Topical corticosteroids and cycloplegics remain first line treatment for pediatric uveitis and periocular depot glucocorticoid injections are considered for persistent disease. Topical therapy alone is often insufficient to control anterior segment disease and is inadequate to treat posterior segment uveitis. In one series, systemic treatment was required in 46% of pediatric uveitis patients at an ophthalmology referral center.1 When children with uveitis require systemic immunosuppressive therapy, management is often shared by ophthalmologists and rheumatologists.
Because the autoimmune mechanisms responsible for inflammatory arthritis and uveitis are thought to be similar, immunomodulating drugs prescribed for inflammatory arthritis are often used to treat chronic uveitis. Oral NSAIDs are sometimes used in the initial management of chronic uveitis.2,3 Methotrexate is a widely employed steroid-sparing agent despite limited data to support its use in pediatric uveitis.4,5 Cyclosporine,6 mycophenolate mofetil (MMF)7 and other immunosuppressive medications are also reportedly efficacious.
Recent case series have described successful use of anti-tumor necrosis factor alpha (anti-TNFα) therapy in management of childhood uveitis. Three anti- TNFα agents are currently commercially available: etanercept (Enbrel®, Immunex, Thousand Oaks, CA, USA), infliximab (Remicade,® Centocor, PA, USA) and adalimumab (Humira®, Abbott Laboratories, Abbott Park, IL, USA). Etanercept is a subcutaneously-administered molecule which binds serum and membrane-bound TNFα. Both infliximab and adalimumab are monoclonal anti–TNFα antibodies. Infliximab is a chimeric antibody administered intravenously while adalimumab is fully humanized and is administered subcutaneously.
Published data concerning use of etanercept to treat pediatric uveitis have yielded mixed results. In a series of 10 children with refractory uveitis treated prospectively with etanercept for three months, ocular inflammation improved in 10/16 affected eyes, resolved in four of 16 eyes, and worsened in one. Seven non-responders improved with higher dose etanercept.8 In an update to this series, four children remained on etanercept with good control of uveitis for over one year.9 The only randomized, placebo-controlled trial of etanercept to treat juvenile idiopathic arthritis-associated uveitis involved 12 children and showed no significant reduction in ocular inflammation at six months.10
Data on adalimumab in pediatric uveitis are limited to two retrospective analyses. In one report, 18 children treated with adalimumab and background immunosuppression for a median of 17 months had fewer uveitis recurrences during adalimumab therapy than in the two years prior to treatment; other ocular outcome measures were not assessed.11 In another series, 14 children were treated with adalimumab for a mean of 18 months; 81% demonstrated decreased anterior chamber flare and 65% achieved sustained control of inflammation. Incomplete information was provided regarding concomitant immunosuppressive treatment.12
In contrast to the mixed results observed with etanercept, case series reporting use of infliximab in childhood uveitis tend to show more favorable results. A retrospective analysis of 17 uveitic children treated with infliximab (10–20 mg/kg) for 14 months (range three to 34 months) described positive response in all patients and two uveitis recurrences during treatment. In this series, six of 17 children were treated concomitantly with methotrexate.13 In another report, all six children treated with infliximab (5–18 mg/kg) and background immunosuppression for eight to 19 months all had a favorable response and there was only one uveitis recurrence during infliximab therapy.14
A separate publication described a series of six children with juvenile arthritis-associated anterior uveitis treated with infliximab (5–10 mg/kg) and background immunosuppression for three to 26 months; anterior cell inflammation improved in all children.15 In a series of 21 children treated with either infliximab or etanercept for active uveitis, Saurenmann et al, found that 86% experienced improvement with anti-TNF therapy, but the response was better with infliximab and some children responded to infliximab after failing etanercept.16 Outcome measures were limited to visual acuity and ability to wean topical or oral corticisteroids or other immunosuppressives. No randomized, placebo-controlled trials have been performed to assess efficacy of infliximab in pediatric uveitis.
