Abstract
Background
In cranioplasty patients split cranial bone provides excellent structural support and fundamentally “replaces like with like.” However, traditional teaching in craniofacial surgery is that cranial bone cannot be split before the age of 3 years because of the lack of diploic space. The authors have found this not to be the case and describe their experience with splitting cranial bone in children with craniosynostosis younger than 3 years.
Methods
The authors completed a retrospective review of 418 cranioplasties performed between 1997 and 2013 by a single surgeon on patients younger than 3 years with syndromic and nonsyndromic craniosynostosis. Average patient age at the time of the procedure was 328 days. The youngest patient was 58 days old.
Results
Of the 418 cranial procedures performed in this study, cranial bone could be split and used as bone graft in every case. Although the presence of Lückenschädel prevented a complete split of the inner table from the outer table of the bone flap, split cranial bone grafting could still be performed, providing significant grafting material to foster reconstruction. No complications from split cranial harvest were observed.
Conclusions
Contrary to popular belief and the misconception perpetuated by the Plastic Surgery In-Service Examination, the cranium of children younger than 3 years can indeed be safely and predictably split between the inner and outer cortex. This important finding provides the craniofacial surgeon with a valuable, expanded source of rigid bone for cranial vault remodeling in the pediatric patient population.
CLINICAL QUESTION/LEVEL OF EVIDENCE
Therapeutic, IV.
Autologous bone grafts are the sine qua non in pediatric cranioplasty.1–4 They are preferred over alloplastic materials because of their high biocompatibility and strength profiles; commensurate expansion with the rapidly growing brain; and their ability to promote osteoconduction, osteoinduction, and osteogenesis.5,6 Furthermore, the use of alloplastic materials is inherently associated with higher rates of infection and extrusion, especially when there is direct communication with the paranasal sinuses.7–9 In the first year of life, the osteogenic potential of the dura allows smaller cranial defects to be left open; however, the bone derived from dural regeneration tends to be uneven and unpredictable in thickness and irregular in contour. In addition, larger defects tend not to fully reossify, thus necessitating coverage with a graft.10 Common sources of autologous bone for grafting of the craniofacial skeleton include the cranium, ribs, and iliac crest, but the cranium is uniquely suited for this purpose because of its favorable cross-sectional anatomy and its ability to recapitulate the native bone in both form and function. It exists as a tripartite structure consisting of a resilient inner and outer cortex separated by an intervening diploic space. The diploic space is filled with soft cancellous bone that facilitates splitting of the cranium into separate bone fragments that are similar in size, shape, and contour. These bone fragments can then be used as grafts in the reconstruction of the cranial vault, essentially doubling the amount of autologous bone available.11 As a semirigid material, split cranial bone is easily contoured in young patients and fundamentally “replaces like with like.”
A recurring theme in the literature is that cranial bone is too thin for splitting in both infants and children younger than 3 years.1,12–14 The prevailing concept is that the inner and outer table of the cranium cannot be separated before formation of the diploë—a statement often repeated in textbooks.15,16 As a result, particulate bone grafting is often touted as the procedure of choice in these young patients, given its relative ease of harvest, minimal morbidity, and ability to reossify larger calvarial defects because of the underlying dural osteogenic potential.1,10,17 Although particulate bone grafting may be a useful technique in certain situations, it does not afford the structural integrity or resistance to resorption often called for in cranial vault reconstruction. Split cranial bone grafting is used exclusively in our practice at the University of Michigan. Our experience with split cranial bone grafting has led us to revisit the common dictum that cranial bone cannot be split before 3 years of age, because we routinely perform this technique in patients as young as 2 months.
