Abstract
The Dartmouth Center for Cancer Nanotechnology Excellence – one of nine funded by the National Cancer Institute as part of the Alliance for Nanotechnology in Cancer – focuses on the use of magnetic nanoparticles for cancer diagnostics and hyperthermia therapy. It brings together a diverse team of engineers and biomedical researchers with expertise in nanomaterials, molecular targeting, advanced biomedical imaging and translational in vivo studies. The goal of successfully treating cancer is being approached by developing nanoparticles, conjugating them with Fabs, hyperthermia treatment, immunotherapy and sensing treatment response.
Keywords: immunotherapy, magnetic hyperthermia, magnetic nanoparticles
The Dartmouth Center for Cancer Nanotechnology Excellence (DCCNE), led by principle investigator (P.I.) Ian Baker and Co-P.I. Keith Paulsen, is one of nine centers funded by the National Cancer Institute as part of the Alliance for Nanotechnology in Cancer. Unlike other centers, DCCNE has a single focus on cancer therapy. Research in magnetic nanoparticle (mNP) hyperthermia and mNP-induced immunotherapy via an externally applied alternating magnetic field (AMF) are supported by mNP production and mNP therapy monitoring efforts. Impetus for the center resulted from a series of internally funded studies that produced novel iron/iron oxide nanoparticles [1–4], efficacy data in a murine xenograft model of human breast cancer [5,6] and innovative technologies for magnetic nanoparticle imaging and temperature measurement [7–9].
The DCCNE is a collaboration between faculty and researchers in Dartmouth’s Thayer School of Engineering, Geisel School of Medicine and the Norris Cotton Cancer Center. The DCCNE research program consists of three cores (Nanoparticle Development, Production and Characterization; Toxicology, Pathology and Biodistribution; Biostatistics, Data Analysis and Computation) supporting four original research projects (Optimization of nanoparticle targeting in ovarian and breast cancer; Spectroscopic quantification of ligand binding in vivo; Optimization of magnetic nanoparticle breast cancer treatment; Magnetic nanoparticle immunotherapy against ovarian cancer, later melanoma). Key results from recent DCCNE activities are outlined below.
The Nanoparticle Core
Although the DCCNE began with newly developed mNPs [1–4], particles were needed those heat well at low AMFs (<300 Oe): the use of high AMFs is problematic since significant eddy current heating of tissue can occur. Generating localized heat at low field strengths (<300 Oe) allows treatment of deep-seated tumors without causing overexposure of nontargeted organs, tissues and skin from eddy current heating. Thus, the Nanoparticle Core, led by Ian Baker and Katsiaryna Kekalo, developed a new generation of high-performance mNPs which exhibit efficient heating at low AMF: in some cases more than eight-times higher specific absorption rate, a measure of the heating efficiency, relative to other mNPs (see Figure 1).
Figure 1.
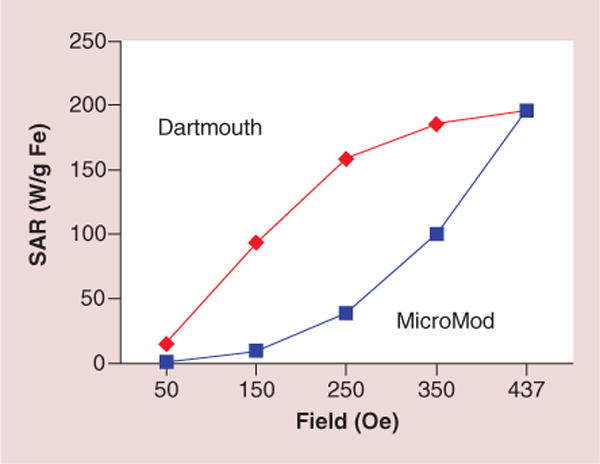
Specific absorption rate, a measure of the heating efficiency, of Dartmouth- and commercial magnetic nanoparticles at the same concentration as a function of alternating magnetic field strength at 164 kHz.
The Dartmouth mNPs consist of small single crystals of iron oxide (2–5 nm) gathered into aggregates (20–40 nm) with polysaccharide embedded within their structure (see Figure 2).
Figure 2.
