Abstract
Objective
Descriptions of mandibular distraction osteogenesis for tissue replacement after oncologic resection or for defects caused by osteoradionecrosis have been limited. Previous work demonstrated radiation decreases union formation, cellularity and mineral density in mandibular distraction osteogenesis. The authors posit that intermittent systemic administration of parathyroid hormone will serve as a stimulant to cellular function, reversing radiation-induced damage and enhancing bone regeneration.
Methods
Twenty male Lewis rats were randomly assigned to three groups: group 1 (radiation and distraction osteogenesis, n = 7) and group 2 (radiation, distraction osteogenesis, and parathyroid hormone, n = 5) received a human-equivalent dose of 35 Gy of radiation (human bioequivalent, 70 Gy) fractionated over 5 days. All groups, including group 3 (distraction osteogenesis, n = 8), underwent a left unilateral mandibular osteotomy with bilateral external fixator placement. Distraction osteogenesis was performed at a rate of 0.3 mm every 12 hours to reach a gap of 5.1 mm. Group 2 was injected with parathyroid hormone (60 μg/kg) subcutaneously daily for 3 weeks after the start of distraction osteogenesis. On postoperative day 40, all left hemimandibles were harvested. Biomechanical response parameters were generated. Statistical significance was considered at p ≤ 0.05.
Results
Parathyroid hormone–treated mandibles had significantly higher failure load and higher yield than did untreated mandibles. However, these values were still significantly lower than those of nonirradiated mandibles.
Conclusions
The authors have successfully demonstrated the therapeutic efficacy of parathyroid hormone to stimulate and enhance bone regeneration in their irradiated murine mandibular model of distraction osteogenesis. Anabolic regimens of parathyroid hormone, a U.S. Food and Drug Administration–approved drug on formulary, significantly improve outcomes in a model of postoncologic craniofacial reconstruction.
The mandible is often included in the radiation field during treatment of head and neck cancers.1,2 Osteoradionecrosis affects approximately 5 percent of patients and is a debilitating complication for these patients treated with radiation therapy for head and neck cancers.3 Those stricken with osteoradionecrosis often have severe limitations in mandibular function and are at heightened risk for pathologic fracture. Quality of life is often affected in patients with osteoradionecrosis, including limitations related to loss of dentition and altered aesthetics, all of which result in limitation of social interaction. Clearly, a management strategy that avoids this condition and ensures that patients have the ability to remain as productive members of society is both desirable and warranted.
For grade IV osteoradionecrosis that does not respond to medical treatment options, a segmental I mandibulectomy is typically indicated. Currently, there are not many reconstructive options for these patients—the criterion standard for reconstruction following a segmental mandibulectomy after irradiation is osseous free tissue transfer or soft tissue in combination with a reconstruction bar/plate. Studies have shown that free tissue transfer procedures have superior efficacy in preventing plate exposure and wound infection, leading to the increased use of these procedures.4,5 However, free tissue transfer requires the harvest of bony and soft-tissue substrate from a distant donor site, and these procedures are characterized by longer operative times, multiple comorbidities, and increased complication rates.
Distraction osteogenesis, the gradual separation of two osteogenic fronts to stimulate new bone formation, presents an alternative to free tissue transfer. One of the limitations to the immediate translation of distraction osteogenesis to surgical practice for the purpose of oncologic reconstruction is the immense difficulty in healing previously radiated tissue.6 The effects of ionizing radiation on the regeneration of bone within a fracture site include decreased osteocyte number, suppressed osteoblast activity, and diminished vascularity in murine models of distraction osteogenesis.7 As the success of distraction osteogenesis (and in general, bone healing) is predicated on resident vascularity and cellularity, the direct cellular impairment and vascular injury caused by adjuvant radiation therapy are potential obstacles to successful distraction osteogenesis reconstruction following segmental mandibulectomy.
Parathyroid hormone is a major regulator of Ca2+ homeostasis and exerts both anabolic and catabolic effects on bone homeostasis, and is currently used clinically for the treatment of osteoporosis. Continuous infusions of the hormone (or its active portion, amino acids 1 to 34) lead to greater bone resorption, whereas intermittent daily dosing has an anabolic effect and leads to increased bone mass, osteoblast generation and mobilization, and improved fracture healing.8
Our goal is to investigate the effects of parathyroid hormone and radiotherapy in murine models of distraction osteogenesis by using metrics of biomechanical response. Our previous research indicates significant improvement of union quality and radiomorphometric parameters given an anabolic dosing regimen of parathyroid hormone following irradiation and mandibular osteogenesis. Our hypothesis is that intermittent systemic administration of parathyroid hormone will serve as a stimulant to cellular function that will act to reverse a human-equivalent dose of radiation–induced damage and enhance bone regeneration. Specific quantitative analysis of bone will allow us to compare outcomes of irradiated animals and tissues that have been treated with those that have not been treated with parathyroid hormone. In addition, we will gauge the efficacy of parathyroid hormone to remediate radiation damage by comparing our results to those of nonirradiated animals who underwent distraction osteogenesis without irradiation.