The impetus for reporting our experience with splitting cranial bone in pediatric cranioplasty patients is from a question on the 2012 Plastic Surgery In-Service Examination. The question asked the examinee to identify the most appropriate material for reconstruction of a 12-cm2 cranial defect in a 21-month-old child who had previously undergone a cranial vault remodeling procedure. Both particulate bone and split calvaria were offered as possible answers, but particulate bone was considered the most correct, citing that cranial bone is difficult to split before the age of 4 years. We found this explanation to be at great odds with our experience. For the past 17 years, the senior author (S.R.B.) has assigned the task of splitting cranial bone to the residents in all of his pediatric cranioplasties. Despite several recent publications demonstrating that cranial bone can indeed be split in children younger than 3 years,18,19 the application of this powerful technique in infants and children has regrettably become ingrained in craniofacial surgery as a surgical myth, on par with the prohibition of the use of epinephrine in the finger. In this study, we demonstrate the technique of splitting cranial bone in children younger than 3 years.
PATIENTS AND METHODS
Study Design
After institutional review board approval, a database search was performed using the Current Procedural Terminology codes 21175 and 61559 to identify all patients who underwent cranial vault remodeling for craniosynostosis performed by the senior author (S.R.B.) between November of 1997 and June of 2013. All operative records were reviewed, and those cases using split cranial bone in patients younger than 3 years were identified. Patient demographics, type of craniosynostosis, intraoperative complications, and revision rate were extracted from the clinical record.
Operative Technique
For both syndromic and nonsyndromic craniosynostosis patients, our preferred treatment plan involves a collaborative effort between the neurosurgeon and the craniofacial surgeon. Similar to other institutions, we have found that a multidisciplinary approach greatly reduces complications and facilitates improved patient care.20,21 Our pediatric cranioplasties for patients with craniosynostosis always begin with a craniotomy performed by the neurosurgeon followed by reshaping of the calvaria by the craniofacial surgeon. No additional operative time needs to be dedicated to splitting cranial bone because this occurs immediately after the craniotomy while the neurosurgeon is continuing to dissect the dura from the calvaria to allow for barrel-staving and out-fractures. Before the start of the case, a separate back table should be set up for splitting cranial bone. The only tools required are a single green towel and a series of small (2 to 6 mm) osteotomes that can be straight or curved. From a technical standpoint, it is particularly important to use sharp osteotomes, as the thin calvaria is more likely to fracture under the manipulation of a dull osteotome blade.
After the neurosurgeon performs the craniotomy, the excised pieces of calvaria are passed off the operative field to the resident. The neurosurgeon then continues to operate as the resident begins to split bone on the back table. We recommend holding the pieces of bone in the resident's nondominant hand to provide counterpressure against the osteotome, which is being controlled by the resident's dominant hand. Grasping the bone with a green towel while the bone is being split can enhance grip and protect the resident's hands against injury should the osteotome slip. Initially, a 2-mm straight osteotome is insinuated between the cortices of the cut bone edge (Fig. 1). (See Video, Supplemental Digital Content 1, which displays the frontal bone from a 5-month-old boy undergoing total cranial vault remodeling for sagittal synostosis, http://links.lww.com/PRS/B6. A sharp 2-mm osteotome is inserted all the way around the cut edge. Then, the surgeon switches to a wider osteotome and continues to separate the inner cortex from the outer cortex, attacking on a broad front.) The principle of “attacking on a broad front” is then followed to avoid inadvertent fracture to one of the cortices, which is usually caused by advancing the osteotome too far along a narrow path. The inner and outer cortices are separated with the use of firm, precise pressure combined with a gentle side-to-side rocking motion that allows the osteotome blade to cut through the soft osseous tissue constituting the nascent diploic space. A mallet is used sparingly and most times is not required at all. Smaller osteotomes are progressively replaced by larger osteotomes as the resident works around the margins of the cut bone edge toward the center of the graft. A curved osteotome can be used when the contour of the cranial bone demands it, but we have found that separation is technically easier when straight osteotomes are used. From our experience, unless there is an overwhelming amount of Lückenschädel, there is always a clear plane between the inner and outer cortex. We have found this to be the case even among the youngest patients treated. Following the split, the two halves of cranial bone can then be used as a graft in reconstruction of the cranial vault (Figs. 2 and 3).