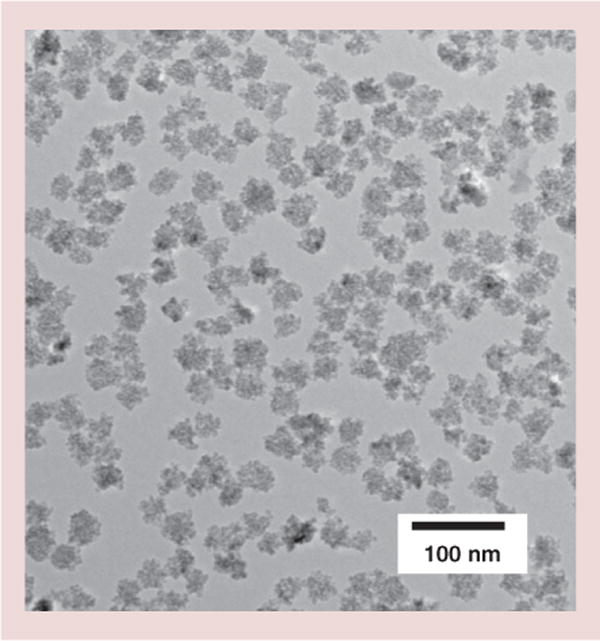
Transmission electron micrograph of Dartmouth magnetic nanoparticles.
A wide variety of mNPs variants have been produced, some containing trace quantities of other metals that enhance heating behavior (Co, Mn, Zn) or enable ultrasensitive detection and quantification (Eu). As part of the Nanoparticle Core, the Dartmouth electron microscope facility, led by Charles Daghlian, characterizes the mNPs and contributes to studies such as analysis of subcellular mNP localization.
Biodistribution; Biostatistics, Data Analysis & Computation Core
The Biodistribution, Biostatistics, Data Analysis and Computation Core is led by Eugene Demidenko, who provides comprehensive statistical analysis of all DCCNE data, and Fridon Shubitidze, who investigates the effects of AMF activation on mNPs of various shapes and sizes. The latter calculations of particle–particle, particle–core and particle–aggregate interactions showed that irregularly shaped mNPs produce higher specific absorption rates than spherical mNPs. These studies, conducted in close collaboration with the Nanoparticle Core, informed the design and development of the customized Dartmouth mNPs [10]. While mNP hyperthermia has progressed significantly, the conventional approach of mNP activation using an AMF coil has inherent limitations. For example, excessive risk of damaging normal tissues occurs when treating deeply seated tumors, such as those associated with pancreatic and colorectal cancers. In an effort to address this barrier to clinical translation, Shubitidze and colleagues developed an innovative AMF guiding and focusing system that may enable application of mNP hyperthermia to the treatment of deep seated tumors. Preliminary studies illustrated that the AMF guiding and focusing system can: precisely direct AMF to a particular volume; minimize eddy currents and associated off-target side effects; and achieve high specific heating in tumor masses.
Toxicology, Pathology & Biodistribution Core
This core, led by Lionel Lewis, provides animal toxicology/toxicokinetic studies, animal pathology-histopathology, mNP biodistribution studies and pharmacokinetic data analysis/modeling. An important function of the core is the analysis of mNP dissemination and organ uptake following their intratumoral and/or systemic administration in vivo.
Project 1: Optimization of nanoparticle targeting in ovarian & breast cancer
Karl Griswold and Tillman Gerngross lead the efforts of the molecular targeting group under this project. The interdisciplinary team, spearheaded in the laboratory by Christian Ndong, has examined active molecular targeting as a means to selectively localize mNPs to cancer cells, with an eye toward preferential tumor accumulation in complex in vivo settings. Their systematic approach began with engineering Fabs from clinically validated, anticancer, monoclonal antibodies, such as trastuzumab, pertuzumab and farletuzumab. These Fab-targeting moieties were designed to have reduced size while maintaining the binding specificity of the full-length monoclonal antibodies. Additionally, the Fabs were engineered to enable high fidelity, site-specific coupling to the surface of chemically activated mNP. This site-specific conjugation ensures proper orientation of the antibodies on the mNP surface, thereby maximizing the binding potential of the antibody–mNP conjugates. Rigorous quality control measures were implemented throughout the studies to ensure that the antibodies maintained functionality both before and after coupling to the nanoparticle substrates. Using a diverse panel of cell lines, the Project 1 researchers showed that targeted mNPs bound human cancer cells in a receptor dependent fashion [11,12]. Moreover, the antibody-mediated targeting resulted in rapid mNP internalization by cancer cells, and this facile intracellular accumulation has important implications for both diagnostic and therapeutic applications of mNPs (see Figure 3).
