Abstract
Importance
Interventions encouraging primary care patients’ engagement with their clinicians to address depression could improve outcomes but foster unnecessary treatment.
Objective
Determine whether a depression engagement video (DEV) or a tailored interactive multimedia computer program (IMCP) improves initial depression care without increasing unnecessary anti-depressant prescribing.
Design
Randomized controlled trial comparing three interventions (DEV, IMCP, and control) conducted in two patients groups (depressed, defined by a Patient Health Questionnaire [PHQ]-9 score ≥5, and non-depressed [PHQ-9<5]) conducted between June 2010 and March 2012.
Setting
Primary care offices at 7 sites in 2 cities.
Participants
Depressed (N=559) and non-depressed (N=308) adult patients of 135 primary care clinicians.
Intervention(s)
DEV targeted to gender and income; IMCP tailored to individual patient characteristics; a sleep hygiene video (control).
Main Outcome Measure(s)
Depressed patients: composite measure of antidepressant recommendation and/or mental health referral (primary outcome); 12-week mental health, measured by the PHQ-8 (secondary outcome). Non- depressed patients: clinician-reported prescribing and patient-reported antidepressant recommendation (primary outcomes, pre-specified 3.5% non-inferiority margins).
Results
Depressed patients: composite care outcome rates were 18%, 26%, and 16% respectively in the DEV, IMCP, and control groups (cluster-adjusted DEV-control difference = 1.1% [95% CI −6.7 to 8.9, P=.79]; IMCP-control = 9.9% [95% CI 1.6 to 18.2, P=.02]). Twelve-week PHQ-8 effects were not significant: DEV- control = −0.2 points (95% CI −1.2 to 0.8); IMCP – control = 0.9 (95% CI −0.1 to 1.9). Non-depressed patients: clinician-reported antidepressant prescribing in the DEV and IMCP groups was non-inferior to control (DEV-control = −2.2%, 90% CI −8.0 to 3.498, non-inferiority (NI) P=.0499; IMCP-control = −3.3%, 90% CI −9.1 to 2.4, NI P=.02); patient-reported antidepressant recommendation did not achieve non-inferiority: DEV-control = 0.9% (90% CI −4.9 to 6.7; NI P=.23); IMCP-control = 0.3% (90% CI −5.1 to 5.7; NI P=.16).
Conclusions and Relevance
A tailored IMCP increased antidepressant recommendation and/or mental health referral among depressed patients but had no effect on 12-week mental health. The possibility that the IMCP and DEV increased patient-reported antidepressant recommendations among non-depressed patients could not be excluded. Further research is needed on the benefits and harms of these interventions.
Despite progress, depression in primary care remains under-recognized and under-treated.1-5 Barriers to improvement include system, clinician, and patient factors. System-level interventions are effective in increasing recognition and treatment of depression, but these interventions are difficult to disseminate.4,6 Clinician behavior is difficult to change.7 Patients are potentially attractive targets for intervention,8 but they may have difficulty articulating their distress and signaling openness to treatment for depression.9-11 Marketing strategies such as direct-to-consumer advertising encourage patients to report depression symptoms and accept depression treatment12,13 but these interventions may also promote overprescribing.13-17 More selective approaches are needed.
In shaping messages to influence health-related behavior, researchers have applied 2 approaches. Targeting involves segmenting a general population into smaller, more homogeneous units based on observable factors such as age, gender, or place of residence.18 Tailoring uses information elicited from the respondent, often through an interactive computerized interface, to craft messages specific to that person.19
We examined whether targeted and tailored communication strategies, respectively, could enhance patient engagement and initial care for patients with depression symptoms. We also examined the extent to which each intervention promoted prescribing or recommendation of anti-depressant medication, depression-related discussion, and antidepressant requests among patients who were not depressed. We developed 2 interventions for use in primary care: a depression engagement video (DEV) targeted to gender and income, and an interactive multimedia computer program (IMCP) tailored to the characteristics, interests, and problems of the individual patient.
Enrolled patients were categorized into 2 groups according to whether or not they had significant depression symptoms (Patient Health Questionnaire [PHQ]-9 score ≥5 defined depressed patients and PHQ-9 score < 5 defined non-depressed patients). Within each of these 2 groups (depressed patients and non-depressed patients) we compared the effectiveness of each intervention with an attention control (sleep hygiene video). Among depressed patients, we hypothesized that each intervention would increase delivery of depression treatments (primary outcome), encourage patients to ask questions about depression, and lead to improved mental health 12 weeks later as compared to the control group. Among non-depressed patients, we hypothesized that each intervention would not increase antidepressant prescribing or recommendations (primary outcomes), depression-related discussion, patient requests for antidepressants, or clinician time and burden as compared to the control group.
METHODS
Design overview
The trial was designed as a randomized controlled trial comparing 3 interventions: a targeted depression engagement video (DEV) designed to encourage patient participation in depression-related discussion and care, a tailored interactive multimedia computer program (IMCP), and an attention control. We report separately on results for depressed and non-depressed patient groups, defined by PHQ-9 score ≥5 and <5, respectively. Study procedures and protocol have been detailed elsewhere.20 Ethics approval was obtained from the Institutional Review Boards at all performance sites.
Sampling
Patients and clinicians were recruited from primary care clinics affiliated with the University of California, San Francisco (UCSF); the San Francisco Veterans Affairs Medical Center; the University of California, Davis (UCD) Ambulatory Care Center; the UCD Primary Care Network; the Northern California (Sacramento) Veterans Affairs Health System; Kaiser Permanente, Sacramento; and Sutter Medical Group, Sacramento.
We recruited primary care clinicians through email announcements and in-person presentations. Clinicians were told that the study was a randomized trial of an intervention designed to improve communication about common physical and mental health symptoms in primary care. Although not blinded to patients’ participation in the study, clinicians were not alerted to patients’ group assignments. All clinicians agreed to enroll up to 12 of their patients.
Patient enrollment
Eligible patients were aged 25-70 years, could read and understand English, use a computer, and were not currently taking antidepressant medication (with the exception of low dose tricyclics for pain or sleep). We studied working aged adults because of the social and economic burden of depression in this age group.21 In all recruitment settings except UCSF urgent care, eligibility screening was conducted by telephoning patients who were scheduled for a routine primary care visit in the next 1-2 weeks. Patients were told that the study was designed to improve care for patients with common symptoms including sleep problems, depression, and chronic pain. Research staff made up to 3 attempts to reach each patient. Patients were selected for telephoning from each clinic's appointment lists in random order until daily quotas were filled. Patients with significant depression symptoms based on the PHQ-822 (used in lieu of the PHQ-9 for telephone screening) were over-sampled. Eligible patients who provided preliminary verbal consent were invited to a research appointment 1 hour prior to the upcoming “index” visit. At the UCSF urgent care clinic only, patients were approached directly by research assistants in waiting rooms, without any prior telephone screening. Patients were offered an incentive of $20-$35 for completing index visit procedures and an additional $10 for completing the 12-week follow-up telephone interview.