MATERIALS AND METHODS
Experimental Groups and Model
Twenty 12-week-old male Lewis rats were assigned randomly to one of three experimental groups preoperatively: group 1, rats that would receive preoperative radiation followed by distraction (n = 7); group 2, rats that would receive preoperative radiation followed by distraction and parathyroid hormone (n = 5); and group 3, rats that would undergo distraction alone (n = 8).
The rats in groups 1 and 2 underwent fractionated irradiation to the left hemimandible followed by a 2-week recovery period before surgery. All three experimental groups then underwent surgical osteotomy and placement of a distraction device with the osteotomy gap closed. All three groups were then subjected to a 4-day latency period followed by distraction, 0.3 mm twice daily to a 5.1-mm total gap width. Parathyroid hormone (60 μg/kg) and vehicle (0.9% normal saline) were administered subcutaneously daily to group 2 beginning the on first day of distraction, for 21 days. The other two groups received vehicle injections for the same length of time. All three experimental groups underwent 28 days of regenerate consolidation after the last day of distraction.
Preoperative Animal Care
The male Lewis rats weighed approximately 350 g and were housed three animals per cage in a pathogen-free, restricted-access facility on arrival to our laboratory. Animals in groups 1 and 2 were fed hard chow and water ad libitum during a 3-day acclimation period and during radiotherapy. Group 3 animals were fed hard chow and water ad libitum during the 3-day acclimation period. The diet was changed in all groups to moist chow 4 days preoperatively. All animal procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Michigan Animal Care and Use Committee.
Irradiation
Radiotherapy was performed at the Irradiation Core of the University of Michigan Cancer Center. Radiotherapy was delivered with a Phillips RT250 orthovoltage unit (250 kV, 15 mA) (Kimtron Medical, Woodbury, Conn.). Dosimetry was carried out using an ionization chamber connected to an electrometer system. The group 1 and 2 rats were irradiated after being anesthetized with isoflurane. Induction was begun at 4%, after which the rat was maintained at 1.5%. They were placed right side down, to expose the left mandible. A lead shield was used to protect the remainder of the animal. A total of 35 Gy of radiation was delivered per rat in five fractions over 5 days at a rate of 7 Gy per fraction, which is the bioequivalent dose of 70 Gy in humans.9
Surgical Procedure
Our surgical distraction procedure has been described and published before,10 but briefly, preoperatively, animals were given prophylactic gentamicin (5 mg/kg subcutaneously) 1 hour before incision. In addition, buprenorphine (0.03 mg/kg subcutaneously) and lactated Ringer's solution (25 cc/kg subcutaneously) were given for pain control and hydration, respectively. General anesthesia was induced with an isoflurane/oxygen mixture, and the animal's ventral submental hair was shaved in preparation for surgery. The animal was then placed in supine position and prepared and draped in sterile fashion, and the procedure commenced under sterile conditions.
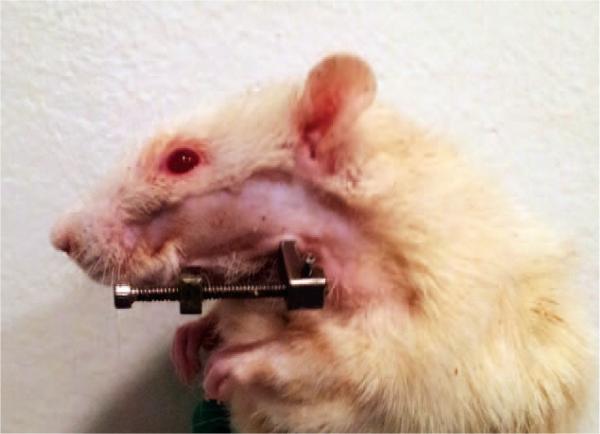
After standard preparation and draping with the animal on its dorsum and the neck extended, a 1.5-cm midline incision was placed ventrally from the anterior submentum to the neck crease. Skin flaps were elevated, exposing the anterolateral mandible and avoiding the mental nerve. After predrilling holes bilaterally 0.5 mm posterior to the symphysis, a 1.5-inch stainless steel threaded pin was inserted transversely across the anterior mandible. On each side, an 8-mm incision was made through the masseter, down to and inline with the inferior border of the mandible, approximately 2.5 mm anterior to the angle. After predrilling 2 mm superior to the inferior border and 4 mm anterior to the angle, bilateral stainless steel threaded pins were inserted in a buccal-to-lingual direction and secured with a custom titanium washer and nut. The pin ends were brought externally through the skin for the posterior fixator placement with titanium cap screws. The right mandible was fixed rigidly, whereas the left mandible was fixed with a distraction screw for postoperative manipulation (Fig. 1).