Figure 3. Transmission electron micrograph of farletuzufab magnetic nanoparticle conjugates binding to KB tumor cells, which overexpress α-folate receptor.
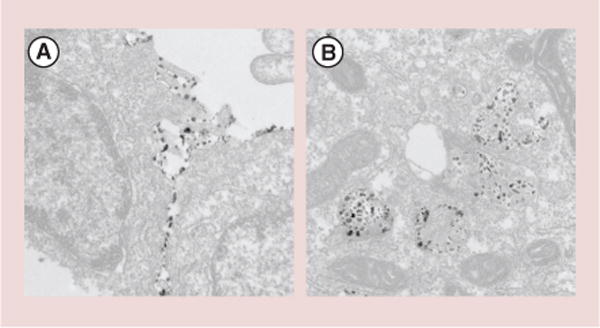
(A)Antibody-targeted magnetic nanoparticle binding to the extracellular plasma membrane at the interface between two cells. (B) Targeted magnetic nanoparticles that have been internalized into intracellular vesicles following binding on the cell surface.
Building upon their in vitro studies of cancer cell line selectivity, the team collaborated with other DCCNE projects and scientific cores to test the performance of their mNPs in murine xenograft models of human cancer. In orthotopic breast and ovarian cancer models, systemically administered, antibody-targeted mNPs exhibited significant tumor tissue accumulation, whereas nontargeted control mNPs failed to show any detectable association with malignant masses [11,12]. Importantly, the previously observed cellular internalizing properties of the targeted mNP translated into the more complex in vivo systems, underscoring the potential utility of the antibody mNP constructs. The team is now concluding follow-up experiments with more sophisticated bispecific antibody mNP conjugates.
Project 2: Spectroscopic quantification of ligand binding in vivo
John Weaver leads efforts to develop methods of characterizing the microenvironment surrounding mNPs using magnetic spectroscopy of Brownian motion (MSB). The technique exploits the high sensitivity available from measuring the harmonics of the magnetization induced by magnetic mNPs in an AMF to measure the mNP response in vivo. Larger mNPs that relax via Brownian mechanisms are used to maximize the number of environmental factors that influence the spectra. The approach allows facile measurement of temperature [13–15], viscosity [16,17], relaxation time [18], mNP uptake in cells [19], matrix stiffness [20], bound state [21–25] and mNP number [26]. Tumor microenvironment characteristics can be measured using the shape of the spectra, because it reflects the ability of the mNPs to align with the alternating field. The mNPs rotational freedom is reflected by the inflection of the magnetization as the saturated mNPs are flipped to the opposite direction. mNPs that are relatively free to move have a very sharp transition inducing high signals at the higher harmonics. mNPs that are more inhibited have a slower, smoother transition resulting in lower signals at the higher harmonics. At present, the team is most interested in using mNP rotational freedom to measure protein–protein binding interactions [17–18,22,27]. By functionalizing the mNPs with binding ligands to specific analytes, an mNP ‘sandwich’ can be formed around each analyte molecule, and precise concentration measurements can be made from the MSB spectra [24].
Project 2 investigators have further improved the MSB sensitivity by measuring the magnetization perpendicular to the AMF generated using a small static field in that direction [28]. The perpendicular magnetization is geometrically isolated from the applied AMF, allowing smaller signals to be measured. The sensitivity is high enough that biologically relevant analyte concentrations can be measured in vivo [25]. The group has been exploring inflammatory cytokine measurements, because many diseases and conditions have inflammatory components. IL-6 has been the first target, as it is a very common, innate immune marker and it reaches higher concentrations than most cytokines.
Penetration of nanoparticles into tumor tissue is limited by pressure, leakage and convection rates from the vascular space to the interstitial compartments of the lesion. Delivery of agents to bind to cell surface receptors can be tracked by ratiometric measurement, and systematic study of the factors that influence binding and how they can be affected has been the focus of the team led by the project’s coinvestigator, Brain Pogue. This group uses dual wavelength fluorescence imaging of labeled particles and conjugates to develop methodologies for quantitative in vivo imaging. They have examined delivery and binding to a number of cancer epithelial cells, both in solid tumors and breast cancer lymphatic metastases, as well as targeted binding to endothelial cells in melanoma tumors (see Figure 4).
Figure 4. Lymph node images using fluorescent dyes.