Treatment of children with chronic, noninfectious uveitis remains a challenging clinical problem as disease is often refractory to standard therapy and visual morbidity is high. Infliximab has reported short-term efficacy in the treatment of pediatric uveitis. This report describes the course of long-term infliximab therapy for 16 children with chronic uveitis and provides the longest follow-up to date regarding infliximab therapy for pediatric uveitis.
METHODS
We obtained Duke Institutional Review Board approval and identified all children with chronic, noninfectious uveitis treated with infliximab for a minimum of 6 months in the pediatric rheumatology clinic at Duke University Medical Center (DUMC) from July 2001 to February 2006. All children underwent thorough history, physical examination and serologic evaluation to exclude underlying infection or systemic illnesses responsible for uveitis. Children with uveitis and systemic illness were included only if uveitis was the indication for starting infliximab. Ophthalmologic care was provided by ophthalmologists at the Duke Eye Center (nine children), by an ophthalmologist at a nearby academic center (four children) and by community ophthalmologists (three children).
A protein purified derivative (PPD) skin test was negative in all patients prior to initiation of infliximab therapy. The following laboratory tests were monitored at regular intervals, every four to eight weeks: complete blood count, alanine aminotransferase, aspartate aminotransferase, albumin. Acetaminophen and oral or intravenous diphenhydramine were administered prior to each infusion. Infliximab was administered in an outpatient pediatric infusion center where vital signs were monitored every 15–30 minutes.
We collected the following baseline information: age, weight, gender, ethnicity, type and duration of uveitis, other systemic diseases, anti-nuclear antibody, HLA-B27 status, and angiotensin converting enzyme testing results when available, prior treatment with topical, systemic, periocular glucocorticoids, methotrexate and other immunosuppressive medications. If the exact month of uveitis onset was not available, onset was assumed to be January of that year. The date, patient weight and dose for each infliximab infusion and adverse effects were recorded. Visual acuity, anterior chamber inflammatory cell count, and description of posterior segment inflammation for each ophthalmology exam prior to and during infliximab therapy were recorded. Visual acuity measured by Snellen visual acuity chart was converted to logMAR (logarithm of the reciprocal of Snellen visual acuity). A 0.3 change in logMAR was considered to be clinically significant. We recorded initial and final doses of topical and systemic glucocorticoids, methotrexate, and other immunosuppressive medications. After a structured chart review, data were entered into a confidential electronic database.
The outcome variables of primary interest included proportion of patients who achieved zero anterior chamber inflammation or a two-step reduction in anterior chamber inflammation, change in visual acuity as measured by logMAR, uveitis recurrence, and proportion of patients who discontinued topical glucocorticoid therapy. In determining time intervals for data analysis, we selected only those time points at which data were available for 10 or more children. The following scale modified from Hogan et al was used to reconcile different ophthalmologists’ methods of grading anterior chamber inflammation: trace = <5 cells/HPF, 1+ = 5–9 cells/HPF, 2+ = 10–19 cells/HPF, 3+ = 20–50 cells/HPF, 4+ >50 cells/HPF. 17 A uveitis recurrence during the study period was defined as at least two grade change in ocular inflammation leading to a change in local or systemic therapy.
Data were analyzed using simple descriptive statistics. Wilcoxon rank sum paired t-test was used to compare means and Fisher’s exact test was used to compare categorical variables. Kaplan-Meier survival analysis was performed to estimate percentage of eyes remaining free of uveitis recurrence over time. P values less than 0.05 were considered to be statistically significant. SAS Enterprise Guide software was used for statistical analysis (version 8.2, SAS Institute Inc., Cary, NC).
RESULTS
Patient characteristics
Patient baseline characteristics are summarized in Table 1. 29/32 eyes were affected in 16 children. Prior ocular complications included cataracts (seven children), secondary glaucoma (five children), band keratopathy (six children), macular edema (two children), aphakia (one child), and legal blindness (one child). Six children (38%), had a systemic inflammatory disease. Four children had elevated levels ACE levels without clinical evidence of sarcoidosis.