Fig. 1.
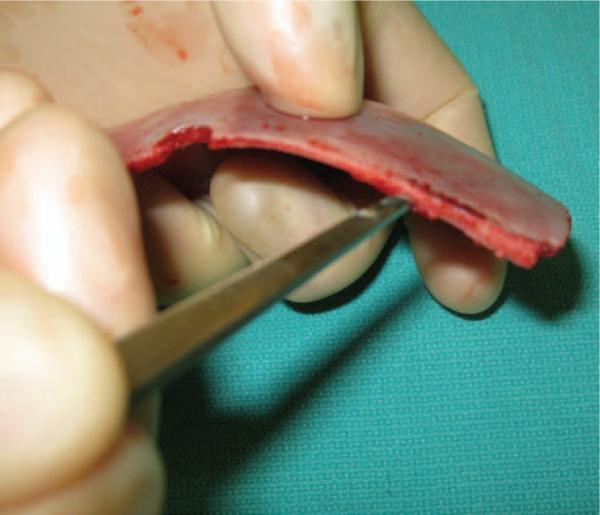
The first step in splitting cranial bone is to gain access to the diploic space. This is accomplished by inserting a 2-mm straight osteotome between the inner and outer cortices along the cut bone edge.
Fig. 2.
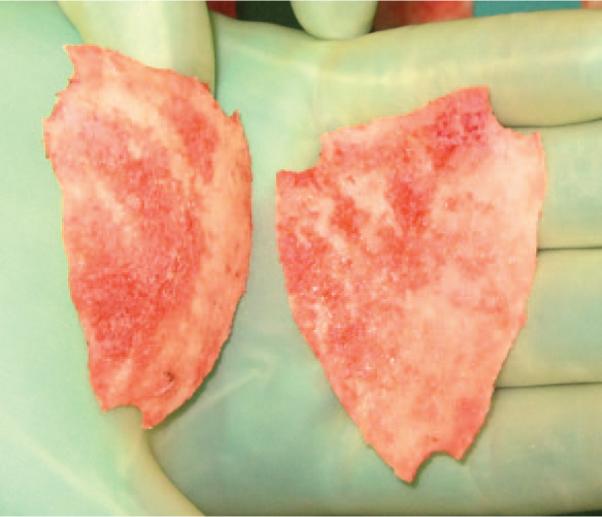
A complete split of the frontal bone in an 8-month-old patient with sagittal synostosis.
Fig. 3.
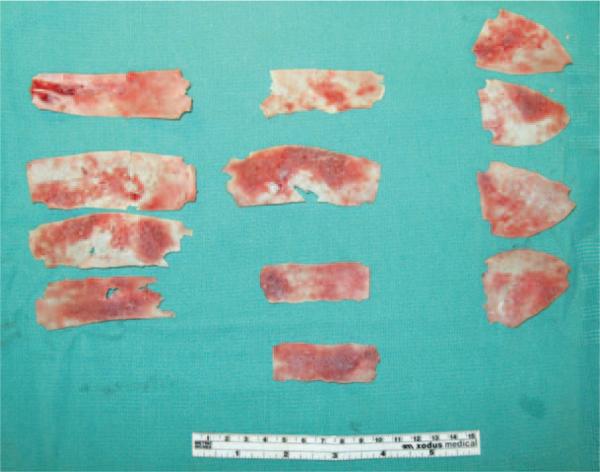
Splitting cranial bone essentially doubles the amount of rigid bone available for reshaping the calvaria.
Split cranial bone grafts used in this cohort are used as inlay grafts to cover defects resulting from the vault expansion. Occasionally, bone is used as an onlay graft in areas such as the frontal bone. The grafts are held in place by drilling holes through the bones and using 0 Vicryl (Ethicon, Inc., Somerville, N.J.) or plating systems to maintain bony contact between the grafts.