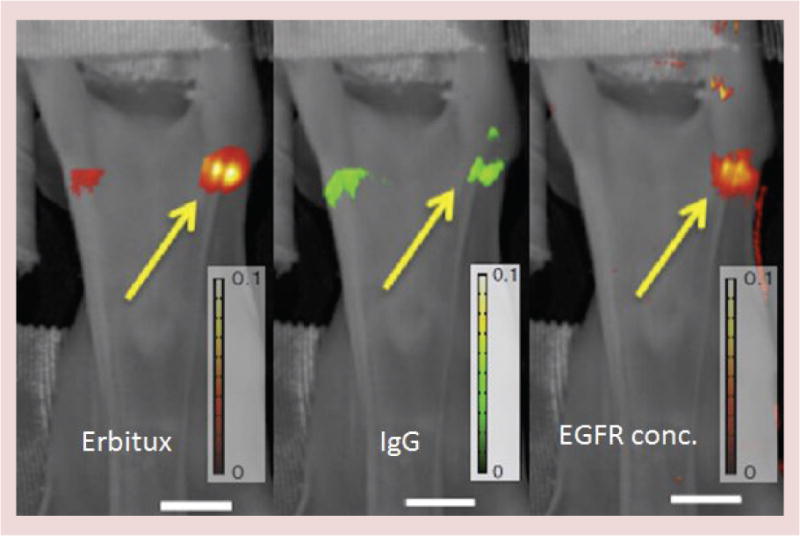
Lymph node imaging of tumors with Eribtux labeled with fluorescent reporter IRDye800 and nonspecific reporter IgG labeled with IRDye680, are shown (left and middle) with the processed image of receptor concentration for epidermal growth factor receptor shown (right). Imaging with this approach shows the ability to detect and quantify metastases down to as few as 200 cells in the lymph node [29]. Scale bar: 1 cm.
EGFR: EGF receptor.
Project 3: Optimization of magnetic nanoparticle breast cancer treatment
This project, led by Jack Hoopes with coinvestigators Peter Kaufman and Lionel Lewis, focuses primarily on cancer treatment with dextran or starch coated iron oxide mNPs, with or without antibodies or polyethylene glycol. The fundamental goal of the research is to develop an understanding of clinically motivated parameters that will enable better in vivo mNP tumor imaging and enhance the efficacy of mNP-AMF hyperthermia treatment. A wide range of experiments have spanned the spectrum from in vitro phantoms to rodent and large animal in vivo models, with experimental designs that are closely coordinated with pending clinical trials in human subjects (work has progressed to the Internal Review Board and US FDA Investigational Device Exclusion/Investigational New Drug-Filing level). Project goals include achieving 80% tumor coverage with >1.0 mg iron/gram tumor following systemic/intra-arterial or controlled release intratumoral mNP injection. The group is also examining the combination of mNP hyperthermia with chemotherapy and radiation therapy as a strategy to increase therapeutic index. Additionally, they are exploring the potential for intracellular mNP deposition to serve as a radiation sensitizer without AMF activation. Preliminary data have demonstrated that mNP hyperthermia can enhance chemotherapy by 1.4×, radiation therapy by 1.6× and AMF-free radiation sensitization has thus far yielded a 1.2× effect when mNPs, located within cancer cells, are exposed to standard dose megavoltage radiation (see Figure 5) [30–32].
Figure 5. Number of days (post-treatment) required for the tumors to reach three-times treatment volume.
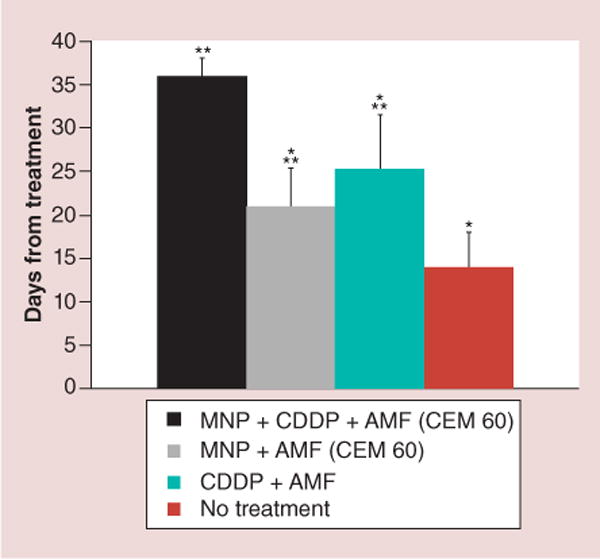
mNP-AMF-cisplatin was 1.7-times more effective than magnetic nanoparticle-alternating magnetic field (36 vs 21 days), 1.4-times more than cisplatin-AMF (36 vs 25 days) and 2.6-times more than no treatment (36 vs 14 days).