Interventions
The targeted DEVs and tailored IMCP were developed based on literature reviews and extensive formative research.23,24 The attention control intervention was a sleep hygiene video produced by HealthiNation.25
The DEVs, produced in collaboration with a marketing firm, were designed to enhance depression recognition and care-seeking by educating patients about depression; emphasizing the importance of disclosing relevant symptoms; and suggesting ways to start a conversation with their primary care provider.9,10,23,26 The marketing firm produced 4 DEV variants targeted to gender and household income. By employing terms and images likely to resonate with the intended audience, targeted messages are generally better attended to and more deeply processed than non-targeted messages.27
The IMCP was developed collaboratively by the study investigators, guided by standard software engineering principles. The IMCP provided patients with feedback tailored to level of depression symptoms (e.g., those with PHQ-9 scores <5 were told they were probably not depressed, whereas those with higher scores were told they might be depressed and were advised to discuss with their clinician), visit agenda (intention to discuss depression and/or depression treatment), depression causal attributions (biological, psychosocial, situational, existential),28,29 treatment preferences (medication, counseling, both, neither),28,30,31 self-efficacy for communicating with healthcare providers,32 and depression stigma.9,33, The IMCP gave users control over knowledge acquisition (“self-tailoring”) by offering links to more detailed material.34 Tailored health messages are better remembered, read, and perceived as relevant and are superior to non-tailored interventions in improving various health behaviors and outcomes across a broad array of patient populations and target conditions,35 including depression.36,37 Screenshots of the DEVs and IMCP are included in the electronic appendix.
Randomization and patient flow
A study Research Assistant met patients1 hour prior to their primary care clinic appointment. Following written informed consent, patients were logged on to a tablet computer for randomization and intervention assignment.
The unit of randomization was the patient. As described previously,20 the computer randomization program stratified subjects into categories defined by self-reported race/ethnicity (because of its association with socio-economic position [a target of the depression engagement video] and to enhance generalizability), gender and site. Within each category, patients were randomly allocated in equal proportions to 1 of 3 study arms, in randomly permuted blocks of 9 subjects, with the size of the blocks not disclosed to research staff during enrollment. After randomization, patients were again asked about current antidepressant use; users were excluded from participation.
After intervention assignment, patients answered additional questions to measure baseline depressive symptom burden (using the PHQ-9)38, and to assess baseline health status. Immediately thereafter, patients were exposed to their randomly assigned intervention: 1 of 4 targeted DEV variants; the tailored IMCP; or the control video. The DEVs and control video were each approximately 3 minutes long. Patients assigned to the IMCP spent a median of 5 minutes with the program (10th percentile, 2 minutes; 90th percentile, 15 minutes).
Following the office visit, subjects completed a computer based post-visit questionnaire including questions about the encounter (i.e., whether they asked about or discussed depression and/or depression-related care; whether the physician recommended an antidepressant or made a mental health referral; and when the physician arranged for primary care follow-up). Clinicians independently completed a brief post-visit questionnaire. Agreement between patient and physician for antidepressant recommendation was 87% and for mental health referral 89%. Patients in the depressed sample (PHQ-9≥5 at index visit) were telephoned 12 weeks later to assess severity of depression symptoms and health status.
Outcome measures
Measures for this study include patient post-visit reports, physician post-visit reports, and 12-week patient follow-up by telephone. Among patients categorized as depressed, we focused on patient reports because of the critical role of patient perceptions in driving health behaviors and assessing outcomes. Among patients categorized as non-depressed, we used both patient and clinician reports.
The primary pre-specified outcome applied to the group of participants categorized as depressed was a composite measure of initial depression care (“composite care measure”) defined as receiving an antidepressant recommendation, a mental health referral, or both during the index visit. Secondary outcomes included: patient-physician communication self-efficacy using a scale modified from Maly et al.32 (sum of 6 items, each scored from 1 [not at all confident] to 5 [very confident]; scale range, 6-30); whether the patient reported asking the provider for information about depression during the visit; 12-week scores on the PHQ-8 (sum of 8 items, each scored from 0 [not at all] to 3 [nearly every day]; scale range, 0-24);22,38-40 and the SF-12 Version 2.0 Mental Health Component Summary Scores (MCS-12) and Physical Health Component Summary Scores (PCS-12) (both scored from 0-100, with higher scores representing better health).41,42
The primary pre-specified outcome applied to non-depressed patients was whether the clinician recommended or prescribed an antidepressant. This was assessed by clinician report of antidepressant prescribing and by patient report of whether the clinician “recommended” a medication for depression. Secondary outcomes among non-depressed patients included: 1) whether depression or depression treatment were discussed (each classified as yes/no/uncertain); 2) whether the patient requested medication for depression during the study visit (yes/no/uncertain); 3) clinician-reported face-to-face visit time (minutes) and 4) clinician-reported visit burden, computed as the sum of 3 items rating the visit in terms of “amount of time required ... amount of effort required.... [and] degree to which you found the visit difficult”, each on a 0-2 scale (0=less than average, 1=about average, 2=greater than average; Cronbach's alpha, 0.79).
Statistical analysis
Details on power calculations, model assumptions, and variable selection have been reported.20 Briefly, we fit clustered data regression models that would allow assessment of the pairwise (intervention versus control) contrasts of interest, while accounting for study design effects arising from the stratified sampling and randomization scheme and for the clustering of patients within clinicians. No adjustments were made for multiple comparisons. The target sample size of 170 per arm for the analyses involving depressed patients was established to provide 80% power under two-sided testing (alpha=5%) to detect standardized pairwise differences of 0.3 (e.g. 15 percentage points for a binary outcome with an expected value of 50%). For analyses of non-depressed patients, the per-group target sample of 102 was established to provide 80% power to reject the inferiority null hypothesis that the rate of antidepressant prescribing in the DEV and IMCP groups would be 3.5 percentage points higher than in the control group, under the alternative hypothesis that the true probability was 1%.
Outcomes were analyzed using Stata Version 12.1.43 Binary outcomes were assessed using logistic regression models, estimated using random effects estimation or, for low event counts, generalized estimating equations. Relative comparisons for binary outcomes were expressed as adjusted odds ratios from models that adjusted for the study design (to minimize omitted covariate bias),44 while absolute comparisons were expressed as cluster-adjusted mean percentage point differences [DIFFs] on the original scale of measurement. Cluster-adjusted mean percentages and differences were estimated via Stata's margins post-estimation command, immediately after fitting simple clustered data logistic regression models. For mixed-effects models, margins were estimated with the random effect for each observation set to 0 (the mean value).
Continuous outcomes were assessed using mixed effect linear regression models with adjustment for stratifiers. In the depressed sample all pairwise contrasts were estimated with 95% confidence intervals and tested with two-sided P-values. In the non-depressed sample, two-sided 90% confidence intervals are reported, equivalent to 1-sided testing of the inferiority null hypothesis. The significance threshold was P<0.05 for all contrasts. For harms, we report non-inferiority [NI] P-values for only the antidepressant prescribing and recommendation outcomes, using pre-specified tolerance margins of 3.5 percentage points. When the NI P-value is less than 0.05, the contrast is statistically significant in favor of non-inferiority at the specified tolerance margin.