Our routine follow-up after the initial postoperative period is a yearly physical examination performed by both the neurosurgeon and the plastic surgeon. Patients are also evaluated yearly by a neuro-ophthalmologist until age 10 years. The decision to perform additional bone grafting is made before the child is school age, with the rationale that by age 6 or 7 years, the chances that a defect will reossify are exceedingly low. Children with syndromes are evaluated yearly by a multidisciplinary craniofacial team until age 21.
RESULTS
Four hundred fifty-two patients underwent cranial vault remodeling procedures for the treatment of craniosynostosis performed by the senior author (S.R.B.) between November of 1997 and June of 2013. Among those 452 cases, 418 were performed on children younger than 3 years. Cranial bone was split in every case, but only in the presence of Lückenschädel was a complete split prevented (n = 4). The average age of the patients at the time of the procedure was 328 days. The age of the youngest patient was 58 days.
Patient demographics are listed in Table 1. Two hundred seventy-three patients were boys and 145 patients were girls. Six percent of cases (n = 26) were syndromic and 94 percent of cases (n = 392) were nonsyndromic. The distribution of craniosynostosis repairs included sagittal (n = 208), metopic (n = 88), unicoronal (n = 57), multiple (n = 53), and lambdoid (n = 12). No intraoperative complications were associated with splitting cranial bone. Even in cases of Lückenschädel, additional bone was obtained for reshaping the calvaria.
Table 1.
Patient Demographics
| Variable | No. (%) |
|---|---|
| Age at the time of repair, days | |
| Mean | 328 |
| Range | 58–1039 |
| Sex | |
| Male | 273 (65) |
| Female | 145 (35) |
| Type of synostosis | |
| Sagittal | 208 (50) |
| Unicoronal | 57 (13) |
| Metopic | 88 (21) |
| Lambdoid | 12 (3) |
| Multiple | 53 (13) |
| Genetic inheritance | |
| Syndromic | 26 (6) |
| Nonsyndromic | 392 (94) |
Postoperatively, we follow the patients with yearly physical examinations and neuro-ophthalmologic examinations. We do not routinely obtain computed tomographic scans in a conscious effort to decrease the patient's exposure to radiation. Thirteen patients (3.1 percent) required revision bone grafting for a persistent cranial defect. This persistent defect was typically found in the region of a reconstruction in which dura was left to regenerate bone. Twelve patients had a bone defect larger than 5 cm2 and one patient had a bone defect smaller than 5 cm2. The patient with the smaller defect in the frontal region underwent surgery because of contour irregularity. The average age at initial craniosynostosis repair in this patient cohort was 400 days (range, 96 to 1039 days). The distribution of craniosynostosis repairs requiring revision bone grafting included sagittal (n = 5), metopic (n = 3), unicoronal (n = 3), and Apert syndrome (n = 2).
DISCUSSION
It is tautologous that cranial bone is the ideal material for reconstructing a cranial defect.1–3,22–24 Compared with bone from extracranial donor sites, cranial bone more accurately recapitulates both the structure and the function of the calvaria, because it is harvested directly from the bone that it is replacing. In situations where cranial bone graft is not available, surgeons must turn to other sources of bone such as rib or iliac crest, or alloplastic materials such as porous polypropylene or methylmethacrylate.1 However, we strongly recommend against using alloplastic material for pediatric cranial vault remodeling, as alloplastic materials cannot expand with the rapidly growing brain and are associated with higher rates of infection and extrusion.7–9,25,26 In cases of craniosynostosis repair, complications are minimized when splitting cranial bone because a craniotomy is already required. Furthermore, this technique does not require additional operative time because the bone is split while the neurosurgeons finish their portion of the procedure. Splitting cranial bone essentially doubles the amount of rigid bone available for reshaping the calvaria, and even in cases of Lückenschädel, in which a complete split is prevented, the amount of rigid bone available for grafting is still expanded. The bone is simple to manipulate and its malleability allows it to be contoured to the natural curvature of the cranial vault. This technique is feasible and easily taught to plastic surgery and neurosurgery trainees, and the morbidity associated with an extracranial donor site is avoided.