*p < 0.003 when compared with mNP-cisplatin-AMF;
**p < 0.02 when compared with no treatment.
AMF: Alternating magnetic field; CDDP: Cis-diamminedichloroplatinum; CEM: Cumulative equivalent minutes; mNP: Magnetic nanoparticle.
The Hoopes group collaborates with the University of Minnesota, Center for Magnetic Resonance Research (Professor Michael Garwood) to assist in the development and in vivo utilization of a novel mNP-imaging technique: SWIFT-MRI [33]. SWIFT-imaging is being coupled with sophisticated radiation treatment planning software and techniques (Eclipse) and AMF-based hyperthermia modeling to achieve an innovative mNP hyperthermia-based planning system. Using a spontaneous canine oral tumor model and various xenograft murine models, the team has demonstrated: in vivo biodistribution and safety/toxicity of different concentrations of intravenously delivered dextran-coated mNP with or without AMF activation [34,35]; that most cancer cells are programmed to endocytose mNPs at a high and rapid level, for example, dextran-coated mNPs are 90% intracellular at 4 h following direct mNP injection into a mouse mammary adenocarcinoma (see Figure 6) [36]; and that pre-mNP low dose radiation and chemotherapy decrease interstitial tumor pressure and significantly improve systemic mNP uptake (2.5× and 3.2×, respectively) (see Figure 7) [37,38]. These latest results suggest that mNP predosing could occur as part of a conventional radiation or chemotherapy regimen.
Figure 6. In vivo observation of iron oxide nanoparticle movement.
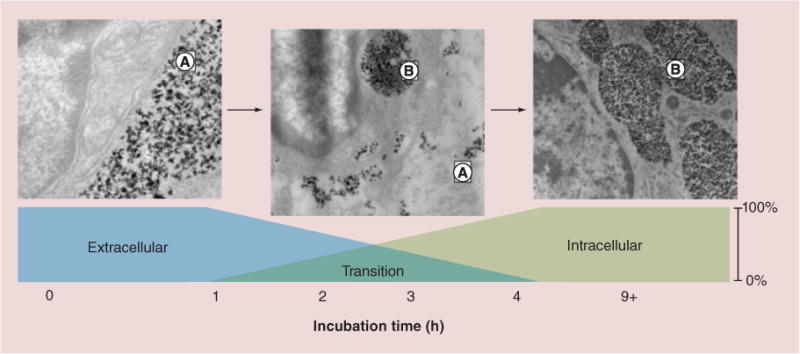
These figures demonstrate movement of iron oxide nanoparticles from the extracellular location (left figure, A) to semi-aggregated intracellular (A) and extracellular (B) locations (center figure) to entirely intracellular aggregation (right figure, B). The intratumoral postinjection time is indicated [36].
Figure 7. Effect of radiation on interstitial tumor pressure and vascular permeability.
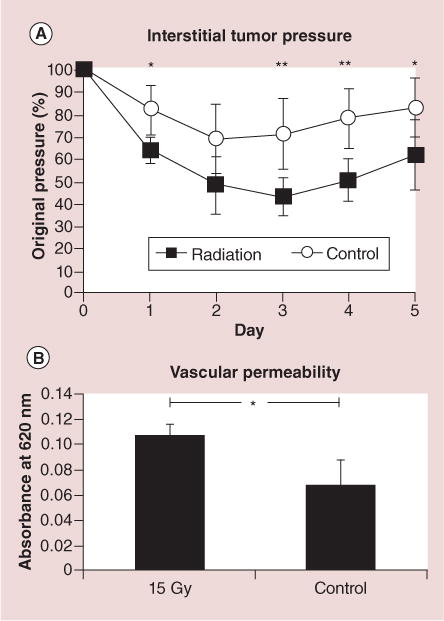
(A)A single 15-Gy fraction of 6-MeV electron radiation significantly decreases interstitial tumor pressure as compared with nonirradiated controls. (B) 3 days following irradiation, vascular permeability is correspondingly increased (Evans blue spectrophotometry assessment).
