-resistance exercise hypotension.
Chronic interventions
Supervised resistance training was performed on three nonconsecutive days per week (Mondays, Wednesdays, and Fridays) during the morning for 12 weeks. The program followed recommendations for resistance training in older population to improve muscular endurance and strength (American College of Sports Medicine 2009b; Garber et al. 2011). The resistance training was a whole-body program, comprised of eight exercises performed in the following order: chest press, knee extension, wide-grip front lat pulldown, leg curl, preacher curl, seated calf raise, triceps pushdown, and abdominal crunches. In two previous weeks, participants were initially submitted to six sessions of familiarization with the equipment and exercises. In the following 12 weeks, participants performed two consecutive sets of 10–15 submaximal repetitions (until moderate fatigue or stopped when it began to be difficult) (Nelson et al. 2007), except for abdominal crunches, which was performed on 20 to 30 repetitions without overload. Participants were instructed to inhale during the eccentric phase and exhale during the concentric phase, while maintaining a constant velocity of movement at a ratio of approximately 1:2 s (concentric and eccentric phases, respectively). Subjects rested for 60 to 90 s between each set and for 2 to 3 min between each exercise. All participants were personally supervised by exercise professionals with substantial experience in resistance training prescription throughout each training session in order to reduce deviations from the study protocol and to ensure subject safety. Instructors adjusted the loads of each exercise according to the participant’s ability and improvements in exercise capacity throughout the study in order to ensure that they were exercising with as much resistance as possible while maintaining proper exercise technique. Progression was planned so that when 15 repetitions were completed in both sets for two consecutive training sessions, weight was increased by 2–5 % for upper limb exercises and by 5–10 % for lower limb exercises in the following session (American College of Sports Medicine 2009b).
The CG performed stretching exercises based on the American College of Sports Medicine recommendations twice a week, during the 12 weeks (Garber et al. 2011). All training sessions lasted 25–30 min and included active stretching exercises for both upper and lower body muscle groups. For each stretching exercise, the movement was held at the maximal stretch position for 20 s, and this procedure was repeated twice. The rest interval between trials was 15 s and a minimum of 30 s between the different exercises. Participants in both groups were instructed to maintain their normal level of physical activity and were asked not to start a new exercise regimen during the study period.
Statistical analysis
The data were stored and analyzed using the Statistical Package for the Social Sciences (SPSS for Windows Version 17.0). Normality was checked by Shapiro-Wilk’s test. Baseline or pre-acute experimental session differences between groups were assessed with an independent sample t test. A two-factor (group vs. training time) ANOVA with repeated measures was employed to analyze the chronic effects of resistance training on anthropometry and dietary intake. The acute experimental session effect on post-session BP values was assessed by ANOVA with repeated measures. The chronic effect of resistance training on BP net change was analyzed by a two-way (group vs. training time) ANOVA with repeated measures. For all ANOVA analyses, when sphericity was violated, as indicated by Mauchly’s test, the Greenhouse-Geisser correction was used. A Fisher’s post hoc test was used when significant F ratios were found for main or interaction effects. For all statistical analyses, significance was accepted at P < 0.05.
Results
General characteristics of the study participants at baseline and after 12 weeks of intervention are shown in Table 1. No intra- or intergroup differences for age, anthropometric variables, and dietary intake were observed (P > 0.05). The effectiveness of this RT program in improving muscular strength (+10 % for upper lower limb and +13 % for lower body limb; P < 0.05), functional fitness (improvements from 8 to 23 %), as well as in reducing rest SBP (−5 mmHg) in this sample has already been showed in previous studies of our research group (Gerage et al. 2013a, b).
Table 1.