Table 1.
Baseline Characteristics of Children with Uveitis at Initiation of Infliximab Therapy
| Total number of children | 16 |
| Sex | |
| Male | 10 (62.5%) |
| Female | 6 (37.5%) |
| Age, median (range) | 11 years (3 to 15 years) |
| Ethnicity | |
| White | 12 (75%) |
| Hispanic | 1 (6%) |
| African American | 3 (19%) |
| Type of uveitis | |
| Anterior | 8 (50%) |
| Intermediate | 4 (25%) |
| Panuveitis | 4 (25%) |
| Systemic diagnoses | |
| Juvenile rheumatoid arthritis | 4 (25%) |
| Psoriatic arthritis | 1 (6%) |
| Other* | 1 (6%) |
| None | 10 (63%) |
| Laboratory tests | |
| ANA positive | 11 (69%) |
| HLA B27 positive | 4 (25%) |
| ACE elevated | 4 (25%) |
| Duration of uveitis, median (range) | 27 months (2 to 109 months) |
| Baseline therapy | |
| Oral glucocorticoids | 11 (69%) |
| Topical glucocorticoids | 16 (100%) |
| Methotrexate | 14 (88%) |
| Other immunosuppressives | 0 |
| Duration MTX treatment, median (range) | 27 months (6 to 109 months) |
| Previous periocular depot glucocoricoids | 6 (38%) |
One patient had a benign, inflammatory tumor of the skin and subcutaneous tissues affecting the lower extremity. Review of biopsy and extensive evaluation revealed no underlying systemic disease.
ACE = angiotensin converting enzyme, ANA = anti-nuclear antibody, HLA = human leukocyte antigen, MTX = methotrexate.
At infliximab initiation, all but three children (81%) were taking methotrexate with median weekly dose of 18.5 mg (range 10 to 25 mg). Twelve were taking methotrexate by subcutaneous injection. Infliximab and methotrexate were initiated concomitantly in two patients due to severity of ocular inflammation. Methotrexate was started after infliximab initiation in one patient, and the reason was not documented. Eleven children (69%) were on daily or alternate day oral prednisone at infliximab initiation and all were using corticosteroid ophthalmic drops. None of the children had been treated with immunosuppressive agents other than methotrexate and glucocorticoids prior to infliximab initiation.
At baseline, 13/16 (81%) children had ≥1+ anterior chamber cells in at least one eye. One child had zero and two trace anterior chamber cells bilaterally at baseline; in these three children, infliximab was initiated in an effort to spare glucocorticoid exposure (all were taking daily prednisone 10–20 mg/day). Of the children with intermediate or panuveitis, two had vitreal inflammation (vitreal cells, vitreal haze or snowbanking) at baseline, two had no vitreal inflammation and in three anterior chamber inflammation and/or band keratopathy obstructed a view of the posterior segment. Median follow up duration was 26 months (range 13 to 54 months).
Response to infliximab treatment
The median initial infliximab dose was 5.1 mg/kg (range 2.9 to 6.9 mg/kg). Doses were administered at weeks 0, 2, and 4 and continued at four week intervals until ocular inflammation had significantly improved or resolved. Initial and subsequent doses of infliximab were determined by the treating rheumatologist and ophthalmologist. The infliximab dose was increased at each visit until ocular inflammation was under control (trace or no inflammatory cells in anterior chamber). Once eyes were quiet, oral and topical corticosteroids were tapered. If possible, the infliximab dosing interval was slowly increased as tolerated. Oral glucocorticoids were discontinued in 10 children. Methotrexate was stopped in one patient and the dose reduced in four patients.
There were no serious infusion reactions, infections or other adverse events reported during a total of 362 infliximab infusions. No significant complications occurred during the course of infliximab treatment. One patient experienced mild bradycardia and flushing during the initial infusion which did not require intervention and did not recur with subsequent infusions. The median maintenance infliximab dose was 8.2 mg/kg (range 2.0 to 12.9 mg/kg). Dosing intervals ranged from four to 10 weeks; the median maintenance interval was 5.6 weeks. The median number of infliximab infusions per patient was 19 and ranged from 12 to 48 doses.