*p < 0.05; **p < 0.01. Error bars show standard deviations [36].
Project 4: Magnetic nanoparticle immunotherapy against ovarian cancer & melanoma
This project, led by Steve Fiering with coinvestigators Jose Conejo-Garcia at the University of Pennsylvania, and Mary Jo Turk, was originally focused on ovarian cancer, but later began to work on melanoma. Cancer immunotherapy is a major focus in cancer therapy research. One undeveloped approach is to treat a known tumor prior to surgery to change the tumor microenvironment and stimulate antitumor immunity against the identified disease as well as known or occult metastases. The strategy tested in this project involves mild hyperthermia treatment of the primary tumor.
Using mNPs and an AMF to treat poorly immunogenic intradermal murine melanoma (B16F10) with mild hyperthermia (43°C 30 min, CEM of 30), generates sufficient T-cell-mediated antitumor immune response to significantly suppress the growth of tumors on mice rechallenged with the same tumor [39]. The antitumor immunity is systemic, since rechallenge is suppressed either on the same flank as the original treated tumor or the on contralateral flank. The system was developed to model clinical feasibility, such that 3 days after hyperthermia, the treated tumors were surgically removed, as was done with the control group that received no hyperthermia in the primary tumor. Four days after surgery, new tumors were inoculated on the flanks of both the hyperthermia treatment and control groups, and tumor growth was monitored on the following days (see Figure 8). Importantly, the use of mNPs and AMF enabled precise and uniform temperature control throughout the tumor, a requirement for stimulating an effective immune response. Additionally, the team showed that the immune response itself was mediated by T cells, since elimination of CD8+ T cells reversed the protective effects. These studies demonstrate the potential to leverage mNP-AMF hyperthermia as a presurgery treatment that stimulates systemic antitumor immunity following surgical resection of the primary tumor.
Figure 8. Mild hyperthermia of a primary tumor stimulates systemic antitumor immune response that inhibits growth of tumor rechallenge.
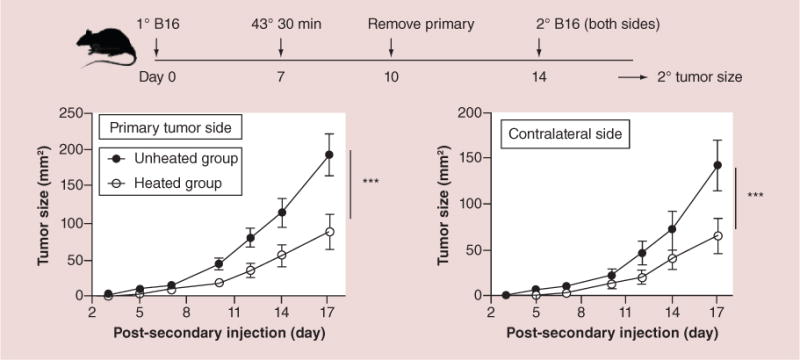
Experimental plan is the top timeline, 2° B16 refers to rechallenge to test systemic immune response. Lower left growth curve is for rechallenge on the same side as the primary tumor removed previously, lower right growth curve is for rechallenge on the contralateral side. Note that rechallenge tumors on either side are suppressed equally, demonstrating true systemic immune response.
***p < 0.001.
Acknowledgments
The DCCNE has contributed to the education of a large number of undergraduate students, graduate students and postdoctoral fellows. The achievements of DCCNE trainees are reflected in the center’s numerous publication and patents, see http://engineering.dartmouth.edu/dccne/.
Financial disclosure: This work was supported in part by the Dartmouth Center for Cancer Nanotechnology Excellence funded by NCI grant U54CA151662-01.
Footnotes
Disclaimer
The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing official policies, either expressed or implied of the NCI or the US Government.
Competing interests disclosure:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
- 1.Zeng Q, Baker I, Loudis JA, Liao Y, Hoopes PJ, Weaver JB. Fe/Fe oxide nanocomposite particles with large specific absorption rate (SAR) for hyperthermia. App Phys Lett. 2007;90:233112–233114. [Google Scholar]
- 2.Zhang G, Liao Y, Baker I. Surface engineering of core/shell iron/iron oxide nanoparticles from microemulsions for hyperthermia. Mater Sci Eng C Mater Biol Appl. 2010;30:92–97. doi: 10.1016/j.msec.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng Q, Baker I, Loudis JA, Liao YF, Hoopes PJ. Synthesis and heating effect of iron/iron oxide composite and iron oxide nanoparticles. Proc Soc Photo Opt Instrum Eng. 2007;6440:64400H. doi: 10.1117/12.708182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iron/Iron Oxide Nanoparticle and Use Thereof. US20110104073 A1. US patent application. filing date: sep 13, 2010; priority date: Jan 18th (2007).