Contrary to popular belief, the calvaria of children younger than 3 years can be split into an inner and outer cortex. The common misperception in craniofacial surgery is that the inner and outer table of the cranium cannot be separated before the recognized full formation of the diploë, a notion attributable to a study by Koenig et al.27 in 1995. This study reviewed computed tomographic scans of 96 patients and correlated age with temporal bone thickness. The authors performed a regression analysis that purportedly allowed one to predict the presence or absence of the diploic space based on the age of the child. The article recommended that “split cranial bone grafting is not to be planned before the age of three years because the presence of the diploic space is not reliable and the skull is thin.” This dictum has led many to use particulate bone grafting as an alternative, which allows for a greater expansion of the cranial bone and minimal morbidity.17 Although we agree that particulate bone grafting may be a useful technique in certain situations, it does not afford the structural integrity or resistance to resorption often called for in cranial vault reconstruction. Our preference, therefore, is to use split cranial bone because it provides a more stable immediate coverage. Furthermore, although this article is not meant to compare one technique with another, our revision rate of 3.1 percent for overall craniosynostosis repair is comparable or better to that reported with other techniques in the literature.17
Our experience with splitting cranial bone in craniosynostosis patients suggests that the diploic space forms at a much earlier age than previously thought, because we routinely split cranial bone in patients as young as 2 months. However, we are not the first to report this technique in children younger than 3 years, as others have also championed this technique. Barone and Jimenez19 reported a much smaller series in which they were able to split cranial bone in patients as young as 13 months following full-thickness craniotomy. Our study is much larger and our technique differs from theirs in that the authors used a drill to separate the inner and outer cortices. We prefer to use a series of straight and curved osteotomes ranging in size from 2 to 6 mm and then, by hand, insinuate the osteotome between the inner and outer table. We feel this provides more control during the split and helps reduce the incidence of inadvertent fracture to the graft. In another small technical note published by Steinbok and colleagues,18 an interesting technique for splitting cranial bone is demonstrated in infants as young as 9 months who are undergoing fronto-orbital advancement for craniosynostosis. The authors used a Tessier bone bender to initially shear the inner and outer cortex, and then completed the separation with either a periosteal elevator or straight osteotome. The authors also note that they were unable to split cranial bone “when there [was] a marked copper beaten appearance of the calvaria.” We do not use a Tessier bone bender to assist in separation of the cortices, as the progressive increase in size of the osteotome from 2 to 6 mm facilitates this process.
We present this large series not because we feel this technique is novel, but to counter what appears to be an incorrect understanding of the anatomy (i.e., lack of a diploic space) and technical ability (i.e., it cannot be performed) and to finally put to rest an urban legend that has made its way into the Plastic Surgery In-Service Examination. Split cranial bone remains our material of choice for bone grafting in operations for craniosynostosis and for cranioplasty after trauma and other neurosurgical procedures. This technique does require a craniotomy and thus the involvement of a neurosurgeon. Although the diploic space exists as a separable plane between the inner and outer cortex, it is not large enough to permit in situ bone harvest and we agree with exhortations against in situ bone harvest in this very young patient population. Finally, in cases of craniosynostosis repair where there is significant evidence of Lückenschädel, and not enough bone can be split, the use of particulate bone graft is a potential alternative that can be used as a complementary procedure to the technique we have outlined.
CONCLUSIONS
Contrary to prior reports in the literature, we present our experience with splitting cranial bone in craniosynostosis patients younger than 3 years. A clear plane exists between the inner and outer cortex that can be separated using a series of small osteotomes. We infer this plane to be the diploic space, and this technique expands the amount of rigid bone available for cranial vault remodeling in this pediatric patient population.