Models adjusting for strata included the following terms: patient gender, ethnicity, and practice setting [multispecialty group, faculty/resident practice, health maintenance organization, or VA clinic]), and (in analyses of depressed patients) baseline PHQ-9 category (5-9 vs. ≥10). The post-visit patient-physician communication self-efficacy outcome analysis also adjusted for pre-visit self-efficacy. For 12-week outcomes (PHQ-8, MCS-12, and PCS-12 scores), 3-level mixed effects models estimated adjusted within-group mean (over-time) differences and between-group differences in mean differences. This approach uses all available data, including baseline data from patients who drop out, to avoid biases that could occur in complete case analysis.45
Although not pre-specified prior to patient enrollment, we hypothesized on clinical grounds prior to examination of the data that the interventions might be particularly effective among patients with more severe depressive symptomatology; this was assessed by conducting analyses stratified by PHQ-9 level. Heterogeneity of treatment effects by baseline depression severity (5-9 versus ≥10) was assessed by fitting a model including the group-by-depression category interaction term (tested with the Wald Chi-square test [2 degrees of freedom]).
RESULTS
Patient Flow and Baseline Characteristics
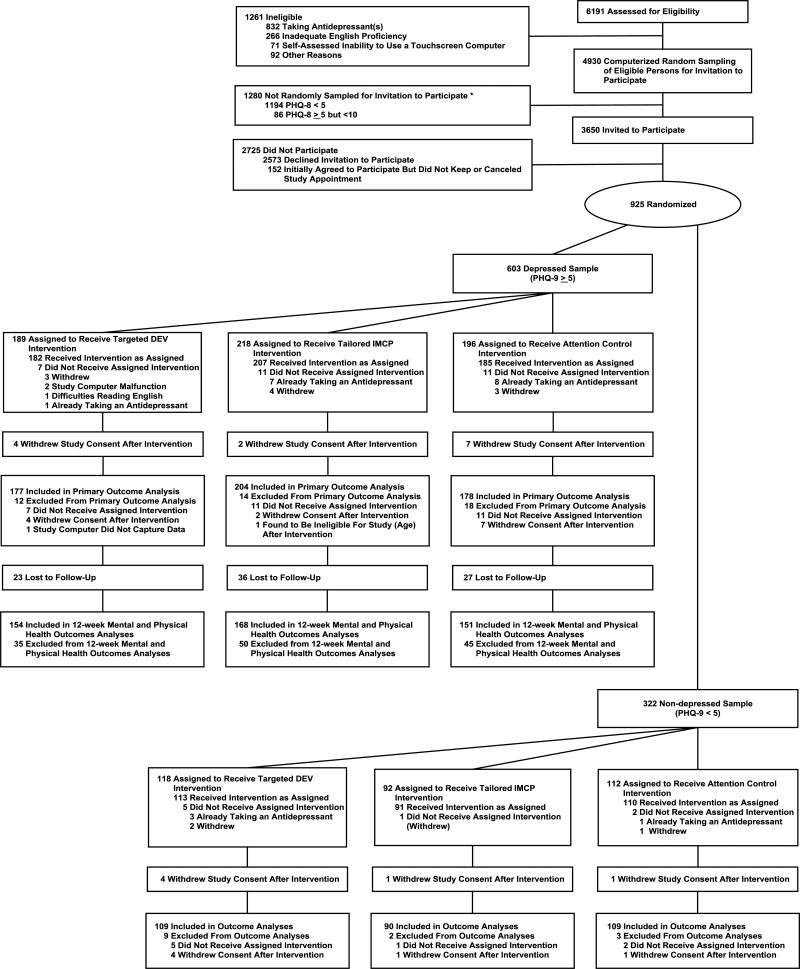
Of 135 consenting clinicians, 124 enrolled at least 1 patient with a PHQ-9 score ≥ 5, and 106 enrolled at least 1 with a PHQ-9 score <5. Figure 1 depicts the flow of patients from screening through 12-week follow-up. Of 6,191 patients assessed for eligibility, 3650 were invited to participate, and 925 (603 in the depressed sample and 322 in the non-depressed sample) were randomized to the DEV, IMCP, or attention control group prior to a primary care office visit. Of the 925 randomized participants, 58 were excluded post-randomization, leaving 867 analyzable subjects: 559 categorized as depressed and 308 as non-depressed sample. Of the 559, approximately 85% completed the 12-week telephone follow-up survey (Figure 1). Subjects were enrolled from June 16, 2010 to November 8, 2011; follow-up was complete by March 31, 2012.
Figure 1. Flow of subjects through study.
Of 6191 patients successfully contacted, 1261 were ineligible due to age, lack of English proficiency, inability to operate a computer, psychosis, or currently taking antidepressants. An additional 1280 subjects (denoted in the Figure by an asterisk) were excluded at random by an automated sampling program designed to maintain a ratio of depressed:non-depressed participants of approximately 5:3 and, within the depressed sample, to slightly over-sample patients with PHQ-8 scores ≥10. Of the remaining 3620 patients, 2725 declined participation or did not keep their appointments, leaving 925 (25.5%) who underwent randomization at the index visit. A total of 58 patients (44 in the depressed sample and 14 in the non-depressed sample) were excluded post-randomization. For the effectiveness analysis, 559 patients were included in the primary analysis and 473 were available for follow-up at 12 weeks. For the harms analysis, 308 patients were included.
PHQ-9 = Patient Health Questionnaire-9
DEV = Depression engagement video
IMCP = Interactive multimedia computer program
Within both the depressed and non-depressed samples, participants assigned to the 3 experimental groups were similar in gender, age, race/ethnicity, family income, depression symptoms, and baseline self-efficacy for communicating with the physician about mental health issues (Table 1). In the depressed sample, the DEV group had a higher mean baseline MCS-12 score than the IMCP or control group (P=.01).
Table 1.
Baseline characteristics of participants (n=559 in depressed sample, n=308 in non-depressed sample).