- 5.Hoopes PJ, Strawbridge RR, Gibson U, et al. Intratumoral iron oxide nanoparticle hyperthermia and radiation cancer treatment. Proc Soc Photo Opt Instrum Eng. 2007;6440:64400K. doi: 10.1117/12.706302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giustini AJ, Petryk AA, Cassim SM, Tate JA, Baker I, Hoopes PJ. Magnetic nanoparticle hyperthermia for cancer treatment. NanoLife. 2010;1:17–32. doi: 10.1142/S1793984410000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver JB, Rauwerdink AM, Sullivan CR, Baker I. Signal dependence on frequency in magnetic particle imaging. Med Phys. 2007;34:2361. [Google Scholar]
- 8.Weaver JB, Rauwerdink AM, Sullivan CR, Baker I. Frequency distribution of the nanoparticle magnetization in the presence of a static as well as a harmonic magnetic field. Med Phys. 2008;35:1988–1994. doi: 10.1118/1.2903449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver JB, Baker I, Hansen E. System and method for use of nanoparticles in imaging and temperature measurement. 7994786. United States patent. filing date: Aug 9 (2011).
- 10.Shubitidze F, Kekalo K, Stigliano R, Baker I. Magnetic nanoparticles with high specific absorption rate of electromagnetic energy at low field strength for hyperthermia therapy. J Appl Phys. 2015;117:094302. doi: 10.1063/1.4907915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ndong C, Tate JA, Kett WC, et al. Tumor cell targeting by iron oxide nanoparticles is dominated by different factors in vitro versus in vivo. PLoS ONE. 2015;10(2):e0115636. doi: 10.1371/journal.pone.0115636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NDong C, Toraya-Brown S, Kekalo K, et al. Antibody-mediated targeting of iron oxide nanoparticles to the Folate receptor alpha increases tumor cell association in vitro and in vivo. Int J Nanomed. 2015;10:2595–2617. doi: 10.2147/IJN.S79367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver JB, Rauwerdink AM, Hansen EW. Magnetic nanoparticle temperature estimation. Med Phys. 2009;36(5):1822–1829. doi: 10.1118/1.3106342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perreard I, Reeves D, Zhang X, Kuehlert E, Forauer E, Weaver J. Temperature of the magnetic nanoparticle microenvironment: estimation from relaxation times. Phys Med Biol. 2014;59(5):1109. doi: 10.1088/0031-9155/59/5/1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rauwerdink AM, Hansen EW, Weaver JB. Nanoparticle temperature estimation in combined AC and DC magnetic fields. Phys Med Biol. 2009;54(19):L51–L55. doi: 10.1088/0031-9155/54/19/L01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauwerdink AM, Weaver JB. Viscous effects on nanoparticle magnetization harmonics. J Magn Mag Mater. 2010;322(6):609–613. [Google Scholar]
- 17.Rauwerdink AM, Weaver JB. Harmonic phase angle as a concentration-independent measure of nanoparticle dynamics. Med Phys. 2010;37(6):2587–2592. doi: 10.1118/1.3426294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver JB, Kuehlert E. Measurements of magnetic nanoparticle relaxation times. Med Phys. 2012;39(5):2765–2770. doi: 10.1118/1.3701775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giustini AJ, Perreard I, Rauwerdink AM, Hoopes PJ, Weaver JB. Noninvasive assessment of magnetic nanoparticle–cancer cell interactions. Integr Biol (Camb) 2012;4(10):1283–1288. doi: 10.1039/c2ib20130e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver JB, Rauwerdink KM, Rauwerdink AM, Perreard IM. Magnetic spectroscopy of nanoparticle Brownian motion measurement of microenvironment matrix rigidity. Biomed Tech (Berl) 2013;58(6):547–550. doi: 10.1515/bmt-2013-0012. [DOI] [PubMed] [Google Scholar]
- 21.Rauwerdink AM, Giustini AJ, Weaver JB. Simultaneous quantification of multiple magnetic nanoparticles. Nanotechnology. 2010;21(45):455101. doi: 10.1088/0957-4484/21/45/455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rauwerdink AM, Weaver JB. Measurement of molecular binding using the Brownian motion of magnetic nanoparticle probes. Appl Phys Lett. 2010;96(3):033702. [Google Scholar]