General characteristics and dietary intake of the sample measured at baseline and after 12 weeks of intervention in the control (CG) and the training (TG) groups (mean ± standard deviation)
| TG (n = 14) | CG (n = 14) | |||
|---|---|---|---|---|
| Baseline | After 12 weeks | Baseline | After 12 weeks | |
| Age (years) | 65.5 ± 5.0 | 66.2 ± 4.1 | ||
| Body mass (kg) | 57.3 ± 6.5 | 57.2 ± 6.5 | 61.1 ± 11.7 | 61.6 ± 12.2 |
| Height (cm) | 156.7 ± 5.1 | 156.5 ± 4.9 | 157.5 ± 7.1 | 157.6 ± 7.0 |
| BMI (kg/m2) | 23.9 ± 2.9 | 24.0 ± 2.6 | 25.1 ± 3.4 | 25.4 ± 3.8 |
| Energy (kcal/day) | 1440 ± 197 | 1432 ± 179 | 1379 ± 160 | 1420 ± 159 |
| Carbohydrates (g/kg) | 3.7 ± 0.7 | 3.6 ± 0.8 | 3.1 ± 0.7 | 3.3 ± 0.9 |
| Protein (g/kg) | 1.1 ± 0.3 | 1.0 ± 0.3 | 0.9 ± 0.3 | 1.0 ± 0.3 |
| Lipids (g/kg) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.2 |
Values are expressed as mean ± standard deviation. No intra- or intergroup differences for age, anthropometric variables and dietary intake were observed (P > 0.05)
BMI body mass index
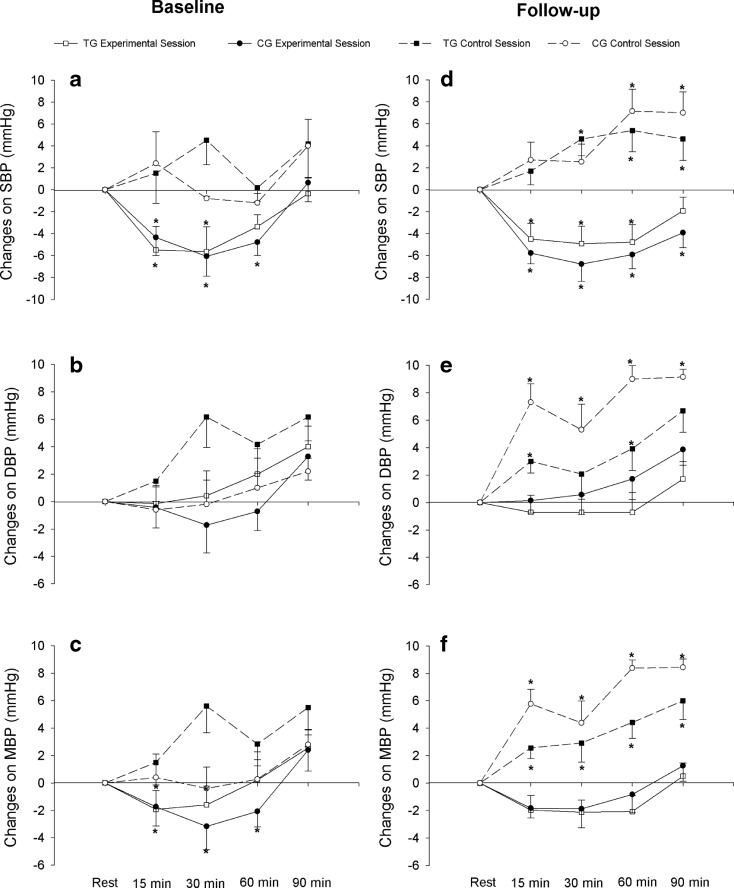
Figure 2 illustrates changes in SBP, DBP, and mean BP (MBP) after a single resistance exercise session and after a control session compared with rest. At baseline, we observed a significant main effect of time (F = 3.74; P < 0.001) for SBP (Fig. 2a) with acute resistance exercise. In both groups, there was a reduction from 15 to 60 min after the end of the session, when compared to rest. For DBP (Fig. 2b) and MBP (Fig. 2c), no main effect or interaction group vs time was observed (P > 0.05).
Fig. 2.
Systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean blood pressure (MBP) for training group (TG = 14) and control group (CG = 14), in resistance exercise and control sessions at baseline (a, b, and c) and after 12 weeks of intervention - follow up - (d, e, and f). *P < 0.05 vs. rest
After 12 weeks of intervention, in post-training measurement, acute resistance exercise reduced only SBP (Fig. 2d, F = 3.43; P < 0.001), from 15 to 60 min of post-resistance exercise in TG and from 15 to 90 min in CG. On the other hand, for DBP (Fig. 2e) and MBP (Fig. 2f), no main effect or interaction group vs. time was found (P > 0.05). SBP, DBP, and MBP responses following the control session at baseline did not change significantly (P > 0.05) from rest in both groups. However, following the control session after 12 weeks of training, a significant main effect of time (P < 0.05) was observed, with higher values of SBP, DBP, and MBP compared to rest for both groups.