Fig. 1.
Rat with bilateral fixator with unilateral distraction device implanted surgically into the mandible. The distraction device is visible.
A vertical osteotomy was made in the left hemimandible approximately 2 mm anterior to the titanium washer using a 10-mm micro–reciprocating blade (Stryker, Portage, Mich.) attached to a power saw (Stryker). The osteotomy extended from the inferior mandible border superiorly to the sigmoid notch along the anterior aspect of the coronoid process. The external fixator device was adjusted to ensure reduction and hemostasis of the osteotomy edges (Fig. 2). After reduction of the osteotomy edges, the wounds were irrigated, hemostasis was verified, soft tissue was approximated using 4-0 Vicryl (Ethicon, Inc., Somerville, N.J.) suture, and the midline incision was closed with staples. The animal was caged under a heat lamp and monitored with additional hydration by means of subcutaneous lactated Ringer's solution, approximately 25 to 35 cc/kg, and observed for approximately 1 hour.
Fig. 2.
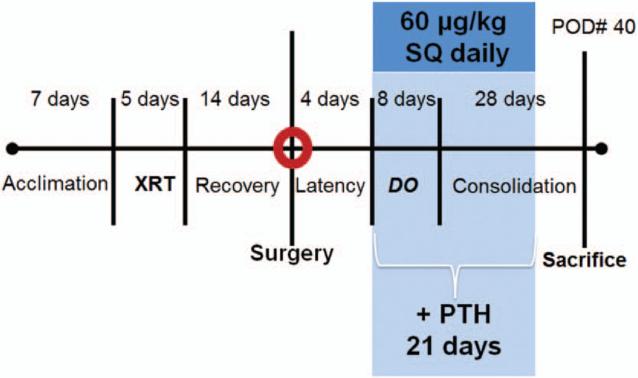
Timeline from acclimation, irradiation, surgery, distraction, consolidation, and then euthanasia after postoperative day 40. XRT, irradiation; PTH, parathyroid hormone; DO, distraction osteogenesis; POD, postoperative day; SQ, subcutaneously.
Postoperative Animal Care
All experimental groups were housed one animal per cage and fed moist chow with Hill's high-calorie a/d diet (Columbus Serum, Columbus, Ohio) and water ad libitum. Both groups of animals were given two postoperative doses of gentamicin (5 mg/kg subcutaneously every 12 hours). Buprenorphine was continued (0.03 mg/kg) with 10 cc of lactated Ringer's solution subcutaneously every 12 hours through postoperative day 4 and as needed thereafter. Bactrim was given with the moist chow postoperatively, prophylactically against postoperative wound infection, until the animals were killed. Pin care was performed with Silvadene (Monarch Pharmaceuticals, Inc., Bristol, Tenn.) every other day. Maxillary incisors were clipped weekly because of overgrowth from cross-bite and staples removed at postoperative day 10. Weights were monitored daily and diets adjusted as needed.
Distraction Protocol
Animals in all three groups underwent distraction after 4 days of latency. One 180-degree clockwise turn of the distraction screw corresponded to a 0.3-mm separation of the osteotomy fronts. A total of 17 half-turns were performed every 12 hours starting in the pm of postoperative day 4, resulting in a 5.1-mm distraction gap. No analgesic or sedation was required during the distraction. After 28 days of consolidation, all animals were killed (postoperative day 40). See Figure 2 for the timeline of the experiment.
Mechanical Testing
Preparation of Samples
Hemimandibles were split between the incisors and frozen at −20°C until testing in lactated Ringer's solution. The freeze/thaw cycle has no effect on the biomechanical integrity of the mandibles. Just before potting, mandibles were thawed in water at room temperature. For improved adhesion of the potting medium to the specimens, small reinforcement wires were inserted perpendicularly through the hemimandibles at both existing anterior and posterior pin-site holes. In addition, a new hole was drilled through the condyle at the posterosuperior aspect for insertion of another support wire. The 0.8-mm stainless steel reinforcement wires measure approximately 1.5 cm in length.
