Abstract
Objective
Consuming alcohol prior to a meal (an apéritif) increases food consumption. This greater food consumption may result from increased activity in brain regions that mediate reward and regulate feeding behavior. Using functional magnetic resonance imaging, we evaluated the blood oxygenation level dependent (BOLD) response to the food aromas of either roast beef or Italian meat sauce following pharmacokinetically controlled intravenous infusion of alcohol.
Methods
BOLD activation to food aromas in non-obese women (n=35) was evaluated once during intravenous infusion of 6% v/v EtOH, clamped at a steady-state breath alcohol concentration of 50 mg/dL, and once during infusion of saline using matching pump rates. Ad libitum intake of roast beef with noodles or Italian meat sauce with pasta following imaging was recorded.
Results
BOLD activation to food relative to non-food odors in the hypothalamic area was increased during alcohol pre-load when compared to saline. Food consumption was significantly greater, and levels of ghrelin were reduced, following alcohol.
Conclusions
An alcohol pre-load increased food consumption and potentiated differences between food and non-food BOLD responses in the region of the hypothalamus. The hypothalamus may mediate the interplay of alcohol and responses to food cues, thus playing a role in the apéritif phenomenon.
Keywords: Apéritif, Functional Magnetic Resonance Imaging, Obesity, Olfaction, Alcohol, Hypothalamus, Ghrelin, Leptin, Reward Value
INTRODUCTION
Appearing in the late 19th century, the word „apéritif' derives from the Latin “aperire”, to open the appetite, as alcohol was thought to “open” the stomach. Epidemiological research on the relationship between alcohol intake and weight gain is contradictory (for review see 1). However, the preponderance of evidence documents that alcohol ingestion just prior to a meal is associated with increased food consumption as demonstrated in a number of studies conducted in both men (2-4), and women (5). An apéritif also leads to higher energy intake compared to soda (6), or liquids containing high levels of fat, protein, or carbohydrates (5). Given the rise in reported alcohol consumption (7), particularly wine, in the United States, overeating following alcohol consumption may contribute to weight gain and significant health-related consequences.
While increased food consumption following an apéritif appears replicable, the mechanism remains unclear. Most commonly proposed is that an apéritif promotes consumption by increasing appetite (food desire), although no studies have indicated a significant increase in self-reported hunger following an apéritif (2-6, 8). “Placebo” effects from apéritif “lore” appear unlikely, as disguised alcohol delivery still leads to greater energy intake (5, 8-10).
This study uses functional magnetic resonance imaging (fMRI) to determine how alcohol intoxication affects the brain, hypothesizing that alcohol potentiates the response to food stimuli in brain regions that mediate reward and/or feeding (ventral striatum, ventral frontal cortex, hypothalamus). Specifically, we used blood oxygenation level dependent (BOLD) fMRI to study responses to food aromas (roast beef or Italian meat sauce) and an inedible control odor (Douglas fir). Two fMRI sessions were conducted at lunchtime: once during pharmacokinetically controlled intravenous infusion of alcohol at a clamped, constant brain exposure (50mg% target, similar to prior behavioral, non-imaging studies investigating this effect (2-4)), and once during saline infused at the same rates, with subjects eating a late lunch after both sessions.
MATERIALS & METHODS
Subjects
Thirty-five non-obese (body mass index [BMI] 18.8-25.2 kg/m2), non-vegetarian, non-smoking women (86% Caucasian), who performed normally on the 20-item Smell Identification Test (11) were recruited (Table 1). Women were excluded if pregnant/breast-feeding within the past 6 months, had diagnosed diabetes or fasting blood glucose ≥126 mg/dl, self-reported symptoms consistent with (or a known history of) DSM-IV Axis-I psychiatric or neurological disorders, or food preferences inconsistent with the odorants or meals used in the study. All subjects gave informed consent, and the study was approved by the Indiana University Institutional Review Board.
Table 1.
Subject Characteristics
| (n = 35) | Mean | (SD) |
|---|---|---|
| Age | 24.9 | (4.3) |
| Education (yrs.) | 15.9 | (1.1) |
| Height (cm) | 165.3 | (4.8) |
| Weight (kg) | 59.3 | (4.8) |
| BMI | 21.7 | (1.8) |
Breakfast
This study was conducted over two randomized, non-contiguous, single-blind, “Alcohol” and “Saline” days (see Figure S1). Subjects reported to the Indiana Clinical Research Center (ICRC) at approximately 6:30am. A uniform breakfast was provided at 7:40am (see Table S1) with portions adjusted to account for 20% of the subject's daily energy requirement for weight maintenance (12). Subjects consumed 88.6%±8.8% (±SD) of the total energy offered at breakfast.