As summarized in Table 2, at 12 months 64% (9/14) of children had zero anterior chamber inflammation and 79% (11/4) achieved either zero or a two-step reduction in anterior chamber inflammation. Mean visual acuity as measured by LogMAR did not change significantly during the first 12 months of follow up as shown in Table 3. Two children underwent cataract removal during infliximab therapy. As demonstrated in Table 4, all patients were using glucocorticoid ophthalmic drops at baseline, while 69% (11/16) had discontinued topical glucocorticoids at 12 months.
Table 2.
Proportion of children achieving zero inflammation or two-step reduction in anterior ocular inflammation after 3, 6, 9, and 12 months of infliximab therapy
| Baseline | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|
| No. patients (%) with zero ocular inflammation | 1 (6%) | 7 (44%) | 9 (60%) | 5 (45%) | 9 (64%) |
| Comparison to baseline, P value | N/A | 0.04 | 0.002 | 0.03 | 0.001 |
| No. patients (%) with zero inflammation or two-step reduction in ocular inflammation | 1 (6%) | 12 (75%) | 10 (67%) | 9 (82%) | 11 (79%) |
| Comparison to baseline, P value | N/A | 0.0001 | 0.0006 | 0.0001 | 0.00000 |
| Total no. patients for whom data available | 16 | 16 | 15 | 11 | 14 |
No. = number, N/A = not applicable
Table 3.
Change in LogMAR over time during infliximab therapy for pediatric uveitis
| Baseline | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|
| Mean LogMAR | 0.30 | 0.28 | 0.29 | 0.30 | 0.30 |
| No. of patients | 15 | 14 | 13 | 11 | 13 |
| Comparison to baseline, P value | N/A | 0.90 | 0.96 | 0.99 | 0.99 |
No. = number; N/A = not applicable
Table 4.
Proportion of children discontinuing opthalmic glucocorticoid drops during infliximab therapy for uveitis
| Baseline | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|
| No. (%) patients off topical glucocorticoids | 0 (0%) | 6 (37%) | 7 (44%) | 8 (50%) | 11 (69%) |
| Total | 16 | 16 | 16 | 16 | 16 |
| Comparison to baseline, p value | N/A | 0.08 | 0.04 | 0.02 | 0.006 |
No. = Number, N/A = not applicable
Of the seven children with intermediate uveitis, panuveitis or pars planitis, two had no evidence of vitreal inflammation at baseline or during follow up. Two had few anterior vitreal cells at baseline that cleared within one month of infliximab therapy and remained absent. In three subjects, baseline vitreal inflammation was difficult to gauge due to anterior chamber inflammation and/or band keratopathy; these three subjects all had a few snowballs in the inferior vitreous which were visualized once the anterior chamber inflammation improved. These findings remained stable throughout follow up. No new chorioretinal lesions or neovascularization were described in any of the children.
Three children did not improve immediately, necessitating additional intervention to achieve control of severe ocular inflammation. These two children required periocular depot triamcinolone acetonide injections within one month of starting infliximab, and one child was also prescribed a brief course of oral prednisone.