Potting
Pots were cylinders machined out of aluminum, measuring 2.0 cm in diameter and 1.8 cm deep. The potting medium is a Cerrobend bismuth alloy (50 percent bismuth, 27 percent lead, 13 percent tin, and 10 percent cadmium; melting point, 70°C) which, when melted, becomes an easy-to-use, conforming liquid. The posterior aspect of the mandible was potted first. The mandible was positioned inside the pot with the inferior edge of the mandible perpendicular to the top edge of the pot. The depths were determined by (and varied according to) the location of the osteotomy. Pots were placed as close to the osteotomy as possible without crossing into or over the posterior osteotomy border. Therefore, only the posterior potting depth varied between specimens, whereas the top edge of the pot was always perpendicular to the inferior edge of the mandible, regardless of regenerate gap size or angle. After correct positioning was achieved, potting medium was poured into the pot and allowed to adhere to both the pot and the specimen. Care was taken to ensure that the support pins were fully buried in the medium, and that the medium was spread uniformly around the bone. To speed the adhesion, a cool-air gun was used until the metal was hard (tested by pressing with forceps). Pots (with one-half potted mandible) were then further cooled in an ice bath for approximately 60 seconds. The anterior portion of the mandible was potted next. Before potting, the posterior pot was aligned precisely with the new anterior pot. Anterior depth was determined anatomically by fully burying the third molar in the potting medium. Inferior edges of mandibles were again perpendicular to the top edge of the pot. After correct positioning, the potting medium was added as above and cooled similarly. Potted mandibles were stored temporarily in an ice bath until testing.
Tension Testing
Potted anteroposterior hemimandibles were loaded to failure using tension at a constant displacement rate of 0.5 mm/second. We used a servohydraulic testing machine (858 Mini Bionix II; MTS Systems Corporation, Eden Prairie, Minn.). A 10-lb load cell (Sensotec, Columbus, Ohio) was used to measure load applied to the hemimandibles. Load head displacement was monitored with an external linear variable differential transducer (Howard A. Schaevitz Technologies, dba Macro Sensors, Pennsauken, N.J.). The load and displacement data were acquired using the TestStar IIs system (version 2.4; MTS Systems) at a sampling frequency of 200 Hz through an A/D system (Labview; National Instruments, Austin, Texas). Load-displacement curves were analyzed for yield and maximum load, and then stiffness and postyield displacement were calculated and recorded.
Outcome Metric Definitions
Ultimate load translates to the peak load sustained during testing of the mandible. Failure load is the load that was being experienced by the mandible at the moment when it finally failed and separated into two separate pieces. Failure loads are typically marginally lower than ultimate loads, as microfractures that occur before failure typically relieve some of the force on the bone.
Yield is the transition point between nonplastic and plastic deformation. When experiencing loads under the yield point, the bone will return to its original shape and state (nonplastic or elastic deformation). However, when experiencing loads greater than the yield point, the bone will be irrevocably altered, and the normal shape cannot be reclaimed. Thus, the yield point can be thought of as the maximum load before permanent damage to the bone occurs.
Statistical Analysis
Rats were assigned randomly to the three groups (distraction osteogenesis, irradiation/distraction osteogenesis, and parathyroid hormone/irradiation/distraction osteogenesis). A two-tailed analysis of variances was used for statistical analysis. The Levene test of homogeneity of variances was performed to determine the appropriate post hoc test. For homogenous variances, a Tukey post hoc test was used. For non-homogenous variances, a Games-Howell post hoc test was used. Analysis of variance was used, as it is known to be fairly robust against small sample sizes and potentially non-Gaussian distributions. Values of p < 0.05 were considered significant. Animals were excluded from the experiment only if they had gross infection on their left side. The sample sizes needed to test our hypotheses correlating with our specific aims were determined using a power analysis, under the assumption that the data would be evaluated using a general linear model with associated analysis of variance with a desired power of 0.8 with differences between groups of greater than 25 percent. All statistical analysis was performed using IBM SPSS version 20 (IBM Corp., Armonk, N.Y.).
RESULTS
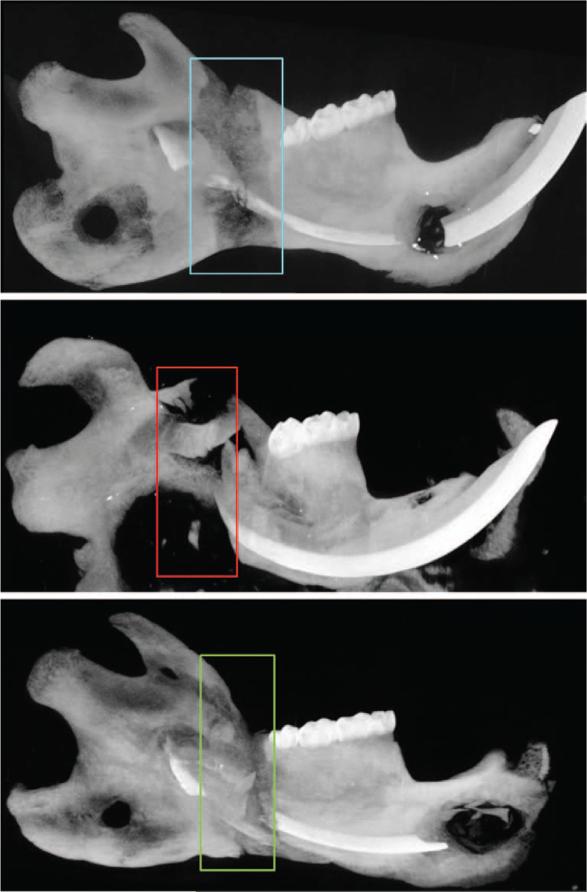
Animals in all three treatment groups tolerated the protocol. Animals treated with radiation demonstrated expected clinical signs of radiation treatment, ranging from alopecia to mucositis. Postoperatively, animals lost weight initially but then gained weight once distraction was complete and in the consolidation phase. None of the animals experienced device dislodgment, and the fixators remained stable until the animals were killed on postoperative day 40 (Fig. 2). Gross examination and maximum intensity projections revealed three different levels of bony union (Fig. 3).