Lunch
Following breakfast, subjects remained in the ICRC until escorted to imaging at 1:00pm. Following imaging, subjects were escorted back to the ICRC to break their fast under nursing supervision with a late lunch (~3:00–4:00pm), and were presented with a casserole chosen by the subject prior to the study day. The choices were either pasta with ground beef and Italian tomato sauce (5.34 kJ/gm) or noodles with shredded beef and gravy (5.48 kJ/gm) served in a deep, black, slanted bowl to minimize the appearance of excess food (see also, 13) and served with water (550ml, consumed ad libitum) . Subjects were instructed to eat over a 30min period until full with the same meal offered on both study days. Between breakfast and lunch, subjects were monitored by the ICRC nursing staff, and allowed ad libitum water (volume documented) and sedentary activity (reading, television, computing, etc.), excluding sleep.
Behavioral Assessments
Odorant Perception
Prior to imaging, odorant intensity was assessed on a horizontally oriented 100mm labeled magnitude scale (see Figure S2; 14) until each odorant's intensity was within 4mm of the others. Odorant pleasantness and representativeness were rated on horizontally oriented Likert-type scales (see Figure S2; 15).
Hunger
Subjects rated general hunger, as well as hunger for the subject's chosen food type, using a vertically oriented 100mm scale (see Figure S2; 14, 16). Both hunger types were assessed in the scanner, once prior to the IV infusion (T0), and at five time points (~15(T1), 45(T2), 60(T3), 70(T4), and 120(T5) min) following the infusion's onset.
Subjective Effects of Alcohol
Subjects rated their subjective impressions of each infusion (perceived anxiety, intoxication, numbness and tingling, feeling of being high, and perceived number of alcoholic drinks) on visual analog scales of 100mm at the same time points as the hunger ratings (see Figure S3).
Procedures
Analytes
Blood samples (drawn into EDTA tubes with DPPIV (50μM final) and AEBSF (1 mg/ml final) inhibitors added) for 26 subjects were obtained intravenously just prior to and following infusion, with nine samples missing due to blood draw difficulty. Glucose was measured by the glucose oxidase method (Randox Daytona clinical analyzer), insulin by radioimmunoassay, and active ghrelin by ELISA (Millipore Linco, St. Charles, MO).
Alcohol Infusion
Subjects were intravenously infused with either alcohol (6% vol/vol) in saline or saline alone (placebo) in a pseudo-randomized, counter-balanced order. Infusion pump rates were computer-controlled (17, 18), with the infusion profile customized for each individual to achieve the same time-course of breath alcohol concentration (BrAC) for all subjects: a linear ascension to 50mg% in 15min, followed by constant exposure at 50mg% throughout BOLD imaging. Saline infusion employed the same pump-rate profile as the individual's alcohol session. BrAC was measured prior to and after imaging, and during a brief imaging break (between T4-T5) using a forensic grade breath meter.
Olfactory Stimuli
All odorants were delivered to each subject as previously described (19). Two classes of odorants (International Flavors & Fragrances, Union Beach, NJ) were presented: (i) food odors (FO) of Italian meat sauce (20% solution in 1,2-propanediol diluent, Sigma-Aldrich, St. Louis, MO) and roast beef (5% solution in diluent); and (ii) the odor of an inedible object (IEd), Douglas fir (undiluted). Diluent alone served as an odorless sham control odor (CO).
Olfactory Paradigm
Brain responses were assessed using three, non-consecutive 6:27min BOLD fMRI scans. OptSeq2 (http://surfer.nmr.mgh.harvard.edu/optseq/) generated three pseudorandom, mixed-event odor stimulation sequences (inter-stimulus interval average 11.5sec; range 8-22sec), each presented once per session in a pseudo-randomized fashion using E-Prime 2.0 (Psychology Software Tools Inc., Sharpsburg, PA). Each odorant was presented 8 times per scan. Subjects inhaled at a verbal prompt, exhaled following a tone, and reported detecting odors on a trackball (left-click=yes, right-click=no; HHSC-TRK-2; Current Designs, Philadelphia, PA)
Imaging Procedures and Analysis
Image Acquisition
Subjects were imaged on a Siemens 3T Magnetom Trio-Tim (Erlangen, Germany) scanner using a 12-channel head coil. A high-resolution anatomic volume (1.0×1.0×1.2 mm3 voxels, 3D magnetization prepared rapid gradient echo; MPRAGE) was utilized to position functional, BOLD contrast sensitive data (gradient echo, echo-planar imaging, repetition/echo time 2250/29ms, flip angle 78°, field-of-view 220×220mm, 39 interleaved 3mm thick slices, 2.5×2.5×3.0 mm3 voxels, acceleration factor 2). Subjects kept their eyes closed during imaging, with head movement and motion-related artifacts minimized by using foam pads and real time prospective acquisition motion correction (20). Functional scans with any BOLD volume displacement >0.9mm (21), or a fraction of outliers >0.19 (3dToutcount, AFNI; see 22), were rejected from analysis to prevent the influence of gross peak and mean head motion, respectively. These parameter values (>2 standard deviations of the overall sample mean) are consistent with those in previous data scrubbing literature (see 23, 24). To maintain equal power across sessions, the scan with the highest level of motion was also dropped from the other session. The imaging results thus reflect 25 subjects with three BOLD scans per session, and 10 with two BOLD scans per session.