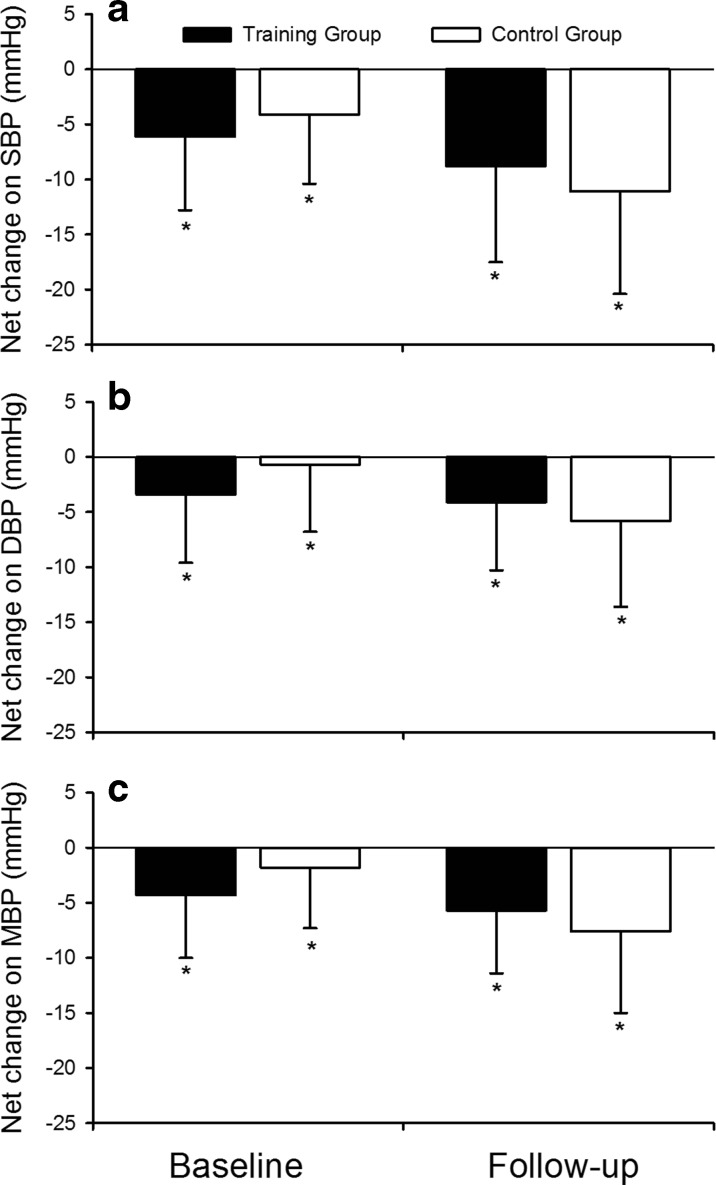
Figure 3 presents the post-resistance exercise net changes (Δ resistance exercise session − Δ control session) for SBP, DBP, and MBP at baseline and after 12 weeks of training. Similar levels of post-resistance exercise hypotension were observed for SBP, DBP, and MBP when analyzing TG and CG, both at baseline and after 12 weeks of training (P < 0.05). In addition, training programs did not change the magnitude or pattern of post-exercise hypotension in any of the groups (P > 0.05). Considering the net change, participants that presented post-exercise hypotension were classified as responders. Thus, at baseline, we found 52 and 48 % responders for TG and CG, respectively, according to SBP responses; 86 and 79 % for DBP; and 86 and 93 % for MBP, without differences between groups (P > 0.05). On the other hand, after 12 weeks of training, we observed 100 % of responders for SBP, 100 and 93 % for TG and CG, respectively, for DBP, and 100 % for MBP, without differences between groups (P > 0.05).
Fig. 3.
Net changes for systolic blood pressure ((SBP), a), diastolic blood pressure ((DBP), b), and mean blood pressure ((MBP), c) for training group (TG, n = 14) and control group (CG, n = 14) at baseline and after 12 weeks of resistance training (follow up). *P < 0.05 vs. rest
Discussion
The major finding of our investigation was that a single session of resistance exercise significantly decreased post-exercise SBP, DBP, and MBP in normotensive older women. Moreover, 12 weeks of resistance training did not affect the pattern or magnitude of the post-resistance exercise BP in these participants. These results are in disagreement with our previous hypothesis that resistance training would minimize the post-exercise BP responses in older, normotensive women.