Supplementary Material
Video. Supplemental Digital Content 1 displays the frontal bone from a 5-month-old boy undergoing total cranial vault remodeling for sagittal synostosis, http://links.lww.com/PRS/B6. A sharp 2-mm osteotome is inserted all the way around the cut edge. Then, the surgeon switches to a wider osteotome and continues to separate the inner cortex from the outer cortex, “attacking on a broad front.”
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. No internal or external funding was received.
Supplemental digital content is available for this article. Direct URL citations appear in the text; simply type the URL address into any Web browser to access this content. Clickable links to the material are provided in the HTML text of this article on the Journal's Web site (www.PRSJournal.com).
REFERENCES
- 1.Goldstein JA, Paliga JT, Bartlett SP. Cranioplasty: Indications and advances. Curr Opin Otolaryngol Head Neck Surg. 2013;21:400–409. doi: 10.1097/MOO.0b013e328363003e. [DOI] [PubMed] [Google Scholar]
- 2.Posnick JC, Goldstein JA, Armstrong D, Rutka JT. Reconstruction of skull defects in children and adolescents by the use of fixed cranial bone grafts: Long-term results. Neurosurgery. 1993;32:785–791. doi: 10.1227/00006123-199305000-00011. discussion 791. [DOI] [PubMed] [Google Scholar]
- 3.Stal S, Netscher DT, Shenaq S, Spira M. Reconstruction of calvarial defects. South Med J. 1992;85:812–819. doi: 10.1097/00007611-199208000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Edwards MS, Ousterhout DK. Autogeneic skull bone grafts to reconstruct large or complex skull defects in children and adolescents. Neurosurgery. 1987;20:273–280. doi: 10.1227/00006123-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Oppenheimer AJ, Mesa J, Buchman SR. Current and emerging basic science concepts in bone biology: Implications in craniofacial surgery. J Craniofac Surg. 2012;23:30–36. doi: 10.1097/SCS.0b013e318240c6d9. [DOI] [PubMed] [Google Scholar]
- 6.Fearon JA, Munro IR, Bruce DA. Observations on the use of rigid fixation for craniofacial deformities in infants and young children. Plast Reconstr Surg. 1995;95:634–637. discussion 638. [PubMed] [Google Scholar]
- 7.Singh KA, Burstein FD, Williams JK. Use of hydroxyapatite cement in pediatric craniofacial reconstructive surgery: Strategies for avoiding complications. J Craniofac Surg. 2010;21:1130–1135. doi: 10.1097/SCS.0b013e3181e482c6. [DOI] [PubMed] [Google Scholar]
- 8.Marchac D, Greensmith A. Long-term experience with methylmethacrylate cranioplasty in craniofacial surgery. J Plast Reconstr Aesthet Surg. 2008;61:744–752. doi: 10.1016/j.bjps.2007.10.055. discussion 753. [DOI] [PubMed] [Google Scholar]
- 9.Matic D, Phillips JH. A contraindication for the use of hydroxyapatite cement in the pediatric population. Plast Reconstr Surg. 2002;110:1–5. doi: 10.1097/00006534-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kantorova VI. The role of the dura mater in induced regeneration of cranial vault bones. Sov J Dev Biol. 1972;3:371–376. [PubMed] [Google Scholar]
- 11.Sahoo NK, Rangan M. Role of split calvarial graft in reconstruction of craniofacial defects. J Craniofac Surg. 2012;23:e326–e331. doi: 10.1097/SCS.0b013e31825434db. [DOI] [PubMed] [Google Scholar]
- 12.Hassanein AH, Couto RA, Kurek KC, Rogers GF, Mulliken JB, Greene AK. Experimental comparison of cranial particulate bone graft, rhBMP-2, and split cranial bone graft for inlay cranioplasty. Cleft Palate Craniofac J. 2013;50:358–362. doi: 10.1597/11-273. [DOI] [PubMed] [Google Scholar]