Imaging Processing and Analysis
Imaging preprocessing (SPM8, Wellcome Department of Imaging Neuroscience, University College, London, UK) included slice-time acquisition correction, rigid-body realignment, and co-registration. Each subject's MPRAGE image was segmented into tissue type; parameters from this nonlinear transformation were used to convert the subject's structural MRI and realigned, co-registered BOLD volumes into Montreal Neurological Institute (MNI) stereotactic space. The resulting normalized volumes were interpolated to 1mm/side isotropic voxels and smoothed by a 6mm full-width at half-maximum isotropic Gaussian kernel for all areas anterior of y=−50.
Responses to discrete, 2sec odorant or sham (odorless diluent) presentations were convolved with a standard hemodynamic response function, and its time and dispersion derivatives. The six movement parameters from realignment were included as regressors while a high-pass filter (1/128 Hz cut-off) was applied to remove low frequency noise. This “first-level” analysis estimated within-subject odorant effects by assessing contrasts of interest that compared a given odor to the CO. This approach avoided relative FO and IEd comparisons and permitted an independent assessment of the baseline IEd response (see also 13).
The primary contrast of interest was [FO>IEd]Alcohol>Saline, where brain responses to food aromas during alcohol infusion were compared to IEd aromas under saline infusion in previously defined regions of interest (ROIs). To define the hypothalamus, we used an a priori hypothalamic area observed in an earlier study (25), centering an 8mm radius spherical ROI around MNI coordinate [0, −4, −8], within which we performed family-wise error (FWE) corrections. We also tested for [FO>IEd] effects within previously defined ROIs of the ventral striatum (26), ventromedial prefrontal cortex (27), and posterolateral orbital cortex (28). FWE corrections were performed within each of these hypothesized ROIs for [FO>IEd] contrasts. Finally, and as a validity check, we also evaluated the positive effect of all odors > CO to assure activation in the brain's olfactory system (piriform and orbital cortex).
RESULTS
All Subjects
Food consumption
No subject finished the provided lunch (~ 1,100g; 6280 kJ) on either test day. Meal choice was unrelated to consumption on either alcohol or saline days (t-test, ps>0.34), with subjects who chose Italian meat sauce and pasta consuming amounts that did not differ from those who choose to eat beef and noodles (alcohol; mean±SD: 567±43g vs. 608±37g: saline; 529±37g vs. 572±25g). Collapsed across meal type, subjects ate more after alcohol than saline (paired t, p=0.04).
Hunger ratings
Session (EtOH, Saline) × Time (T0–T5) linear mixed models of both General and Preferred Food Hunger showed main effects of Time (ps<0.001), but not Session (ps>0.09), nor Session × Time interactions (ps>0.73). The main effect of Time reflected a general increase in hunger as imaging progressed.
Subjective Effects from Alcohol
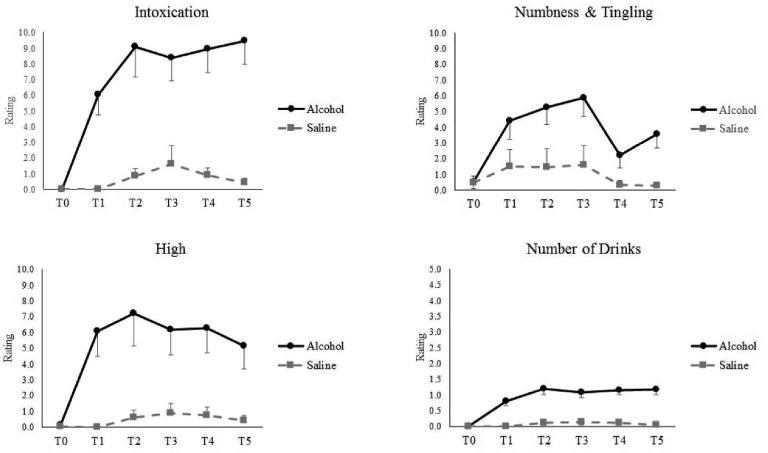
As analyzed in a 2 (Session) × 6 (Time) linear mixed model, significant main effects of both Session and Time for perceived intoxication, number of drinks, high, and numbness/tingling (ps<0.001; Figure 1) emerged. As intended, alcohol infusion induced a clear subjective sense of alcohol exposure.