At baseline, when all participants were untrained, our results indicated significant mean reductions in SBP (−5.1 mmHg), DBP (−2.0 mmHg), and MBP (−3.1 mmHg) after a single session of resistance exercise when the net effect was considered. The magnitude of these changes in BP are within the range of values that has been observed in younger and middle-aged normotensive healthy subjects (Queiroz et al. 2009, 2013, 2014) and slightly lower (especially for DBP) than that observed in normotensive/pre-hypertensive overweight and obese middle-aged women (−7 mmHg for SBP and −6 mmHg for DBP) (Tibana et al. 2013). It is not clear why a lower magnitude of post-exercise hypotension was observed when compared with Tibana and colleagues’ investigation (Tibana et al. 2013). A possible explanation could be related with nutritional status of the participants included in these two studies, given that the exercise protocol adopted in both studies was very similar. In our study, the majority of women had normal weight or slightly overweight, while only overweight or obese women were included in Tibana and colleagues’ study (Tibana et al. 2013). Probably, endocrine, neural, autonomic, and cardiac alterations related to obesity lead obese participants to be more sensitive to post-exercise BP responses (Bastien et al. 2014), but this hypothesis needs to be confirmed in future studies.
Regarding the chronic effect of resistance training on post-exercise BP responses, previous studies have shown that the decrease in BP observed after a single session of resistance exercise is attenuated after a period of training (Costa et al. 2010; Moraes et al. 2012; Mota et al. 2013). Moraes et al. (2012) observed that the acute decreases in post-resistance exercise BP were attenuated after 12 weeks of resistance training in hypertensive men. Mota et al. (2013) did not find post-exercise hypotension in the fourth month of resistance training in hypertensive older women. Costa et al. (2010) also observed greater decreases in acute BP in hypertensive untrained individuals compared to trained older women in a cross-sectional study. Nevertheless, our results indicate that resistance training did not affect the magnitude of the decreases in BP after an acute session of resistance exercise in normotensive older women. As the methodological approach of the present study included a control group and an acute control session for both groups, it is feasible that the divergence between studies is related to the more robust design of our study, although differences in the sample characteristics, such as sex (men vs. women) and levels of BP (hypertensive vs. normotensive), cannot be excluded. In addition, this divergence could be attributed to different resting BP.
The resistance exercise protocol is unlikely to be the reason for differences between studies, since the current and previous studies (Costa et al. 2010; Moraes et al. 2012; Mota et al. 2013) adopted similar protocols (7–10 exercises, 3 sets of 8 to 12 repetitions with 60–80 % 1RM). On the other hand, the period of acute and chronic resistance exercise sessions was different between studies. In our study, data collection occurred in the morning, while the other investigations were performed in the afternoon (Moraes et al. 2012; Mota et al. 2013). Brito (2013) observed that post-exercise hypotension was higher when the exercise was performed in the morning compared to the afternoon in pre-hypertensive adult men, which may explain the divergence between studies.
The magnitude of DBP reduction in our study was lower than that found in middle-aged women with fibromyalgia (−8 to −13 mmHg) after 12 weeks of resistance training (Kingsley et al. 2011). In addition, the intervention period also did not affect the acute BP response after a single bout of resistance exercise. The greater age of the sample in our study is the more likely explanation for this divergence, since aging has been associated with arterial stiffness and consequently increases in peripheral vascular resistance (O’Rourke and Hashimoto 2007).
Our results indicated that acute BP reductions are observed after a resistance exercise program session even if the training program changes the resting BP. Practical application of these results is particularly important for older adults, since the maintenance of the acute decrease in BP during part of the day can be useful to decrease cardiovascular workload (Halliwill et al. 2013). Additionally, a chronic resistance training program that promotes several benefits for health, including increase in bone mineral density (Marques et al. 2012), muscle mass (Cadore et al. 2013), strength, and functional capacity (Cadore et al. 2013; Gerage et al. 2013a), does not seem to influence this phenomenon. It is important to highlight that we showed in previous studies the effectiveness of this resistance training protocol for improving muscular strength (+10 % for upper lower limb and +13 % for lower body limb; P < 0.05), functional fitness (improvements from 8 to 23 %), as well as for reducing rest BP (−5 mmHg for SBP) in this same sample (Gerage et al. 2013a, b).