Fig. 3.
Representative maximum intensity projections. On dissection, gross examination revealed that all distraction osteogenesis group mandibles had solid, bony unions. All parathyroid hormone–treated mandibles also had grossly apparent unions, whereas the irradiation/distraction osteogenesis group did not. (Above) Mandibular distraction osteogenesis without irradiation. Complete bony bridging across the distraction gap is shown. (Center) Mandibular distraction osteogenesis with irradiation. Minor bridging across the distraction gap, and erosion of the posterior pin site and angle of the mandible are shown. (Below) Distraction osteogenesis with irradiation and parathyroid hormone therapy. Restoration of bony bridging to the distraction gap is shown.
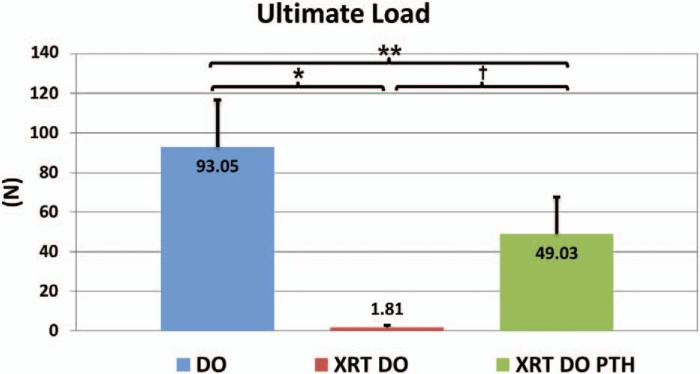
Biomechanical testing showed that our first metric, ultimate load, was vastly improved by parathyroid hormone therapy (distraction osteogenesis/irradiation/parathyroid hormone, 49.03 ± 16.12 N) compared with our nontreated, irradiated group (distraction osteogenesis/irradiation, 1.81 ± 0.50 N; p < 0.001), a significant 2600 percent increase in strength. Parathyroid hormone–treated mandibles achieved 52.7 percent of the strength of nonirradiated, distracted mandibles (distraction osteogenesis, 93.05 ± 18.10 N; p < 0.05), and this difference was also significant (Fig. 4).
Fig. 4.
Ultimate load, or the peak load sustained by the mandible during the test (*p < 0.05, **p < 0.05, and †p < 0.05). DO, distraction osteogenesis; XRT, irradiation; PTH, parathyroid hormone.
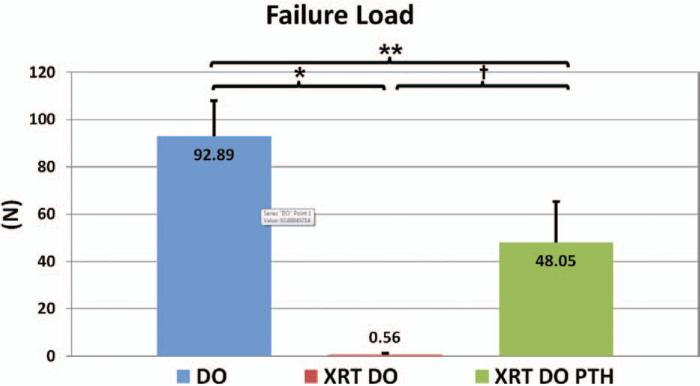
This is also reflected in failure load (Fig. 5). This metric tells a story similar to that of ultimate load. Again, parathyroid hormone therapy (distraction osteogenesis/irradiation/parathyroid hormone, 48.05 ± 20.21 N) vastly improves the failure load of these mandibles compared with irradiated mandibles not treated with parathyroid hormone, by over 8000 percent (distraction osteogenesis/irradiation, 0.56 ± 1.59 N; p < 0.001). The control, nonirradiated, untreated mandibles (distraction osteogenesis, 92.89 ± 17.66 N; p < 0.05) had significantly higher failure loads than parathyroid hormone–treated mandibles.
Fig. 5.
Failure load, or the load being sustained contemporaneous with the breaking of the mandible (*p < 0.05, **p < 0.05, and †p < 0.05). DO, distraction osteogenesis; XRT, irradiation; PTH, parathyroid hormone.