Figure 1.
Subjective Effects of Alcohol as reported both pre- and during alcohol and saline infusion sessions. T0 = Pre-Infusion baseline; T1 – T5 = Intra-Infusion assessments. Intensity (FO=12.2, SE=1.0; IEd=12.1, SE=0.9), pleasantness (FO = 7.0±0.2; IEd = 7.6±0.2), and representativeness (FO = 6.8±0.2; IEd = 7.6±0.2) (for details, see Methods).
Metabolic Response
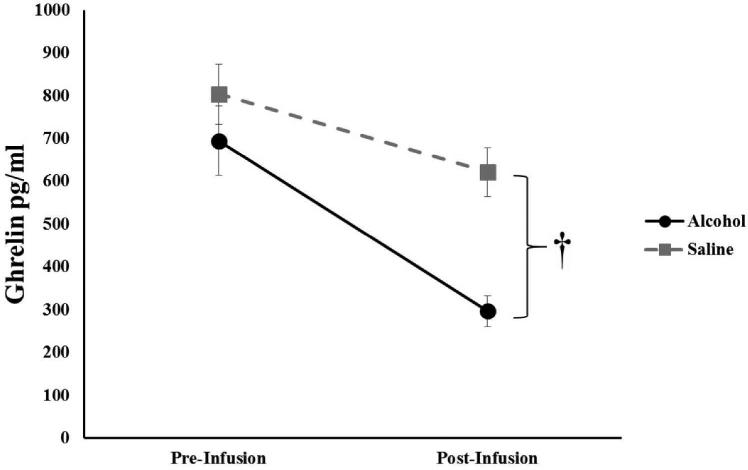
Linear mixed models (Session [EtOH, Saline] × Time [Pre-Infusion, Post-Infusion]) of plasma glucose, insulin, and ghrelin (analyzed separately) revealed main effects of Time (ps<0.002) for all, with each decreasing when compared to the pre-infusion measurement; with no effect of Time observed for leptin (p=0.83). A significant effect of Session (p<0.001) was present for ghrelin, with alcohol significantly decreasing ghrelin (p<0.001), but not saline (p=0.12; Figure 2). A significant effect of Session was present for leptin (p<0.001); however, post-hoc analysis revealed no significant effect of alcohol infusion (p>0.05).
Figure 2. Circulating Ghrelin.
Mean circulating ghrelin pre- and post-intravenous infusion of either alcohol or saline, with ghrelin showing a marked reduction following alcohol administration. †, p < 0.001.
Subjective Responses to Aromas
The odors of food and the inedible object were perceived as equally intense, pleasant, and representative (ANOVA, ps=0.32).
BOLD activation
Olfactory sensory stimulation
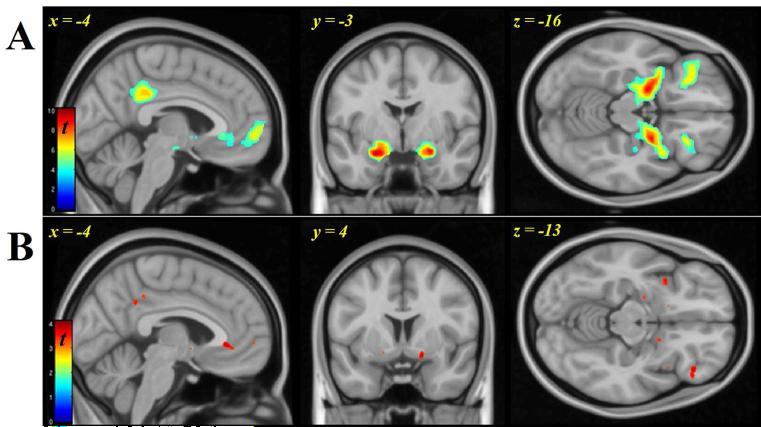
The olfactory sensory paradigm was effective, as primary (piriform) and associative (orbitofrontal) olfactory cortices, along with medial prefrontal cortex, activated robustly to odorant presentation versus CO (Figure 3A; Table S2). The only significant difference in olfactory regions between the alcohol and saline infusions was limited to a small (0.072 ml) region in frontal piriform cortex (Saline>Alcohol, [26, 4, −12], p<0.001) with 3% of the entire 2.49 ml piriform volume activated under alcohol. Furthermore, both piriform regions still contained peaks under alcohol at pFWE<0.05, whole brain corrected (as they did under saline).