| Depressed Samplea | Non-depressed Samplea | |||||
|---|---|---|---|---|---|---|
| DEV (n = 177) | IMCP (n = 204) | Control (n = 178) | DEV (n = 109) | IMCP (n = 90) | Control (n = 109) | |
| Female, n (%) | 94 (53.1) | 110 (53.9) | 99 (55.6) | 65 (59.6) | 52 (57.8) | 66 (60.6) |
| Age (years) , mean (SD) | 50.6 (11.7) | 50.5 (12.4) | 50.6 (11.1) | 54.5 (10.8) | 53.7 (12.1) | 53.5 (12.0) |
| Race/ethnicity, n (%) | ||||||
| White, non-Hispanic | 89 (50.3) | 110 (53.9) | 90 (50.6) | 75 (68.8) | 55 (61.1) | 67 (61.5) |
| Hispanic or Latino | 32 (18.1) | 30 (14.7) | 26 (14.6) | 8 (7.3) | 13 (14.4) | 14 (12.8) |
| Black, not Hispanic | 37 (20.9) | 49 (24.0) | 44 (24.7) | 13 (11.9) | 12 (13.3) | 13 (11.9) |
| Other | 19 (10.7) | 15 (7.4) | 18 (10.1) | 13 (11.9) | 10 (11.1) | 15 (13.8) |
| Income level, n (%) | ||||||
| Less than $35,000 | 79 (44.6) | 89 (43.6) | 77 (43.3) | 31 (28.4) | 17 (18.9) | 24 (22.0) |
| $35,000 or more | 98 (55.4) | 115 (56.4) | 101 (56.7) | 78 (71.6) | 73 (81.1) | 85 (78.0) |
| College or graduate degree, n/N (%) | 59/176 (33.5) | 75/202 (37.1) | 74/178 (41.6) | 64/109 (58.7) | 51/89 (57.3) | 68/108 (63.0) |
| Living with spouse or partner, n/N (%) | 92/176 (52.3) | 75/202 (54.0) | 93/178 (52.2) | 68/109 (62.4) | 53/89 (59.6) | 72/108 (66.7) |
| Practice settingc, n (%) | ||||||
| Multispecialty group practice | 78 (44.1) | 81 (39.7) | 61 (34.3) | 52 (47.7) | 38 (42.2) | 53 (48.6) |
| Faculty/resident practice | 50 (28.2) | 57 (27.9) | 69 (38.8) | 39 (35.8) | 37 (41.1) | 36 (33.0) |
| Health maintenance organization | 24 (13.6) | 26 (12.8) | 19 (10.7) | 6 (5.5) | 6 (6.7) | 5 (4.6) |
| Veterans Affairs (VA) clinic | 25 (14.1) | 40 (19.6) | 29 (16.3) | 12 (11.0) | 9 (10.0) | 15 (13.8) |
| City of care, n (%) | ||||||
| Sacramento | 134 (75.7) | 152 (74.5) | 127 (71.4) | 78 (71.6) | 59 (65.6) | 80 (73.4) |
| San Francisco | 43 (24.3) | 52 (25.5) | 51 (28.7) | 31 (28.4) | 31 (34.4) | 29 (26.6) |
| PHQ-9 score at index visit, mean (SD) (range 0-27, higher=more depressed) | 10.0 (4.6) | 10.8 (4.8) | 10.6 (4.5) | 1.7 (1.5) | 1.9 (1.5) | 1.9 (1.5) |
| PHQ-9 category at index visit, n (%) | ||||||
| 0-4 (non-depressed) | -- | -- | -- | 109 (100.0) | 90 (100.0) | 109 (100.0) |
| 5-9 (mild depression) | 103 (58.2) | 99 (48.5) | 89 (50.0) | -- | -- | -- |
| 10-14 (moderate depression) | 43 (24.3) | 66 (32.4) | 56 (31.5) | -- | -- | -- |
| ≥15 (moderately severe to severe depression) | 31 (17.5) | 39 (19.1) | 33 (18.5) | -- | -- | -- |
| SF-12 at enrollment (nonmissing #) | (n=172) | (n=201) | (n=178) | (n=109) | (n=88) | (n=108) |
| MCS-12 (range 0-100, higher=better health), mean (SD) | 43.4 (11.8)b | 40.0 (10.3)b | 40.8 (12.3)b | 54.5 (7.2) | 55.6 (6.8) | 55.5 (6.6) |
| PCS-12 (range 0-100, higher=better health), mean (SD) | 38.7 (14.1) | 38.5 (13.5) | 38.2 (13.0) | 46.7 (11.6) | 48.1 (10.9) | 46.3 (11.8) |
| Self-efficacy for patient-physician interactions regarding mental health, mean (SD) (range 6 to 30, higher=greater self-efficacy) | 20.9 (6.0) | 21.3 (6.2) | 20.8 (6.2) | 22.9 (4.8) | 23.4 (4.7) | 23.5 (5.1) |
Abbreviations: CI, confidence interval; DEV, depression engagement video; IMCP, interactive multimedia computer program; MCS-12, SF-12 Mental Health Component Summary score; PCP, primary care provider; PHQ-9, Patient Health Questionnaire-9; PCS-12, SF-12 Physical Health Component Summary score; SD, standard deviation
Note: The depressed sample was defined by a baseline PHQ-9 score ≥5, the non-depressed sample by a baseline PHQ-9 score <5.
Due to rounding, percentages might not sum to 100.
P=.01 ANOVA for an all-way comparison (within effectiveness study group)
The multispecialty group category includes the UC Davis Primary Care Network and Sutter Medical Group. The faculty/resident practice category includes UC San Francisco affiliated clinics and the UC Davis Ambulatory Care Center. The health maintenance organization category includes participating clinics from Kaiser Permanente. The Veterans Affairs category includes the Veterans Affairs Medical Center in San Francisco, California and the Northern California VA Health System.
Results in Depressed Patients
Intervention effects on the primary composite outcome (antidepressant recommendation and/or mental health referral)
Rates of receipt of the composite care measure were 18%, 26%, and 16% in the DEV, IMCP, and control groups, respectively (cluster-adjusted mean percentage point difference [DIFF] comparing DEV to control, 1.1, 95% CI −6.7 to 8.9, P=.79; DIFF comparing IMCP to control, 9.9, 95% CI 1.6 to 18.2, P=.02, Table 2). Mixed effects models confirmed the superiority of the IMCP over control (adjusted odds ratio [AOR] 1.81, 95% CI 1.04 to 3.16, Table 2). The IMCP odds ratios were of similar magnitude (albeit not statistically significant) with respect to the two components of the primary outcome (AOR for antidepressant prescribing, 1.85, 95% CI 0.95 to 3.59, P=.07; AOR for mental health referral, 1.76, 95% CI 0.97 to 3.18, P=.06). In stratified analyses, the IMCP effect was significant in those with at least moderate symptoms (AOR 2.42, 95% CI 1.11 to 5.30) but not in those with mild symptoms (AOR 1.10, 95% CI 0.44 to 2.75) (Table 2). The IMCP-by-depression severity interaction term was non-significant (P=.31).
Table 2.
Effects of DEV and IMCP (versus control) on receipt of composite care measure (antidepressant prescription and/or mental health referral) in depressed sample.
| DEV | IMCP | Control | DEV vs. Control | IMCP vs. Control | ||
|---|---|---|---|---|---|---|
| n/N (%) | n/N (%) | n/N (%) | Cluster-adjusted mean percentage point difference (95% CI)a | Adjusted odds ratio (95% CI)b | Cluster-adjusted mean percentage point difference (95% CI) | Adjusted odds ratio (95% CI)b |
|
All subjects with PHQ-9 score ≥ 5 (N=559)
| ||||||
| 31/177 | 53/204 | 29/178 | 1.1 (−6.7, 8.9) | 1.16 (0.63, 2.12) | 9.9 (1.6, 18.2) | 1.81 (1.04, 3.16) |
| (17.5) | (26.0) | (16.3) | P value = .79 | P value = .64 | P value = .02 | P value = .04 |
|
PHQ-9 score 5-9 (N=291) | ||||||
| 8/103 | 13/99 | 10/89 | −3.4 (−11.8, 4.9) | 0.61 (0.23, 1.66) | 1.9 (−7.4, 11.2) | 1.10 (0.44, 2.75) |
| (7.8) | (13.1) | (11.2) | P value = .42 | P value = .34 | P value = .69 | P value = .83 |
|
PHQ-9 score ≥10 (N=268) | ||||||
| 23/74 | 40/105 | 19/89 | 12.5 (−2.8, 27.9) | 1.86 (0.79, 4.38) | 19.4 (5.1, 33.8) | 2.42 (1.11, 5.30) |
| (31.1) | (38.1) | (21.3) | P value = .11 | P value = .15 | P value = .008 | P value = .03 |
Abbreviations: CI, confidence interval; DEV, depression engagement video; IMCP, interactive multimedia computer program; PCP, primary care provider
Cluster-adjusted mean percentage point differences estimated via Stata's margins post-estimation command following a simple (unadjusted) mixed-effects logistic regression model that included fixed effects for study arm and random effects for physicians to adjust inferences for nesting of multiple patient observations within 124 physicians. Margins were estimated with the random effect for each observation set to 0 (the mean value). P values are for the Wald test of the null hypothesis that the contrast = 0.