The present study has some limitations. A longer period of monitoring, both in laboratory and ambulatory settings, could provide information on the long-term effect of a resistance training program on post-exercise BP. On the other hand, a short period of monitoring (90 min of recovery) could mask important responses, which could be different over time (Queiroz et al. 2009), and also hinder us knowing how long this acute response may last. Moreover, we were unable to control the temperature at the moment of measurements and the sodium intake of the participants throughout the study, factors that might have influenced, at least in part, our results. Another limitation is the absence of a pure control group comprised of sedentary participants, since this group was submitted to a stretching program that could have promoted some cardiovascular adaptations, although this was not observed in our study. Although all the women were post-menopausal, we did not control how long they were in this phase; however, a recent study observing the effect of a resistance training program on post-resistance exercise responses did not find significant differences between pre- and post-menopausal women (Cardoso et al. 2014).
In contrast, it is important to highlight the strengths of our study. We only included older adults and this is remarkable, since aging is related to an increased cardiovascular risk (Seals et al. 2014) and with adverse cardiovascular system changes, such as increased arterial stiffness and BP (Hickson et al. 2010). Thus, our findings make post-exercise hypotension even more relevant at this age. Additionally, our study included a control session, which allowed us to identify whether the BP drop observed after the resistance exercise session could actually be attributed to the applied exercise protocol. Another important point was the inclusion of a control group, which allowed us to attribute the chronic results to the training protocol adopted. Finally, to our knowledge, this is the first study that analyzed the effect of a resistance training program on post-exercise BP in combination with dietary assessment of the participants, emphasizing that it did not change over time, which is relevant considering the influence of this factor on responses of BP (Houston 2013; Saneei et al. 2014).
In conclusion, our findings suggest that a single bout of resistance exercise decreases BP, and these responses are not altered by 12 weeks of resistance training in normotensive older women. This further emphasizes the importance of resistance training for improving health, considering also the acute and chronic cardiovascular benefits of the elderly women.
Acknowledgments
We would like to express thanks to all the participants for their engagement in this study, the Coordination of Improvement of Higher Education Personnel (CAPES/Brazil) for the master scholarship conferred to A.M.G. and M.A.N., and the National Council of Technological and Scientific Development (CNPq/Brazil) for the grants conceded to E.S.C. and R.M.R.D. This study was supported (Protocol 15466) by the Araucária Foundation for the Support of Scientific and Technological Development of Paraná (FAADCT/Brazil).
Conflict of interest
The authors declare that they have no competing interests.
References
- American College of Sports Medicine position stand Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine position stand Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Cable NT, George K. The relationship between baseline blood pressure and magnitude of postexercise hypotension. J Hypertens. 2005;23:1271–1272. doi: 10.1097/01.hjh.0000170392.92073.33. [DOI] [PubMed] [Google Scholar]
- Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56:369–381. doi: 10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Brito LC (2013) Hipotensão pós-exercício aeróbico e seus mecanismos hemodinâmicos e neurais em pré-hipertensos: influência da fase do dia e associação com a regulação endócrina circadiana. Universidade de São Paulo
- Cadore EL, Rodriguez-Manas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res. 2013;16:105–114. doi: 10.1089/rej.2012.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso GA, Silva AS, de Souza AA, Dos Santos MA, da Silva RS, de Lacerda LM, Motae MP. Influence of resistance training on blood pressure in patients with metabolic syndrome and menopause. J Hum Kinet. 2014;43:87–95. doi: 10.2478/hukin-2014-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JBY, Gerage AM, Gonçalves CGS, Pina FLC, Polito MD. Influence of the training status on the blood pressure behavior after a resistance training session in hypertensive older females. Rev Bras Med Esporte. 2010;16:103–106. [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP. American College of Sports Medicine position stand. quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Gerage AM, Forjaz CL, Nascimento MA, Januario RS, Polito MD, Cyrino ES. Cardiovascular adaptations to resistance training in elderly postmenopausal women. Int J Sports Med. 2013;34:806–813. doi: 10.1055/s-0032-1331185. [DOI] [PubMed] [Google Scholar]