Figure 3.
A.) Olfactory response (both infusates and odors) in amygdala/piriform, and lateral orbitofrontal cortex. B.) Food Odor (FO) > Odor of Inedible object (IEd): Activation in anterior cingulate/medial prefrontal regions (sagittal view), amygdala/piriform and ventral striatum (coronal and axial), right orbitofrontal cortex and bilateral insula (axial). Display threshold puncorr < 0.001. The colorbar scales indicate t-statistic values for 35 subjects.
Comparison of food and inedible odors on BOLD response
The effect of odor type on BOLD response was evaluated by contrasting the FO against IEd [FO>IEd], collapsing across infusates (Figure 3B; Table S2). Compared to the IEd, FO produced greater activation within the piriform/amygdala, and in right orbitofrontal, ventromedial prefrontal, insula, ventral striatum, and anterior cingulate cortex (peak voxel height, p<0.001, uncorrected).
Alcohol effects on food odor BOLD response
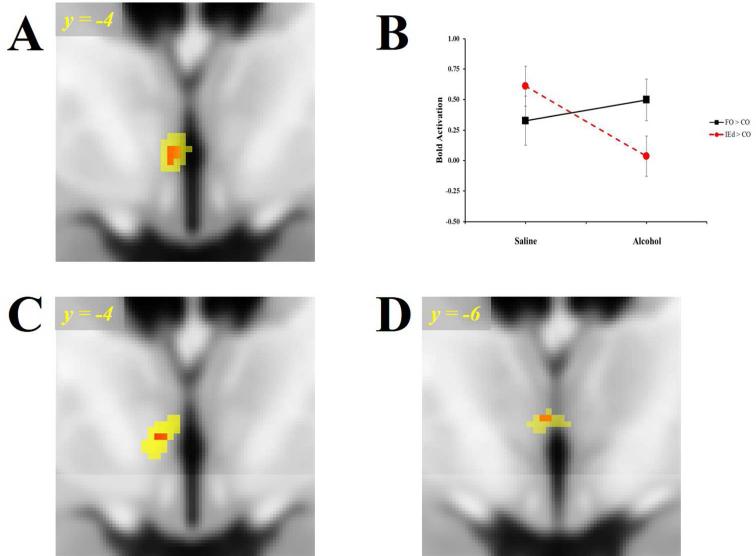
To test the hypothesis that alcohol potentiates the brain's responses to food odors, we evaluated the contrast of: [FO>IEd]Alcohol>Saline resulting in a significantly increased left (dorsal) hypothalamic response to food aromas (Figure 4A; Table 2; peak effect, pFWE=0.03 correcting for the a priori hypothalamic ROI, a second, non-significant, cluster appeared in the right hypothalamus; pFWE=0.11). Mean responses extracted from this functional hypothalamic cluster (see Figure 4A) demonstrate that, while activation to the food aromas remains unchanged, responses to the IEd decreased significantly (p=0.006) following alcohol (Figure 4B). No other ROI displayed effects from alcohol.
Figure 4.
A.) Alcohol's Effect on Food Odor BOLD Response [FO > IEd] Alcohol > Saline in the hypothalamus for all subjects (n=35). B.) Plots of mean BOLD responses for all subjects occurring within the Saline and Alcohol infusion sessions as extracted from the functional cluster (Table 2; puncorr < 0.005; yellow) in Panel A. C.) Differential Odor Response [FO > IEd]Alcohol in the Hypothalamus in 13 AEM subjects. D.) 3-Way Interaction BOLD Response AEM>AEL [FO > IEd] Alcohol > Saline in the Hypothalamus/Thalamus in 25 subjects. Display threshold, puncorr < 0.005 (yellow), Red color illustrates peak voxel-wise effect, puncorr ≥ 0.001. FO = Food Odors; IEd = Inedible Object Odors; AEM = Alcohol Eat More; AEL = Alcohol Eat Less.
Table 2.
BOLD Activation
| Region | Cluster Size (k) | MNI Coordinates (mm) | Peak Voxel Z | p uncorr | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Alcohol > Saline; FO > IEd (n = 35) | ||||||
| L Hypothalamus | 34 | −3 | −3 | −4 | 3.49* | <0.001 |
| AEM; Alcohol; FO > IEd (n = 13) | ||||||
| L Precuneus | 148 | −2 | −4 8 | 31 | 3.65 | <0.001 |
| R Ventromedial Prefrontal Cortex | 36 | 7 | 51 | −2 | 3.42 | <0.001 |
| L Hypothalamus | 9 | −4 | 0 | −5 | 3.19* | 0.001 |
MNI = Montreal Neurological Institute coordinates in mm. Height threshold p ≤ 0.001
pFWE ≤ 0.05, adjusted for the a priori hypothalamic search region; Ref. 25.