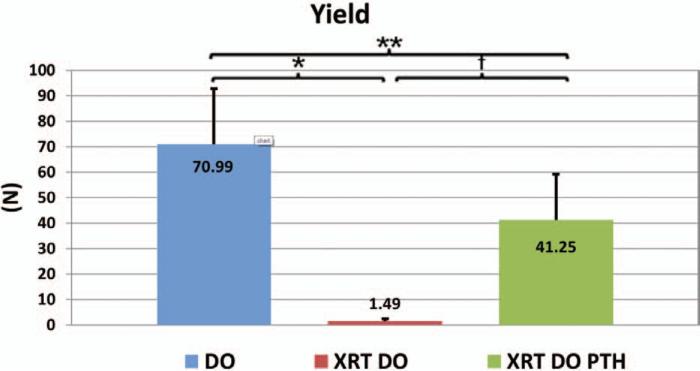
Our findings on the effect of parathyroid hormone on biomechanical integrity were similar for yield as well (Fig. 6). Here, again, parathyroid hormone treatment (distraction osteogenesis/irradiation/parathyroid hormone, 41.25 ± 19.58) significantly improves the yield of irradiated, distracted mandibles (distraction osteogenesis/irradiation, 1.49 ± 1.21; p < 0.001). Yield for control (distraction osteogenesis, 70.99 ± 22.23 N) is significantly greater than for parathyroid hormone–treated mandibles (p < 0.05).
Fig. 6.
Yield, or the transition point from nonplastic deformation to plastic deformation (*p < 0.05, **p < 0.05, and †p < 0.05). DO, distraction osteogenesis; XRT, irradiation; PTH, parathyroid hormone.
DISCUSSION
Our previous data showed a nearly complete mitigation of radiation-induced damage by means of radiomorphometric analysis. However, this was not evident by means of biomechanical testing analysis metrics, where we found significant but not complete remediation of these key metrics of strength, showing that radiomorphometrics alone are not necessarily sensitive enough to be a proxy for strength in all situations. Furthermore, these findings may indicate that parathyroid hormone can assuage the damage to the inorganic portion of the bone, but may benefit from combination with therapies directed specifically at rehabilitating the organic portion of the bone (e.g., the collagenous organization of the mandible).
The pernicious effects of radiation are well known and routinely published. Radiation can devastate local cell population proliferation,11 alter cellular cytokine and chemokine expression, and induce localized vascular degradation (unpublished data) and hypoxia.7 The formation of collagen, a key element of healthy bone, has been shown to be down-regulated in a dose-dependent manner in mouse osteoblasts.11 Radiation has also previously been shown to decrease bone quality, and bone mineral density in particular.12
Mandibular distraction osteogenesis requires the differentiation and proliferation of osteo-progenitor cells after organization of the initial surgical wound.13 It has previously been shown that radiation results in poorer regenerate outcomes in mandibular distraction osteogenesis with regard to cellularity, radiomorphometrics, and biomechanical strength.14–16 Our findings of poorer union quality and decreased bone mineral density and bone volume fraction in the irradiated, distracted animals (group 1) are consistent with these previous outcomes.
The finding that parathyroid hormone improved regenerate outcomes in terms of ultimate load, failure load, and yield in irradiated animals that underwent mandibular distraction osteogenesis is an entirely novel application of parathyroid hormone therapy. Parathyroid hormone is the only U.S. Food and Drug Administration–approved therapy that stimulates bone formation in osteoporotic individuals and has been shown to reverse loss of bone mineral density and prevent fractures in clinical trials.17,18 Parathyroid hormone (1-34), otherwise known as teriparatide, is the active portion of parathyroid hormone and is responsible for calcium homeostasis, and can have contrasting effects on bone. If exposed to bone in brief raised concentrations, parathyroid hormone has an anabolic effect. In contrast, if exposed to bone continuously, parathyroid hormone has a catabolic effect.19 Osteoblasts, when exposed to continuous doses of parathyroid hormone, express RANKL, a surface-bound molecule that activates osteoclastogenesis, leading to bone resorption.20 However, osteoblasts stimulated by intermittent doses of parathyroid hormone secrete the protein insulin-like growth factor, which activates the gene IRS-1 in osteoblast precursors, causing bone formation.21 Parathyroid hormone has been shown to arrest and partially reverse bone loss in animals and humans,22 and clinical trials have shown its benefit in osteoporosis17; it is currently marketed for this purpose as Forteo (Eli Lilly and Company, Indianapolis, Ind.). Animal studies have also demonstrated that intermittent parathyroid hormone (1-34) promotes osteogenesis in fracture healing and enhances the size and mechanical properties of calluses.23–27 Intermittent parathyroid hormone has also been shown to increase strength, stiffness, and bone mineral density in a rat model of long-bone distraction osteogenesis.28
Parathyroid hormone also assists in the activation of survival signaling, preventing apoptosis of residual osteoblasts, and increasing the overall number of cells.29 Conversely, it has been shown that radiation decreases the number and function of osteocytes in mandibular bone14 and further down-regulates pro-osteogenic signaling molecules in bone.30 Thus, it is entirely possible that anabolic regimens of parathyroid hormone assist in restoring the osteogenic pathways specifically negated by radiation. Therefore, an improvement in osteoblast survival, proliferation, and function may explain the striking increases in bone quality and biomechanical parameters resulting from parathyroid hormone administration.