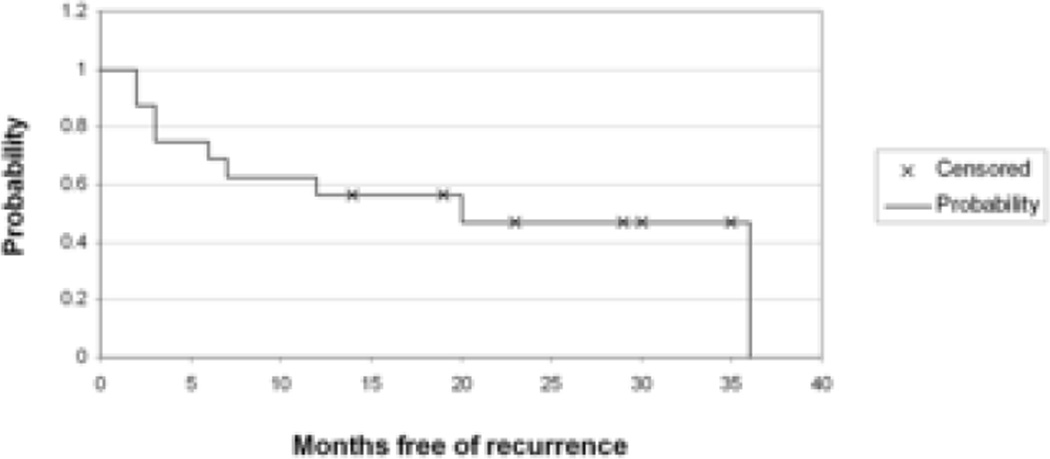
Sixteen uveitis recurrences occurred in 9/16 (56%) of children. Figure 1 shows a Kaplan Meier curve summarizing the proportion of children who remained free of uveitis over time. At six months, 63% remained free of uveitis recurrence (95% confidence interval, 39% to 86%). At 12 months, 58% remained free of uveitis recurrence (95% confidence interval 32% to 82%). While it was difficult to pinpoint timing and etiology given the retrospective nature of the data, two recurrences occurred following cataract surgery and nine developed after intentional or inadvertent increase in interval between infliximab doses. Uveitis recurrences were managed at the discretion of ophthalmologist and rheumatologist and included one or a combination of the following interventions: change in topical glucocorticoids, brief course of oral prednisone, injection of periocular steroids, increase in infliximab dose, and decrease in interval between infliximab infusions, increase in methotrexate dose or transition from oral to subcutaneous administration, addition of MMF. One child experienced a protracted recurrence 16 months into infliximab therapy which ultimately resolved following escalation of infliximab dose, increase in methotrexate dose and addition of MMF. Another child initially improved, allowing reduction in prednisone, but later developed persistent ocular inflammation requiring the addition of MMF. In this patient, poor response to all immunosuppressive therapy ultimately led to an intraocular fluocinolone acetonide implant and discontinuation of infliximab and MMF. Parental concerns about potential side effects led to termination of infliximab another child; this patient experienced a uveitis recurrence three months following infliximab discontinuation.
Figure 1.
Kaplan-Meier Estimate of Time to Uveitis Recurrence in Children Treated with Infliximab.
DISCUSSION
In this series, we found that long-term (median > 2 years) infliximab therapy appeared to be well tolerated in 16 children with chronic uveitis. Treatment with methotrexate and infliximab was associated with resolution of or two-step reduction in anterior chamber cell inflammation at 3, 6, 9, and 12 months of treatment. Additionally, children treated with infliximab had stable visual acuity and were able to discontinue corticosteroid ophthalmic drops. As in most published case series, the majority (15/16) of children were treated with the combination of infliximab and methotrexate. Consequently, any clinical improvement must be attributed to the combination rather than infliximab alone.
In accordance with the recommendations of the Standardization of Uveitis Nomenclature (SUN) study group,18 this series assessed serial anterior chamber cell count and visual acuity at specific intervals in addition to concomitant medication use and uveitis recurrences. Few published case series concerning use of anti-TNF therapy in pediatric uveitis have provided such comprehensive outcome information. Many of the available case series are significantly limited by invalid outcome measures not endorsed by the SUN study group as well as use of initial and final ophthalmologic exam rather than specific time intervals.
The retrospective nature of this study made it difficult to define the timing of response to treatment and recurrences. Ophthalmologic data were not standardized and were provided by several different ophthalmologists, and data concerning posterior chamber inflammation were limited. Because treatment regimens were at the discretion of providers, discrepancies in infliximab dose and interval and use of concomitant immunomodulatory medications occurred and may be confounding variables. Compliance with frequent infusions was necessary for efficacy. Such adherence can be problematic, particularly in adolescent populations.