Adjusted odds ratios estimated in mixed effects logistic regression model with fixed effects to adjust for patient gender, race and baseline PHQ-9 category and practice setting and with random effects to adjust for nesting of patients within 124 physician practices (residual intracluster correlation coefficient = 0.096).
Wald chi-square test (2 d.f.) for heterogeneity of treatment effects by depressive symptom level = 2.32, P=.31.
Intervention effects on patient engagement
The percentage of patients requesting information about depression during the visit was 17.7 (95% CI, 11.4 to 23.9) in the DEV group, 19.5 (95% CI, 13.3 to 25.6) in the IMCP group, and 9.5 (95% CI, 4.9 to 14.1) in the control group. Patients assigned to the DEV and IMCP groups were significantly more likely than control patients to request information about depression (DEV vs. Control DIFF 8.1 percentage points [95% CI 0.9 to 15.4; P=.03] and AOR 2.11 [95% CI 1.12 to 3.98, P=.02]; IMCP vs. Control DIFF 9.9 [95% CI 2.8 to 17.1; P=.006] and AOR 2.19 [95% CI 1.19 to 4.04, P=.01]).
There were no significant intervention effects on self-efficacy for communicating with the physician about mental health issues (adjusted mean difference on the modified Maly scale [95% CI] for DEV vs. Control: 0.22 [−0.75 to 1.19], P=.66; for IMCP vs. Control: 0.01 [−0.88 to 0.90], P=.98).
Intervention effects on 12-week outcomes
Table 3 shows scores on the PHQ-8 (depression), MCS-12 (mental health) and PCS-12 (physical health), by intervention group, at baseline and at 12-weeks follow-up. All 3 outcomes improved significantly from baseline to follow-up regardless of group assignment (P-values all ≤ .01). There were no significant differences between IMCP and. control or between DEV and control at 12-week follow-up (P-values all ≥ .05, Table 3). Similar results were obtained when the sample was restricted to patients with baseline PHQ-9 scores ≥10 (electronic appendix).
Table 3.
PHQ-8, PCS-12, and MCS-12 scores at baseline (n=559) and 12-week follow-up (n=473)
| Intervention Arm | Time | PHQ-8 (n=# nonmissing) Estimate (95% CI) | PCS-12 (n=# nonmissing) Estimate (95% CI) | MCS-12 (n=# nonmissing) Estimate (95% CI) |
|---|---|---|---|---|
| DEV | Baseline | (n=177) 9.7 ( 9.2, 10.3) | (n=172) 38.7 ( 36.7, 40.8) | (n=172) 43.4 ( 41.8, 44.9) |
| 12-weeks | (n=154) 6.8 ( 5.9, 7.7) | (n=153) 41.4 ( 39.4, 43.4) | (n=153) 46.7 ( 44.9, 48.5) | |
| Adjusted Over-time Mean Difference | −2.9 ( −3.7, −2.2) | 2.3 ( 0.8, 3.7) | 3.0 ( 1.1, 4.9) | |
| DEV v. Control Adjusted difference in mean over-time differences | −0.2 ( −1.2, 0.8) | 0.1 ( −1.9, 2.2) | −0.2 ( −2.9, 2.5) | |
| IMCP | Baseline | (n=204) 10.5 ( 9.8, 11.1) | (n=201) 38.5 ( 36.8, 40.3) | (n=201) 40.0 ( 38.7, 41.4 ) |
| 12-weeks | (n=168) 8.6 ( 7.8, 9.4) | (n=166) 40.2 ( 38.3, 42.1) | (n=166) 43.2 ( 41.4, 45.0 ) | |
| Adjusted Over-time Mean Difference | −1.9 ( −2.6, −1.2) | 1.8 ( 0.4, 3.2) | 3.1 ( 1.3, 4.9) | |
| IMCP v. Control Adjusted difference in mean over-time differences | 0.9 ( −0.1, 1.9) | −0.3 ( −2.3, 1.7) | −0.1 ( −2.7, 2.5) | |
| Control | Baseline | (n=178) 10.4 ( 9.8, 11.0) | (n=178) 38.2 ( 36.1, 40.2) | (n=178) 40.8 ( 39.0, 42.6) |
| 12-weeks | (n=151) 7.6 ( 6.8, 8.4) | (n=148) 39.9 ( 37.7, 42.1) | (n=148) 44.1 ( 41.7, 46.4) | |
| Adjusted Over-time Mean Difference | −2.7 ( −3.5, −2.0 ) | 2.1 ( 0.7, 3.6 ) | 3.2 ( 1.3, 5.1) | |
Abbreviations: CI, confidence interval; PHQ-8, Patient Health Questionnaire-8; PCS-12, SF-12 Physical Health Component Summary score; MCS-12, SF-12 Mental Health Component Summary score; DEV, depression engagement video; IMCP, interactive multimedia computer program.
Note: Adjusted mean differences and 95% confidence intervals from mixed-effects linear regression models with statistical adjustments for patient gender, race, practice setting, baseline PHQ-9 category, and random effects for patients and for physician. Confidence intervals for timepoint-specific means are adjusted for clustering by physician, using clustered survey data analysis methods. Compared to non-respondents at 12-weeks, those who completed the 12-week survey were older, more likely to be partnered, to have higher incomes, to have been recruited from the Sacramento area and to have better mental health status. However, attrition was not associated with treatment assignment. The PHQ-8 is scored from 0-24 (higher=more depressed); the PCS-12 and MCS-12 are scored from 0-100 (100=better health).
Results in Non-Depressed Patients
Among non-depressed participants, rates of clinician-reported antidepressant prescribing were 4.8%, 3.6%, and 6.7% in the DEV, IMCP, and control groups, respectively (Table 4). Rates of patient-reported physician recommendations for anti-depressant medication were 5.6%, 4.4% and 4.6%, respectively (Table 4). For the clinician-reported outcome, these results were consistent with non-inferiority (i.e., equivalence) of the two interventions as compared to the control group (non-inferiority P<.05, Table 4). However, using the patient reported measure, the upper confidence limit for the DEV versus control difference extended to 6.7 percentage points (non-inferiority P=.23) and for the IMCP versus control difference to 5.7 percentage points (non-inferiority P=.16). Therefore, the two interventions were not found to be equivalent to the control group for the outcome of patient-reported recommendation for anti-depressant medication. For discussion of depression (in general), discussion of depression treatment (specifically), and patient requests for depression medication, cluster-adjusted mean differences between each of the active interventions and control were consistently less than ±6 percentage points, with upper 90% confidence bounds for differences ranging from 3.7 (patient requests for medication, comparing IMCP to control) to 15.7 (depression discussion, comparing DEV to control) (Table 4). There were no pre-specified inferiority margins for these outcomes. Neither of the two active interventions had significant impact (versus control) on clinician-reported visit burden or clinician-reported visit time (P>.60 for each of the 4 comparisons).