- 13.Rogers GF, Greene AK, Mulliken JB, Proctor MR, Ridgway EB. Exchange cranioplasty using autologous calvarial particulate bone graft effectively repairs large cranial defects. Plast Reconstr Surg. 2011;127:1631–1642. doi: 10.1097/PRS.0b013e31821084f0. [DOI] [PubMed] [Google Scholar]
- 14.Tessier P. Autogenous bone grafts taken from the calvarium for facial and cranial applications. Clin Plast Surg. 1982;9:531–538. [PubMed] [Google Scholar]
- 15.Dufresne CR, Manson PN. Pediatric facial injuries. In: Mathes SJ, editor. Plastic Surgery. Saunders; Philadelphia: 2005. pp. 381–462. [Google Scholar]
- 16.Whitaker LA. Problems and complications in craniofacial surgery. In: Goldwyn RM, editor. The Unfavorable Result in Plastic Surgery: Avoidance and Treatment. Little, Brown; Boston: 1984. pp. 244–245. [Google Scholar]
- 17.Greene AK, Mulliken JB, Proctor MR, Rogers GF. Pediatric cranioplasty using particulate calvarial bone graft. Plast Reconstr Surg. 2008;122:563–571. doi: 10.1097/PRS.0b013e31817d61c1. [DOI] [PubMed] [Google Scholar]
- 18.Steinbok P, Seal SK, Courtemanche DJ. Split calvarial bone grafting in patients less than 1 year of age: Technical note and use in craniofacial surgery for craniosynostosis. Childs Nerv Syst. 2011;27:1149–1152. doi: 10.1007/s00381-011-1447-4. [DOI] [PubMed] [Google Scholar]
- 19.Barone CM, Jimenez DF. Split-thickness calvarial grafts in young children. J Craniofac Surg. 1997;8:43–47. doi: 10.1097/00001665-199701000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Anantheswar YN, Venkataramana NK. Pediatric craniofacial surgery for craniosynostosis: Our experience and current concepts. Part 1. J Pediatr Neurosci. 2009;4:86–99. doi: 10.4103/1817-1745.57327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David DJ. Craniofacial surgery: The team approach. Aust N Z J Surg. 1977;47:193–198. doi: 10.1111/j.1445-2197.1977.tb04267.x. [DOI] [PubMed] [Google Scholar]
- 22.Goodrich JT, Argamaso R, Hall CD. Split-thickness bone grafts in complex craniofacial reconstructions. Pediatr Neurosurg. 1992;18:195–201. doi: 10.1159/000120662. [DOI] [PubMed] [Google Scholar]
- 23.Hardesty RA, Marsh JL. The skull versus the iliac crest: A comparison of graft donor sites. Plast Surg Forum. 1987;20:118–120. [Google Scholar]
- 24.Tessier P. Inferior orbitotomy: A new approach to the orbital floor. Clin Plast Surg. 1982;9:569–575. [PubMed] [Google Scholar]
- 25.Aydin S, Kucukyuruk B, Abuzayed B, Aydin S, Sanus GZ. Cranioplasty: Review of materials and techniques. J Neurosci Rural Pract. 2011;2:162–167. doi: 10.4103/0976-3147.83584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abuzayed B, Tuzgen S, Canbaz B, Yuksel O, Tutunculer B, Sanus GZ. Reconstruction of growing skull fracture with in situ galeal graft duraplasty and porous polyethylene sheet. J Craniofac Surg. 2009;20:1245–1249. doi: 10.1097/SCS.0b013e3181acdfaf. [DOI] [PubMed] [Google Scholar]
- 27.Koenig WJ, Donovan JM, Pensler JM. Cranial bone grafting in children. Plast Reconstr Surg. 1995;95:1–4. doi: 10.1097/00006534-199501000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video. Supplemental Digital Content 1 displays the frontal bone from a 5-month-old boy undergoing total cranial vault remodeling for sagittal synostosis, http://links.lww.com/PRS/B6. A sharp 2-mm osteotome is inserted all the way around the cut edge. Then, the surgeon switches to a wider osteotome and continues to separate the inner cortex from the outer cortex, “attacking on a broad front.”