Subgroup Analyses
Food consumption
Although the main analyses showed subjects as a group ate more after alcohol, individual differences emerged, with a third (34%) eating more following saline. To contrast extreme groups, the sample was subdivided into approximate tertiles based on the percent difference of food consumed following the alcohol and saline sessions. This resulted in: 1) A group who clearly ate more (>15%) following alcohol (“Alcohol Eat More”; AEM, n=13), 2) A group that ate less food (<0%) following alcohol (“Alcohol Eat Less”; AEL, n=12), and 3) An intermediate group (IG; n=10) that ate only marginally more food (0-15%) after alcohol. There were no differences in ghrelin or change in ghrelin between the two extreme subgroups (t-test, ps>0.38).
Hunger ratings
Group (AEM, AEL) × Session (Alcohol, Saline) × Time (T0–T5) linear mixed models showed no differences between the extreme groups in the subjective effects of either hunger or intoxication (ps>0.15).
BOLD activation
The [FO>IEd]Alcohol contrast in the AEM group alone yielded a robust BOLD response within the hypothalamus (Figure 4C; Table 2; peak effect at [-5, -4, -2]; pFWE=0.01 corrected for the hypothalamic ROI), similar to that seen in the [FO>IEd]Alcohol>Saline contrast for all subjects. However, a voxel-wise analysis of the Group x Session x Odor interaction (where the AEM group had a greater [FO>IEd]Alcohol>Saline response compared to the AEL) showed the peak 3-way interaction effect to be in a somewhat more posterior region bordering the hypothalamus and anteroventral thalamus (Figure 4D).
When effects were extracted from the AEM group's [FO>IEd]Alcohol contrast (the reference group/condition; see Figure 4C), alcohol diminished the response to the IEd (p=0.02), but not the FO (p=0.82), when compared to CO.
DISCUSSION
Food intake increased following intravenous alcohol, as did the hypothalamic BOLD response difference between food and non-food aromas. Although the cohort increased their food intake after alcohol as a whole, the sample could be subdivided into two extreme groups: one eating significantly more and one eating less following alcohol. The extreme group that ate more also showed a differential hypothalamic [FO>IEd]Alcohol BOLD effect. Finally, as previously observed (13), the experimental manipulation primarily affected the IEd response, and not that of the food odor.
Numerous studies have demonstrated increased energy intake after an apéritif (2-6, 8). In the current study, administration of alcohol led to a 7% increase in food consumption in the group as a whole. This increase in consumption, while significant, was low compared to studies reporting increases of 9%–30% (2-5). Although a subset of studies masked the presence of alcohol to eliminate the pre-digestive and expectancy effects that may contribute to alcohol's effect on consumption (5, 8-10), ours is the first to bypass the digestive system by infusing the alcohol pre-load directly into the blood stream, and is thus more specific for brain exposure. Circumventing the digestive system may account for the reduced effect of the alcohol pre-load on consumption; however, increased food intake is still present even in the absence of alcohol absorption within the digestive tract. This suggests that the mechanism underlying increased food consumption following an apéritif does not rely entirely on the interplay of alcohol and gut, but includes a contribution of brain exposure to alcohol.
While infusion of alcohol into the blood stream circumvents the direct absorption of alcohol by the gut, it does affect the gut hormone ghrelin in a fashion similar to ingested alcohol (29). In the present study, ghrelin levels declined significantly following alcohol, which comports with oral alcohol's reduction of circulating ghrelin (30, 31). Although it has been suggested that reduced ghrelin results from the energy content of the alcohol, Calissendorff (31) asserted that the reductions are not proportional to the energy provided in the alcohol pre-load. This interplay of alcohol and ghrelin is counterintuitive, with decreased ghrelin typically corresponding to decreased food intake. Leptin was unaffected by alcohol infusion in the current investigation, which stands in contrast to a previous study (32). The metabolic mechanisms underlying the apéritif phenomenon are further complicated by reports that PPY, GLP-1, NPY, CCK, GRP, cortisol, glucose and insulin remain unaffected by alcohol (30-34), with only leptin depressed by alcohol (32).