Table 4.
Potential harms in the non-depressed sample of 308 patients (PHQ-9 score <5)
| Outcome | DEV (n=109) | IMCP (n=90) | Control (n=109) | DEV vs. Control | IMCP vs. Control |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | Cluster-adjusted mean percentage point difference (90% CI)a | Cluster-adjusted mean percentage point difference (90% CI)a | |
| Antidepressant prescribed (clinician reported; n=292 due to 16 missing values)b | 5 (4.8) | 3 (3.6) | 7 (6.7) | −2.2 (−8.0, 3.49) NI P value = .0499 |
−3.3 (−9.1, 2.4) NI P value = .02 |
| Antidepressant recommended (patient reported)b | 6 (5.6) | 4 (4.4) | 5 (4.6) | 0.9 (−4.9, 6.7) NI P value = .23 |
0.3 (−5.1, 5.7) NI P value = .16 |
| Depression discussed (patient reported)c | 51 (47) | 36 (40) | 48 (44) | 3.3 (−9.2, 15.7) | −2.9 (−15.8, 10.0) |
| Depression treatment discussed (patient reported)c | 25 (23) | 14 (16) | 18 (17) | 5.9 (−2.7, 14.5) | −0.8 (−8.9, 7.4) |
| Depression medication requested (patient report)b | 7 (6.4) | 2 (2.2) | 2 (1.8) | 4.6 (−0.05, 9.3) | 0.4 (−3.0, 3.7) |
Abbreviations: CI, confidence interval; IMCP, interactive multimedia computer program; PCP, primary care provider; DEV, depression engagement video; NI, noninferiority
Cluster-adjusted mean percentage point differences estimated via Stata's margins post-estimation command following simple logistic regression models for clustered data, with study arm as the sole fixed effects term in the model, to adjust inferences for the nesting of multiple patient observations within 106 physicians. Clustered data models estimated either via generalized estimating equations or mixed-effects logistic regression (as indicated in table). For mixed-effects models, margins were estimated with the physician random effect for each observation set to 0 (the mean value). Noninferiority P values are for Wald test of the one-sided inferiority null hypothesis that the contrast ≥ 3.5 percentage points.
Logistic regression model estimated using generalized estimation equations (due to small number of outcomes) to adjust for clustering of patients within PCPs.
Logistic regression model estimated with random intercepts to adjust for clustering of patients within PCPs.
COMMENT
Among patients with clinically relevant depression symptoms (i.e., the depressed patient sample), both a depression engagement video (DEV) and a tailored interactive multimedia computer program (IMCP) delivered before a primary care clinician appointment increased patient-reported requests for information about depression, and the IMCP increased the primary composite outcome of antidepressant recommendation or mental health referral, as reported by the patient immediately after the primary care appointment. However, there were no significant improvements in mental health or quality of life at 12-week follow-up in response to either intervention. Among non-depressed patients, we observed no evidence of harm from either intervention for the outcome of physician-reported antidepressant prescribing but we could not exclude harm (that is a higher-rate of antidepressant prescriptions for non-depressed patients associated with each intervention) based on patient-reported antidepressant recommendation. There were no significant adverse intervention effects on other visit processes.
Overall in the depressed sample, assignment to the IMCP, but not the DEV, was associated with a statistically significant 10 percentage point increase in the likelihood of receiving the primary composite outcome of antidepressant recommendation, mental health referral, or both. The estimated intervention effect was statistically significant in the subgroup of patients with PHQ-9 scores of 10 or more (for whom current guidelines endorse prompt provision of medication or psychotherapy)38,46 but not those with lower scores. While clinically plausible, these subgroup analyses were not pre-specified and should be viewed as exploratory, especially since there was no statistically significant interaction between intervention group and PHQ-9 score category.
In considering the mechanism by which the IMCP improved clinical processes of care, we speculate that individualized information about depression and its manifestations may have helped some depressed individuals to identify their personal symptoms and distress as depression and to communicate these insights to providers verbally or non-verbally. In turn, clinicians may have been less deterred by perceptions of depression-related stigma on the part of patients and consequently more disposed to offer treatment. In addition, individualized information about depression treatment may have increased some participants’ receptiveness to anti-depressant medication or psychotherapy. These tentative explanations should be tested in future studies.
Among patients who were depressed, assignment to the DEV or IMCP was associated with a two-fold increased likelihood of asking the treating clinician about depression. However, regardless of intervention group, most patients never broached the topic. The dearth of depression-related discussion could reflect more pressing clinical issues, competing demands,47 or reluctance to raise the issue of depression.
Among depressed participants who participated in the 12-week follow-up telephone interview, depression symptom scores and mental and physical health component scores improved from baseline in all 3 treatment arms. However, neither the DEV nor the IMCP was associated with improved mental or physical health outcomes compared with control. Thus, our interventions did not demonstrate benefit at 12-week follow-up. Translating improvements in initial depression process of care into better clinical outcomes may require reinforcement, clinician support, or systems improvement, and additional research examining the impact of combined interventions is warranted.
Among non-depressed patients (PHQ-9<5), we found small differences in rates of both antidepressant prescribing (as reported by clinicians) and antidepressant recommendations (as reported by patients). Using the patient-reported measure, we could not exclude the possibility that the two interventions increased rates of antidepressant prescriptions by at least 3.5 percentage points among the non-depressed. In judging the overall merits of the IMCP, clinicians and care managers will have to weigh the benefits (improved process of care) against potential risks of overtreatment.
The brevity of both interventions makes them potentially suitable for widespread implementation in health care settings. Patients could complete depression screening questionnaires on touchscreen machines and, if warranted, receive prompts to select an appropriate multimedia program.
There were study limitations. Eligibility and classification into “depressed” and “non-depressed” categories was based on the PHQ-9, a valid measure of depression symptom burden but not a diagnostic instrument. Participants were volunteers recruited from 2 metropolitan regions in Northern California; the generalizability of our findings to other settings and types of patients is unknown. Randomization by patient rather than by clinician or clinic had advantages but may have diluted intervention effects. Although allocation concealment was achieved, full blinding was infeasible. The primary outcome among depressed patients was based on patient report, arguably the most appropriate choice given the goal of patient activation but still subject to reporting bias. Incomplete follow-up could have skewed 12-week outcomes, though the direction of this bias is unpredictable. Finally, this study examined the effectiveness of the interventions in office settings. Administration in a different context (e.g., via the internet) could produce different results.