The median infliximab dose used in this series, 8.2 mg/kg, is higher than doses typically reported to treat Crohn’s disease or adult rheumatoid arthritis (usually 3–5 mg/kg).19, 20 Particularly in children, treatment of ocular inflammation often requires doses of infliximab higher than those usually prescribed in inflammatory arthritis and colitis in order to provide adequate drug penetration and concentration in the protected ocular compartment. The dosing flexibility of infliximab compared to etanercept may explain why infliximab appears be more efficacious than etanercept to treat noninfectious uveitis. It is also possible that differences in the pharmacokinetics or immunologic activity of etanercept and infliximab are important in mediating ocular inflammation. These differences may include infliximab’s ability to bind both soluble and membrane-bound TNF and its ability to bind complement.21 It is also possible that children metabolize infliximab differently than adults although this has not been investigated.
While infliximab shows promise as a therapy for pediatric uveitis, it is important to keep in mind that it is an expensive medication and one with significant potential side effects. Although not observed in this cohort, rare serious infections and increased malignancy rates have been described with infliximab treatment in adults with rheumatoid arthritis.22
Our study is limited by its retrospective nature, heterogeneity of disease and small number of patients; however, it represents one of the largest series describing long-term infliximab therapy in pediatric uveitis and provides important information to pediatric rheumatologists and ophthalmologists concerning the long term tolerability and potential efficacy of infliximab in children with uveitis. Although these results need to be confirmed in a prospective, controlled trial, our data suggest that infliximab may be a beneficial therapy for chronic noninfectious uveitis in children.
Acknowledgements
Funding/support for this project: Dr. Jaffe reported financial support for this project from Core Grant NEI P30EYO572 (Duke Eye Center)
Financial disclosures: Dr. Schanberg reports support from Centocor (manufacturer of Remicade) to fund pediatric rheumatology fellowship training program. One year of Dr. Ardoin’s rheumatology fellowship (2003–2004) was partially funded by Amgen (manufacturer or Enbrel).
Biography
Dr. Stacy Ardoin is a clinical associate in adult and pediatric rheumatology at Duke University Medical Center. After completing medical school, internal medicine and pediatrics residency and internal medicine chief residency at Ohio State University, she completed a fellowship in adult and pediatric rheumatology at Duke. Her primary research involves cardiovascular and neuropsychiatric complications of systemic lupus erythematosus, and she is the recipient of the American College of Rheumatology Physician Scientist Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Contributions of authors: Design of the study (SA, ER, LS, DK, GJ), Conduct of study (SA, ER, LS, DK, GJ), Data collection and management (SA, LS, GJ), Data analysis (SA, ER, LS, GJ), Review of manuscript (SA, ER, LS, DK, GJ), Approval of manuscript (SA, ER, LS, GJ).
Approval from the Duke University Medical Center IRB was obtained prior to undertaking this project and the study was completed in compliance with HIPAA requirements
References
- 1.de Boer J, Wulffraat N, Rothova A. Visual loss in uveitis of childhood. Br J Ophthalmol. 2003;87:879–884. doi: 10.1136/bjo.87.7.879. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olson NY, Lindsley CB, Godfrey WA. Nonsteroidal anti-inflammatory drug therapy in chronic childhood iridocyclitis. Am J Dis Child. 1988;142:1289–1292. doi: 10.1001/archpedi.1988.02150120043036. Medline. [DOI] [PubMed] [Google Scholar]