As the apéritif effect cannot be easily explained by hormonal signaling, our in vivo neuroimaging helps elucidate regional brain mechanisms. While the food aromas presented in this study did activate regions commonly associated with reward (ventral striatal regions, orbitofrontal and ventromedial frontal cortices), these regions did not activate differentially under the alcohol and saline. Rather, the differential BOLD response to FO (as compared to the IEd) under alcohol exposure was limited to the hypothalamus. Further analysis of these data revealed that responses to FO remained unchanged between alcohol and saline infusions; however, activation to the IEd was greatly reduced relative to FO under the alcohol condition. We previously noted a similar finding, speculating that it may not be evolutionarily adaptive to devalue food's presence (13). Thus, our observed devaluation of the IEd may represent a relative shift of motivated attention, with alcohol leading to the devaluation of non-food stimuli, rather than the enhancement of response to the food odor.
The hypothalamus, a region that receives hormonal and neuronal signals that regulate appetite and metabolism, communicates extensively with frontal cortex and limbic regions through both direct connections and indirectly via midbrain nuclei, mediating reward signaling from the cortex and limbic systems. However, little is known about the type of information transmitted, or the anatomical and neurochemical details of how information is conveyed (for review see 35). Two candidates for the transmission of this information are the peptides orexin and melanin-concentrating hormone (MCH), with both present in hypothalamic neurons that project to prefrontal and limbic regions (35). Expression of both increase following alcohol administration, and lead to elevated food consumption in animals (36). These neuropeptides may also explain the difference between increased appetite following acute alcohol administration, and the apparent loss of appetite observed in advanced alcoholics (for review see, 1). Rodents exposed to acute alcohol increase expression of both neuropeptides, while chronic administration of alcohol decreases their levels (37, 38). Orexin and MCH may thus represent one viable mechanism through which an apéritif activates a hypothalamic orexigenic response.
Previous apéritif studies reported only average food intake across subjects. The current study found approximately one-third of the subjects consumed less following alcohol infusion when compared to saline. Therefore, we felt it prudent to compare the BOLD response of extreme groups to assess any differences in neural activation. This comparison yielded a region of increased BOLD response within the hypothalamus slightly more dorsal and posterior to that found in all subjects. Further analysis of this comparison revealed that the differential response was again driven by decreased activation of the IEd, as observed in all subjects.
There are several factors to consider when interpreting the findings of the current study. To control for variance due to gender, we studied only non-obese women who generally consume less food following an alcohol pre-load than do men (5). This may, partially explain the attenuated increase in consumption following alcohol compared to previous apéritif studies that included men. Alcohol can also dampen BOLD sensitivity without changing the underlying neural activation (39, 40). We did observe a small region of primary olfactory (piriform) cortex that responded less under alcohol. Such an effect would, however, only make it harder to show our hypothesized effects. Lastly, due to the spatial resolution of BOLD acquisition, it is not feasible to distinguish various hypothalamic sub-nuclei.
In summary, this study provides new information from neuroimaging on an apéritif's effect on energy intake. We demonstrated that acute brain exposure to alcohol significantly increased food intake in congruence with previous apéritif studies, and that the orexigenic potentiation occurred in the absence of alcohol's orosensory or gut effects. Furthermore, we replicated findings that ghrelin counterintuitively decreases in the presence of alcohol, suggesting that the peptide does not drive the apéritif phenomenon. The alcohol-induced BOLD response contrasting food and non-food aromas increased significantly within the hypothalamus, suggesting a possible site of action for an apéritif's effects. Further research should elucidate the mechanism by which the hypothalamus affects food reward salience.
Supplementary Material
What is already known about this subject?
Oral alcohol ingestion prior to a meal increases caloric intake
Ingesting alcohol prior to a meal does not result in an increase in self-reported hunger
Ingesting alcohol reduces the orexigenic hormone ghrelin
What does this study add?
Given the rise in reported alcohol consumption, particularly wine, in the United States over the past decade, overeating following routine alcohol consumption prior to a meal may contribute to weight gain and significant health-related consequences. This represents an increasingly prevalent clinical concern.
Intravenous alcohol increases food consumption at a subsequent meal in non-obese women, and the alcohol effect to increase caloric intake is heterogeneous, increasing food intake in some subjects, and decreasing it in others.
Hypothalamic activity is involved in mediating the interplay of alcohol and response to food cues.
ACKNOWLEDGEMENTS
DAK, RVC, and SJO conceived the experiments, MD conceived experiments and analyzed data. WJAE conceived and carried out experiments and analyzed data. KRC, CMS, and AJA carried out experiments. JH analyzed data. RDM and CLHA contributed to data interpretation. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Odorants were kindly donated by Stephen Warrenburg, Ph.D. (International Flavors & Fragrances). Special thanks to MR technologists Michele Beal, Traci Day, and Rob Bryant. We also thank Kieren Mather, M.D. for medical oversight.