In summary, among patients with depression evaluated in a primary care setting, the use of a tailored IMCP immediately prior to a primary care visit resulted in increased receipt of the primary composite outcome of antidepressant prescription recommendation and/or mental health referral during the primary care visit, compared to an attention control group. However, the tailored IMCP intervention had no effect on 12-week clinically meaningful outcomes. While there was no evidence of excess antidepressant prescribing among patients with minimal symptoms of depression as determined by the physician reported outcome, potential overtreatment cannot be excluded based on the patient-reported outcome. Further research is needed to determine effects on clinical outcomes, and whether the benefits outweigh possible harms.
Acknowledgements
Funding: The work was supported by grants 1R01MH079387 (Kravitz), K24MH072756 (Kravitz), and K24 K24MH072712 (Duberstein) from the National Institute of Mental Health.
Role of the funding source: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; or preparation, review, or approval of the manuscript or decision to submit the manuscript for publication.
Footnotes
ClinicalTrials.gov Identifier NCT01144104
Conflict of interest disclosures: None of the authors has any conflicts of interest to disclose.
Author contributions: Dr. Kravitz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kravitz, Jerant, Franks, Tancredi, Feldman, Epstein, Duberstein, Bell, Paterniti
Acquisition of data: Kravitz, Feldman, Slee, Cipri, Olson, Hudnut, Kelly-Reif, Dvorak, Turner, Jackson-Triche
Analysis and interpretation of data: Franks, Tancredi, Iosif, Kravitz, Jerant
Drafting of the manuscript: Kravitz, Tancredi, Franks, Jerant
Critical revision of the manuscript for important intellectual content: all authors
Statistical analysis: Tancredi, Franks, Iosif
Obtained funding: Kravitz
Additional contributions: We are grateful to the following individuals, who coordinated or facilitated recruitment and participation of patients in the study: Julia Huerta, MPH (UCD), Dustin Gottfeld, BS (UCD), Ana Fernandez-Lamothe (UCSF), Jeff Kohlwes, MD (UCSF) and Seth Berkowitz, MD (UCSF). All except Dr. Kohlwes were compensated from grant funds for their time. We also wish to thank Robert Burnett, MA, UCD IET-Academic Technology Services, for developing and programming the animations employed in the tailored interactive multimedia computer program. Mr. Burnett was paid from grant funds for his time. Finally, we are indebted to all of the physicians, offices, and patients who participated.
Contributor Information
Richard L. Kravitz, UC Davis Division of General Medicine and Center for Healthcare Policy and Research Sacramento, California.
Peter Franks, UC Davis Department of Family and Community Medicine and Center for Healthcare Policy and Research Sacramento, California.
Mitchell D. Feldman, UC San Francisco Division of General Internal Medicine San Francisco, California.
Daniel J. Tancredi, UC Davis Department of Pediatrics and Center for Healthcare Policy and Research Sacramento, California.
Christina A. Slee, UC Davis Health System Sacramento, California.
Ronald M. Epstein, University of Rochester Departments of Family Medicine, Psychiatry and Oncology Rochester, New York.
Paul R. Duberstein, University of Rochester Departments of Psychiatry and Family Medicine Rochester, New York.
Robert A. Bell, UC Davis Departments of Communication and Public Health Sciences and Center for Healthcare Policy and Research Davis, California.
Maga Jackson-Triche, Northern California VA Health System Sacramento, California.
Debora A. Paterniti, UC Davis Departments of Internal Medicine and Sociology and Center for Healthcare Policy and Research Davis, California.
Camille Cipri, UC Davis Center for Healthcare Policy and Research Sacramento, California.
Ana-Maria Iosif, UC Davis Department of Public Health Sciences Davis, California.
Sarah Olson, UC San Francisco Division of General Internal Medicine San Francisco, California.
Steven Kelly-Reif, The Permanente Medical Group Sacramento, California.
Andrew Hudnut, Sutter Medical Foundation Sacramento, California.
Simon Dvorak, UC Davis IET-Academic Technology Services Davis, California.
Charles Turner, UC Davis IET-Academic Technology Services Davis, California.
Anthony Jerant, UC Davis Department of Family and Community Medicine and Center for Healthcare Research and Policy Sacramento, California.
References
- 1.Berardi D, Menchetti M, Cevenini N, Scaini S, Versari M, De Ronchi D. Increased recognition of depression in primary care. Comparison between primary-care physician and ICD-10 diagnosis of depression. Psychotherapy and psychosomatics. 2005;74(4):225–230. doi: 10.1159/000085146. [DOI] [PubMed] [Google Scholar]
- 2.Lotfi L, Flyckt L, Krakau I, Martensson B, Nilsson GH. Undetected depression in primary healthcare: occurrence, severity and co-morbidity in a two-stage procedure of opportunistic screening. Nordic journal of psychiatry. 2010 Dec;64(6):421–427. doi: 10.3109/08039481003786378. [DOI] [PubMed] [Google Scholar]
- 3.Simon GE, Fleck M, Lucas R, Bushnell DM, Group L. Prevalence and predictors of depression treatment in an international primary care study. The American journal of psychiatry. 2004 Sep;161(9):1626–1634. doi: 10.1176/appi.ajp.161.9.1626. [DOI] [PubMed] [Google Scholar]
- 4.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2002 Dec 11;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 5.Wells KB. Caring for depression in primary care: defining and illustrating the policy context. The Journal of clinical psychiatry. 1997;58(Suppl 1):24–27. [PubMed] [Google Scholar]
- 6.Katon W, Unutzer J, Wells K, Jones L. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. General hospital psychiatry. 2010 Sep-Oct;32(5):456–464. doi: 10.1016/j.genhosppsych.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA : the journal of the American Medical Association. 1999 Oct 20;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 8.Kravitz RL. Beyond gatekeeping: enlisting patients as agents for quality and cost-containment. Journal of general internal medicine. 2008 Oct;23(10):1722–1723. doi: 10.1007/s11606-008-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell RA, Franks P, Duberstein PR, et al. Suffering in silence: reasons for not disclosing depression in primary care. Annals of family medicine. 2011 Sep-Oct;9(5):439–446. doi: 10.1370/afm.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein RM, Duberstein PR, Feldman MD, et al. “I didn't know what was wrong:” how people with undiagnosed depression recognize, name and explain their distress. Journal of general internal medicine. 2010 Sep;25(9):954–961. doi: 10.1007/s11606-010-1367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanter JW, Rusch LC, Brondino MJ. Depression self-stigma: a new measure and preliminary findings. The Journal of nervous and mental disease. 2008 Sep;196(9):663–670. doi: 10.1097/NMD.0b013e318183f8af. [DOI] [PubMed] [Google Scholar]
- 12.Donohue JM, Cevasco M, Rosenthal MB. A decade of direct-to-consumer advertising of prescription drugs. The New England journal of medicine. 2007 Aug 16;357(7):673–681. doi: 10.1056/NEJMsa070502. [DOI] [PubMed] [Google Scholar]
- 13.Block AE. Costs and benefits of direct-to-consumer advertising: the case of depression. PharmacoEconomics. 2007;25(6):511–521. doi: 10.2165/00019053-200725060-00006. [DOI] [PubMed] [Google Scholar]