- 3.Makela AL. Naproxen in the treatment of juvenile rheumatoid arthritis. Scand J Rheumatol. 1977;6:193–205. doi: 10.3109/03009747709095449. Medline. [DOI] [PubMed] [Google Scholar]
- 4.Weiss AH, Wallace CA, Sherry DD. Methotrexate for resistant chronic uveitis in children with juvenile rheumatoid arthritis. J Pediatr. 1998;133:266–268. doi: 10.1016/s0022-3476(98)70232-x. Medline. [DOI] [PubMed] [Google Scholar]
- 5.Foeldvari I, Wierk A. Methotrexate is an effective treatment for chronic uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2005;32:362–365. Medline. [PubMed] [Google Scholar]
- 6.Kilmartin DJ, Forrester JV, Dick AD. Cyclosporine A therapy in refractory noninfectious childhood uveitis. Br J Ophthalmol. 1998;82:737–742. doi: 10.1136/bjo.82.7.737. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorne JE, Jabs DA, Qazi FA, Nguyen QD, Kempen JH, Dunn JP. Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology. 2005;112:1472–1477. doi: 10.1016/j.ophtha.2005.02.020. Medline. [DOI] [PubMed] [Google Scholar]
- 8.Reiff A, Takei S, Sadeghi S, et al. Etanercept therapy in children with treatmentresistant uveitis. Arthritis Rheum. 2001;44:1411–1415. doi: 10.1002/1529-0131(200106)44:6<1411::AID-ART235>3.0.CO;2-O. Medline. [DOI] [PubMed] [Google Scholar]
- 9.Reiff A. Long-term outcome of etanercept therapy in children with treatmentrefractory uveitis. Arthritis Rheum. 2003;48:2079–2080. doi: 10.1002/art.11155. Medline. [DOI] [PubMed] [Google Scholar]
- 10.Smith JA, Thompson DJ, Whitcup SM, et al. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum. 2005;53:18–23. doi: 10.1002/art.20904. Medline. [DOI] [PubMed] [Google Scholar]
- 11.Biester S, Deuter C, Michels H, et al. Adalimumab in the therapy of uveitis in childhood. British J Ophthal. 2007;91:274–276. doi: 10.1136/bjo.2006.103721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vazquez-Cobian LB, Flynn T, Lehman TJ. Adalimumab therapy for childhood uveitis. J Pediatr. 2006;149:572–575. doi: 10.1016/j.jpeds.2006.04.058. Medline. [DOI] [PubMed] [Google Scholar]
- 13.Kahn P, Weiss M, Imundo LF, Levy DM. Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmology. 2006;113:864, e1–e2. doi: 10.1016/j.ophtha.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Rajaraman RT, Kimura Y, Li S, Haines K, Chu DS. Retrospective case review of pediatric patients with uveitis treated with infliximab. Ophthalmology. 2006;113:308–314. doi: 10.1016/j.ophtha.2005.09.037. Medline. [DOI] [PubMed] [Google Scholar]
- 15.Richards JC, Tay-Kearney M, Murrah K, Manners P. Infliximab for juvenile idiopathic arthritis-associated uveitis. Clin Experiment Ophthalmol. 2005;33:461–468. doi: 10.1111/j.1442-9071.2005.01062.x. Medline. [DOI] [PubMed] [Google Scholar]
- 16.Saurenmann RK, Levin AV, Rose JB, et al. Tumor necrosis factor alpha inhibitors in the treatment of childhood uveitis. Rheumatology (Oxford) 2006;45:982–989. doi: 10.1093/rheumatology/kel030. Medline. [DOI] [PubMed] [Google Scholar]
- 17.Hogan MJ, Kimura SJ, Thygeson P. Signs and symptoms of uveitis. Am J Ophthalmol. 1959;47:155–170. doi: 10.1016/s0002-9394(14)78239-x. Medline. [DOI] [PubMed] [Google Scholar]
- 18.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of Uveitis Nomenclature for Reporting Clinical Data. Results of First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumor necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomized phase III trial. Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. Medline. [DOI] [PubMed] [Google Scholar]
- 20.Hanauer SB, Feagan BF, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomized trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. Medline. [DOI] [PubMed] [Google Scholar]
- 21.Mpofu S, Fatima F, Moots RJ. Anti-TNF-alpha therapies: they are all the same (aren’t they?) Rheumatology (Oxford) 2005;44:271–273. doi: 10.1093/rheumatology/keh483. Medline. [DOI] [PubMed] [Google Scholar]
- 22.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. Medline. [DOI] [PubMed] [Google Scholar]