- Gerage AM, Januário RSB, Nascimento MA, Pina FLC, Cyrino ES. Impact of 12 weeks of resistance training on physical and functional fitness in elderly women. Rev Bras CineantropomDesempenho Hum. 2013;15:145–154. [Google Scholar]
- Gordon CC, Chumlea WC, Roche AF. Stature, recumbent length, and weight. In: Lohman TG, Roche AF, Martorell R, editors. Anthropometric standardization reference manual. Champaign: Human Kinetics Books; 1988. pp. 3–8. [Google Scholar]
- Halliwill JR. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc Sport Sci Rev. 2001;29:65–70. doi: 10.1097/00003677-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Buck TM, Lacewell AN, Romero SA. Postexercise hypotension and sustained postexercise vasodilatation: what happens after we exercise? Exp Physiol. 2013;98:7–18. doi: 10.1113/expphysiol.2011.058065. [DOI] [PubMed] [Google Scholar]
- Hickson SS, Butlin M, Graves M, Taviani V, Avolio AP, McEniery CM, Wilkinson IB. The relationship of age with regional aortic stiffness and diameter. JACC Cardiovasc Imag. 2010;3:1247–1255. doi: 10.1016/j.jcmg.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Houston M. Nutrition and nutraceutical supplements for the treatment of hypertension: part I. J Clin Hypertens (Greenwich) 2013;15:752–757. doi: 10.1111/jch.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney MJ, Seals DR. Postexercise hypotension. Key features, mechanisms, and clinical significance. Hypertension. 1993;22:653–664. doi: 10.1161/01.HYP.22.5.653. [DOI] [PubMed] [Google Scholar]
- Kingsley JD, McMillan V, Figueroa A. Resistance exercise training does not affect postexercise hypotension and wave reflection in women with fibromyalgia. Appl Physiol Nutr Metab. 2011;36:254–263. doi: 10.1139/h10-105. [DOI] [PubMed] [Google Scholar]
- Marques EA, Mota J, Carvalho J. Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age (Dordr) 2012;34:1493–1515. doi: 10.1007/s11357-011-9311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins D, Nelson K, Pan D, Tareen N, Norris K. The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: data from NHANES III. J Gend Specif Med. 2001;4(10-13):20. [PubMed] [Google Scholar]
- Moraes MR, Bacurau RF, Simoes HG, Campbell CS, Pudo MA, Wasinski F, Pesquero JB, Wurtele M, Araujo RC. Effect of 12 weeks of resistance exercise on post-exercise hypotension in stage 1 hypertensive individuals. J Hum Hypertens. 2012;26:533–539. doi: 10.1038/jhh.2011.67. [DOI] [PubMed] [Google Scholar]
- Mota MR, Oliveira RJ, Terra DF, Pardono E, Dutra MT, de Almeida JA, Silva FM. Acute and chronic effects of resistance exercise on blood pressure in elderly women and the possible influence of ACE I/D polymorphism. Int J Gen Med. 2013;6:581–587. doi: 10.2147/IJGM.S40628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- Queiroz AC, Gagliardi JF, Forjaz CL, Rezk CC. Clinic and ambulatory blood pressure responses after resistance exercise. J Strength Cond Res. 2009;23:571–578. doi: 10.1519/JSC.0b013e318196b637. [DOI] [PubMed] [Google Scholar]
- Queiroz AC, Rezk CC, Teixeira L, Tinucci T, Mion D, Forjaz CL. Gender influence on post-resistance exercise hypotension and hemodynamics. Int J Sports Med. 2013;34:939–944. doi: 10.1055/s-0033-1337948. [DOI] [PubMed] [Google Scholar]
- Queiroz AC, Sousa JC, Cavalli AA, Silva ND, Jr., Costa LA, Tobaldini E, Montano N, Silva GV, Ortega K, Mion D, Jr., Tinucci T, Forjaz CL (2014) Post-resistance exercise hemodynamic and autonomic responses: comparison between normotensive and hypertensive men. Scand J Med Sci Sports [Epub ahead of print] [DOI] [PubMed]
- Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253–1261. doi: 10.1016/j.numecd.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You’re only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 2014;29:250–264. doi: 10.1152/physiol.00059.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibana RA, Pereira GB, Navalta JW, Bottaro M, Prestes J. Acute effects of resistance exercise on 24-h blood pressure in middle aged overweight and obese women. Int J Sports Med. 2013;34:460–464. doi: 10.1055/s-0032-1323819. [DOI] [PubMed] [Google Scholar]