Our findings would support the potential clinical use of parathyroid hormone as an adjunct therapy if distraction osteogenesis is attempted in bones that have been previously irradiated and where maximizing the ability of bone to regenerate is essential. The addition of parathyroid hormone as a therapeutic intervention during postirradiation mandibular distraction osteogenesis would make this a more feasible and potentially more successful reconstructive option after oncologic resection or in reconstruction for defects after surgical treatment of osteoradionecrosis. This combination of hormonal therapy and tissue-engineering technique has been demonstrated to aid in mineral deposition and vasculogenesis within the regenerate region (unpublished data), which can be used to treat existing instances of abnormalities such as osteoradionecrosis. The therapeutic efficacy of parathyroid hormone to stimulate and enhance bone regeneration in our irradiated model of distraction osteogenesis suggests that its clinical application would result in an improvement in the quality of mandibular union.
Radiomorphometric analysis of bone healing and regeneration has demonstrated the successful ability of parathyroid hormone to attain union in irradiated mandibular distraction osteogenesis. Although these findings are exciting, the biomechanical properties of these regenerated mandibles are not yet ideal. Because of the disparity between our radiomorphometric and biomechanical testing data, we plan to further examine the role of parathyroid hormone on postoncologic reconstruction. Such assays include quantitative histomorphometry and three-dimensional vascular casting and perfusion analysis. Such analysis may shed light on why an apparent remediation of mineralization does not translate into a normalization of mechanical strength. As there is a growing body of work that shows that parathyroid hormone can assist in the recruitment and differentiation of bone marrow stromal cells (the mesenchymal stem cell progenitor of osteoblasts), we hope to examine the effect of intraoperative administration of bone marrow stromal cells with this same parathyroid hormone therapy regimen. Such research has the potential to accelerate the translation and optimization of postoncologic reconstruction and prevent the devastating sequelae of continuing pathologic fractures and osteoradionecrosis.
Footnotes
Disclosure: None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this article.
REFERENCES
- 1.Shah JP, Gil Z. Current concepts in management of oral cancer: Surgery. Oral Oncol. 2009;45:394–401. doi: 10.1016/j.oraloncology.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sedaghat AR, Zhang Z, Begum S, et al. Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope. 2009;119:1542–1549. doi: 10.1002/lary.20533. [DOI] [PubMed] [Google Scholar]
- 3.Jereczek-Fossa BA, Orecchia R. Radiotherapy-induced mandibular bone complications. Cancer Treat Rev. 2002;28:65–74. doi: 10.1053/ctrv.2002.0254. [DOI] [PubMed] [Google Scholar]
- 4.Foster RD, Anthony JP, Sharma A, Pogrel MA. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: An outcome analysis of primary bony union and endosseous implant success. Head Neck. 1999;21:66–71. doi: 10.1002/(sici)1097-0347(199901)21:1<66::aid-hed9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Pogrel MA, Podlesh S, Anthony JP, Alexander J. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg. 1997;55:1200–1206. doi: 10.1016/s0278-2391(97)90165-8. [DOI] [PubMed] [Google Scholar]
- 6.Swennen G, Schliephake H, Dempf R, Schierle H, Malevez C. Craniofacial distraction osteogenesis: A review of the literature. Part 1: Clinical studies. Int J Oral Maxillofac Surg. 2001;30:89–103. doi: 10.1054/ijom.2000.0033. [DOI] [PubMed] [Google Scholar]
- 7.Dudziak ME, Saadeh PB, Mehrara BJ, et al. The effects of ionizing radiation on osteoblast-like cells in vitro. Plast Reconstr Surg. 2000;106:1049–1061. doi: 10.1097/00006534-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Gunness M, Hock JM. Anabolic effect of parathyroid hormone on cancellous and cortical bone histology. Bone. 1993;14:277–281. doi: 10.1016/8756-3282(93)90152-z. [DOI] [PubMed] [Google Scholar]