- 23.Weaver JB, Rauwerdink AM. Quantitation of nanoparticle concentrations in microscopic bound states. Med Phys. 2010;37(6):358. [Google Scholar]
- 24.Zhang X, Reeves DB, Perreard IM, et al. Molecular sensing with magnetic nanoparticles using magnetic spectroscopy of nanoparticle Brownian motion. Biosen Bioelectron. 2013;50(0):441–446. doi: 10.1016/j.bios.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Reeves DB, Shi Y, et al. Toward localized in vivo biomarker concentration measurements. IEEE Trans Magn. 2015;51(2):5100604 1–4. doi: 10.1109/TMAG.2014.2324993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver JB, Zhang X, Toraya-Brown S, Reeves DB, Perreard IM, Fiering S. Magnetic nanoparticle quantitation: compensating for relaxation effects. Med Phys. 2012;39(6):3927. [Google Scholar]
- 27.Rauwerdink AM, Weaver JB. Concurrent quantification of multiple nanoparticle bound states. Med Phys. 2011;38(3):1136–1140. doi: 10.1118/1.3549762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reeves DB, Weaver JB. Magnetic nanoparticle sensing: decoupling the magnetization from the excitation field. J Phys D Appl Phys. 2014;47:045002–045008. doi: 10.1088/0022-3727/47/4/045002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tichauer KM, Samkoe KS, Gunn JR, et al. Microscopic lymph node tumor burden quantified by macroscopic dual-tracer molecular imaging. Nat Med. 2014;20(11):1348–1353. doi: 10.1038/nm.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giustini AJ, Petryk AA, Hoopes PJ. Comparison of microwave and magnetic nanoparticle hyperthermia radiosensitization in murine breast tumors. Proc SPIE. 2011 doi: 10.1117/12.876515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petryk AA, Giustini AJ, Gottesman RE, Kaufman PA, Hoopes PJ. Magnetic nanoparticle hyperthermia enhancement of cisplatin chemotherapy cancer treatment. Int J Hyperthermia. 2013;29(8):845–851. doi: 10.3109/02656736.2013.825014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazur CM, Strawbridge RR, Thompson ES, Petryk AA, Gladstone DJ, Hoopes PJ. Effect of radiation energy and intracellular iron dose on iron oxide nanoparticle enhancement of radiation cytotoxicity. Proc SPIE. 2015:93260P. [Google Scholar]
- 33.Hoopes PJ, Petryk AA, Gimi B, et al. In vivo imaging and quantification of iron oxide nanoparticle uptake and biodistribution. Proc SPIE. 2012:83170R. doi: 10.1117/12.916097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tate JA, Ogden JA, Strawbridge RR, Pierce ZE, Hoopes PJ. Toxicity and biodistribution of activated and non-activated intravenous iron oxide nanoparticles. Proc Soc Photo Opt Instrum Eng. 2009;7181:71810L. doi: 10.1117/12.809830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misra A, Petryk AA, Strawbridge RR, Hoopes PJ. Macroscopic and microscopic biodistribution of intravenously administered iron oxide nanoparticles. Proc SPIE. 2015:93260N. [Google Scholar]
- 36.Giustini AJ, Ivkov R, Hoopes PJ. Magnetic nanoparticle biodistribution following intratumoral administration. Nanotechnology. 2011;22(34):345101. doi: 10.1088/0957-4484/22/34/345101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giustini AJ, Petryk AA, Hoopes PJ. Ionizing radiation increases systemic nanoparticle tumor accumulation. Nanomedicine. 2012;8(6):818–821. doi: 10.1016/j.nano.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petryk AA, Giustini AJ, Gottesman RE, Hoopes PJ. Improved delivery of magnetic nanoparticles with chemotherapy cancer treatment. Proc Soc Photo Opt Instrum Eng. 2013;8584:85840H. doi: 10.1117/12.2007308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toraya-Brown S, Sheen MR, Zhang P, et al. Local hyperthermia treatment of tumors induces CD8+ T cellmediated resistance against distal and secondary tumors. Nanomed Nanotech Bio Med. 2014;10(6):1273–1285. doi: 10.1016/j.nano.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