- 14.Mintzes B, Barer ML, Kravitz RL, et al. How does direct-to-consumer advertising (DTCA) affect prescribing? A survey in primary care environments with and without legal DTCA. CMAJ. 2003 Sep 2;169(5):405–412. [PMC free article] [PubMed] [Google Scholar]
- 15.Kravitz RL, Epstein RM, Feldman MD, et al. Influence of patients' requests for direct-to-consumer advertised antidepressants: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2005 Apr 27;293(16):1995–2002. doi: 10.1001/jama.293.16.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niederdeppe J, Byrne S, Avery RJ, Cantor J. Direct-To-Consumer Television Advertising Exposure, Diagnosis with High Cholesterol, and Statin Use. Journal of general internal medicine. 2013 Mar 6; doi: 10.1007/s11606-013-2379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avery RJ, Eisenberg MD, Simon KI. The impact of direct-to-consumer television and magazine advertising on antidepressant use. Journal of health economics. 2012 Sep;31(5):705–718. doi: 10.1016/j.jhealeco.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Schmid KL, Rivers SE, Latimer AE, Salovey P. Targeting or tailoring? Marketing health services. 2008;28(1):32–37. Spring. [PMC free article] [PubMed] [Google Scholar]
- 19.Jerant A, Sohler N, Fiscella K, Franks B, Franks P. Tailored interactive multimedia computer programs to reduce health disparities: opportunities and challenges. Patient education and counseling. 2011 Nov;85(2):323–330. doi: 10.1016/j.pec.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tancredi DJ, Slee CK, Jerant A, et al. Targeted versus tailored multimedia patient engagement to enhance depression recognition and treatment in primary care: randomized controlled trial protocol for the AMEP2 study. BMC Health Serv Res. 2013;13(1):141. doi: 10.1186/1472-6963-13-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. JAMA : the journal of the American Medical Association. 2003 Jun 18;289(23):3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. Journal of affective disorders. 2009 Apr;114(1-3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Bell RA, Paterniti DA, Azari R, et al. Encouraging patients with depressive symptoms to seek care: a mixed methods approach to message development. Patient education and counseling. 2010 Feb;78(2):198–205. doi: 10.1016/j.pec.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kravitz RL, Epstein RM, Bell RA, et al. An academic-marketing collaborative to promote depression care: A tale of two cultures. Patient education and counseling. 2011 Aug 20; doi: 10.1016/j.pec.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein A. Common Sleeping Problems. HealthiNation. 2011 [Google Scholar]
- 26.Kravitz RL, Paterniti DA, Epstein RM, et al. Relational barriers to depression help-seeking in primary care. Patient education and counseling. 2011 Feb;82(2):207–213. doi: 10.1016/j.pec.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petty R, Cacioppo J. Communication and Persuasion: Central and Peripheral Routes to Attitude Change. Springer; New York, New York: 1986. [Google Scholar]
- 28.Dwight-Johnson M, Sherbourne CD, Liao D, Wells KB. Treatment preferences among depressed primary care patients. J Gen Intern Med. 2000 Aug;15(8):527–534. doi: 10.1046/j.1525-1497.2000.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karasz A, Sacajiu G, Garcia N. Conceptual models of psychological distress among low-income patients in an inner-city primary care clinic. J Gen Intern Med. 2003 Jun;18(6):475–477. doi: 10.1046/j.1525-1497.2003.20636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper-Patrick L, Powe NR, Jenckes MW, Gonzales JJ, Levine DM, Ford DE. Identification of patient attitudes and preferences regarding treatment of depression. J Gen Intern Med. 1997 Jul;12(7):431–438. doi: 10.1046/j.1525-1497.1997.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dwight-Johnson M, Unutzer J, Sherbourne C, Tang L, Wells KB. Can quality improvement programs for depression in primary care address patient preferences for treatment? Med Care. 2001 Sep;39(9):934–944. doi: 10.1097/00005650-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Maly RC, Frank JC, Marshall GN, DiMatteo MR, Reuben DB. Perceived efficacy in patient-physician interactions (PEPPI): validation of an instrument in older persons. Journal of the American Geriatrics Society. 1998 Jul;46(7):889–894. doi: 10.1111/j.1532-5415.1998.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 33.Cooper AE, Corrigan PW, Watson AC. Mental illness stigma and care seeking. J Nerv Ment Dis. 2003 May;191(5):339–341. doi: 10.1097/01.NMD.0000066157.47101.22. [DOI] [PubMed] [Google Scholar]
- 34.Deci EL, Ryan RM. The support of autonomy and the control of behavior. J Pers Soc Psychol. 1987;53(6):1024–1037. doi: 10.1037//0022-3514.53.6.1024. [DOI] [PubMed] [Google Scholar]
- 35.Kreuter M, Farrell D, Olevitch L, Brennan L. Tailoring health messages: customizing communication with computer technology. Erlbaum; Mahwah, NJ: 2000. [Google Scholar]
- 36.Duffy SA, Ronis DL, Valenstein M, et al. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006 Nov;15(11):2203–2208. doi: 10.1158/1055-9965.EPI-05-0880. [DOI] [PubMed] [Google Scholar]
- 37.Ryan GL, Skinner CS, Farrell D, Champion VL. Examining the boundaries of tailoring: the utility of tailoring versus targeting mammography interventions for two distinct populations. Health education research. 2001 Oct;16(5):555–566. doi: 10.1093/her/16.5.555. [DOI] [PubMed] [Google Scholar]
- 38.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 2001 Sep;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals. 2002;32(9):1–7. [Google Scholar]
- 40.Wells TS, Horton JL, Leardmann CA, Jacobson IG, Boyko EJ. A comparison of the PRIME-MD PHQ-9 and PHQ-8 in a large military prospective study, the Millennium Cohort Study. Journal of affective disorders. 2013 May 15;148(1):77–83. doi: 10.1016/j.jad.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 41.Fleishman JA, Selim AJ, Kazis LE. Deriving SF-12v2 physical and mental health summary scores: a comparison of different scoring algorithms. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2010 Mar;19(2):231–241. doi: 10.1007/s11136-009-9582-z. [DOI] [PubMed] [Google Scholar]
- 42.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. 1996 Mar;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Stata Statistical Software [computer program] StataCorp LP; College Station, TX: 2011. [Google Scholar]
- 44.Hauck WW, Anderson S, Marcus SM. Should we adjust for covariates in nonlinear regression analyses of randomized trials? Control Clin Trials. 1998 Jun;19(3):249–256. doi: 10.1016/s0197-2456(97)00147-5. [DOI] [PubMed] [Google Scholar]
- 45.DeSouza CM, Legedza AT, Sankoh AJ. An overview of practical approaches for handling missing data in clinical trials. Journal of biopharmaceutical statistics. 2009 Nov;19(6):1055–1073. doi: 10.1080/10543400903242795. [DOI] [PubMed] [Google Scholar]
- 46. [May 22, 2013];MacArthur Foundation Initiative on Depression and Primary Care. http://www.depression-primarycare.org/.
- 47.Nutting PA, Rost K, Smith J, Werner JJ, Elliot C. Competing demands from physical problems: effect on initiating and completing depression care over 6 months. Archives of family medicine. 2000 Nov-Dec;9(10):1059–1064. doi: 10.1001/archfami.9.10.1059. [DOI] [PubMed] [Google Scholar]