- 9.Tchanque-Fossuo CN, Monson LA, Farberg AS, et al. Dose-response effect of human equivalent radiation in the murine mandible. Part I. A histomorphometric assessment. Plast Reconstr Surg. 2011;128:114. doi: 10.1097/PRS.0b013e31821741d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchman SR, Ignelzi MA, Jr, Radu C, et al. Unique rodent model of distraction osteogenesis of the mandible. Annals Plast Surg. 2002;49:511. doi: 10.1097/00000637-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Gal TJ, Munoz-Antonia T, Muro-Cacho CA, Klotch DW. Radiation effects on osteoblasts in vitro: A potential role in osteoradionecrosis. Arch Otolaryngol Head Neck Surg. 2000;126:1124–1128. doi: 10.1001/archotol.126.9.1124. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell MJ, Logan PM. Radiation-induced changes in bone. Radiographics. 1998;18:1125–1136. doi: 10.1148/radiographics.18.5.9747611. quiz 1242. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy JG, Stelnicki EJ, Mehrara BJ, Longaker MT. Distraction osteogenesis of the craniofacial skeleton. Plast Reconstr Surg. 2001;107:1812–1827. doi: 10.1097/00006534-200106000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Inyang AF, Schwarz DA, Jamali AM, Buchman SR. Quantitative histomorphometric assessment of regenerate cellularity and bone quality in mandibular distraction osteogenesis after radiation therapy. J Craniofac Surg. 2010;21:1438–1442. doi: 10.1097/SCS.0b013e3181ec693f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz DA, Jamali AM, Kakwan MS, et al. Biomechanical assessment of regenerate integrity in irradiated mandibular distraction osteogenesis. Plast Reconstr Surg. 2009;123(Suppl):114S–22S. doi: 10.1097/PRS.0b013e318191c5d2. [DOI] [PubMed] [Google Scholar]
- 16.Fregene A, Jing XL, Monson LA, Buchman SR. Alteration in volumetric bone mineralization density gradation patterns in mandibular distraction osteogenesis following radiation therapy. Plast Reconstr Surg. 2009;124:1237–1244. doi: 10.1097/PRS.0b013e3181b5a42f. [DOI] [PubMed] [Google Scholar]
- 17.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 18.Black DM, Greenspan SL, Ensrud KE, et al. PaTH Study Investigators. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 19.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole KE, Reeve J. Parathyroid hormone: A bone anabolic and catabolic agent. Curr Opin Pharmacol. 2005;5:612–617. doi: 10.1016/j.coph.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Shinoda Y, Kawaguchi H, Higashikawa A, et al. Mechanisms underlying catabolic and anabolic functions of parathyroid hormone on bone by combination of culture systems of mouse cells. J Cell Biochem. 2010;109:755–763. doi: 10.1002/jcb.22454. [DOI] [PubMed] [Google Scholar]
- 22.Chalidis B, Tzioupis C, Tsiridis E, Giannoudis PV. Enhancement of fracture healing with parathyroid hormone: Preclinical studies and potential clinical applications. Expert Opin Investig Drugs. 2007;16:441–449. doi: 10.1517/13543784.16.4.441. [DOI] [PubMed] [Google Scholar]
- 23.Nakazawa T, Nakajima A, Shiomi K, et al. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1-34) on chondrogenesis in a model of experimental fracture healing. Bone. 2005;37:711–719. doi: 10.1016/j.bone.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Takayanagi H, Kim S, Matsuo K, et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 25.Barnes GL, Kakar S, Vora S, Morgan EF, Gerstenfeld LC, Einhorn TA. Stimulation of fracture healing with systemic intermittent parathyroid hormone treatment. J Bone Joint Surg Am. 2008;90(Suppl 1):120–127. doi: 10.2106/JBJS.G.01443. [DOI] [PubMed] [Google Scholar]
- 26.Bostrom MP, Gamradt SC, Asnis P, et al. Parathyroid hormone-related protein analog RS-66271 is an effective therapy for impaired bone healing in rabbits on corticosteroid therapy. Bone. 2000;26:437–442. doi: 10.1016/S8756-3282(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 27.Chow JW, Fox S, Jagger CJ, Chambers TJ. Role for parathyroid hormone in mechanical responsiveness of rat bone. Am J Physiol. 1998;274:E146–E154. doi: 10.1152/ajpendo.1998.274.1.E146. [DOI] [PubMed] [Google Scholar]
- 28.Seebach C, Skripitz R, Andreassen TT, Aspenberg P. Intermittent parathyroid hormone (1-34) enhances mechanical strength and density of new bone after distraction osteo-genesis in rats. J Orthop Res. 2004;22:472–478. doi: 10.1016/j.orthres.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Jilka RL, Brien CAO, Ali AA, Roberson PK, Weinstein RS, Manolagas SC. Formation by actions on post-miotic bone. Bone. 2009;44:275–286. doi: 10.1016/j.bone.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jegoux F, Malard O, Goyenvalle E, Aguado E, Daculsi G. Radiation effects on bone healing and reconstruction: Interpretation of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;190:173–184. doi: 10.1016/j.tripleo.2009.10.001. [DOI] [PubMed] [Google Scholar]