This work funded by NIH grant R01 DK089070 to RVC and DAK, the Indiana CTSI (ULRR025761), and by the Indiana Alcohol Research Center AA07611.
Footnotes
Names for PubMed indexing: Eiler II, Dzemidzic, Case, Soeurt, Armstrong, Mattes, O'Connor, Harezlak, Acton, Considine, Kareken
Supplementary information is available at Obesity's website.
Competing interests: the authors have no competing interests.
REFERENCES
- 1.Suter PM. Is alcohol consumption a risk factor for weight gain and obesity? Critical reviews in clinical laboratory sciences. 2005;42(3):197–227. doi: 10.1080/10408360590913542. [DOI] [PubMed] [Google Scholar]
- 2.Caton SJ, Ball M, Ahern A, Hetherington MM. Dose-dependent effects of alcohol on appetite and food intake. Physiol Behav. 2004;81(1):51–8. doi: 10.1016/j.physbeh.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Caton SJ, Bate L, Hetherington MM. Acute effects of an alcoholic drink on food intake: aperitif versus co-ingestion. Physiol Behav. 2007;90(2-3):368–75. doi: 10.1016/j.physbeh.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Caton SJ, Marks JE, Hetherington MM. Pleasure and alcohol: manipulating pleasantness and the acute effects of alcohol on food intake. Physiol Behav. 2005;84(3):371–7. doi: 10.1016/j.physbeh.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Westerterp-Plantenga MS, Verwegen CR. The appetizing effect of an aperitif in overweight and normal-weight humans. Am J Clin Nutr. 1999;69(2):205–12. doi: 10.1093/ajcn/69.2.205. [DOI] [PubMed] [Google Scholar]
- 6.Buemann B, Toubro S, Astrup A. The effect of wine or beer versus a carbonated soft drink, served at a meal, on ad libitum energy intake. Int J Obes Relat Metab Disord. 2002;26(10):1367–72. doi: 10.1038/sj.ijo.0802069. [DOI] [PubMed] [Google Scholar]
- 7.Organization WH. Global Alcohol Report. 2014 [Google Scholar]
- 8.Hetherington MM, Cameron F, Wallis DJ, Pirie LM. Stimulation of appetite by alcohol. Physiol Behav. 2001;74(3):283–9. doi: 10.1016/s0031-9384(01)00598-4. [DOI] [PubMed] [Google Scholar]
- 9.Yeomans MR, Hails NJ, Nesic JS. Alcohol and the appetizer effect. Behavioural pharmacology. 1999;10(2):151–61. doi: 10.1097/00008877-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Yeomans MR, Phillips MF. Failure to reduce short-term appetite following alcohol is independent of beliefs about the presence of alcohol. Nutritional neuroscience. 2002;5(2):131–9. doi: 10.1080/10284150290019008. [DOI] [PubMed] [Google Scholar]
- 11.Doty RL. In: The Smell Identification TestTM: Administration Manual. 3rd. ed. Sensonics, editor. Haddon Heights; p. NJ1995. [Google Scholar]
- 12.Institute of Medicine (U.S.) IoMUSSCotSEoDRI. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, D.C.: Panel on Macronutrients. p. 2005. [DOI] [PubMed] [Google Scholar]
- 13.Eiler WJ, 2nd, Dzemidzic M, Case KR, Armstrong CL, Mattes RD, Cyders MA, et al. Ventral frontal satiation-mediated responses to food aromas in obese and normal-weight women. Am J Clin Nutr. 2014;99(6):1309–18. doi: 10.3945/ajcn.113.080788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the 'Labeled Magnitude Scale' for measuring sensations of taste and smell. Chem Senses. 1996;21(3):323–34. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- 15.Eiler WJ, 2nd, Dzemidzic M, Case KR, Considine RV, Kareken DA. Correlation Between Ventromedial Prefrontal Cortex Activation to Food Aromas and Cue-Driven Eating: An fMRI Study. Chemosensory Perception. 2012;5(1):27–36. doi: 10.1007/s12078-011-9112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardello AV, Schutz HG, Lesher LL, Merrill E. Development and testing of a labeled magnitude scale of perceived satiety. Appetite. 2005;44(1):1–13. doi: 10.1016/j.appet.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22(1):202–10. [PubMed] [Google Scholar]
- 18.Ramchandani VA, O'Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger J, Jr., et al. A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcohol Clin Exp Res. 1999;23(8):1320–30. [PubMed] [Google Scholar]
- 19.Bragulat V, Dzemidzic M, Bruno C, Cox CA, Talavage T, Considine RV, et al. Food- related odor probes of brain reward circuits during hunger: a pilot FMRI study. Obesity (Silver Spring) 2010;18(8):1566–71. doi: 10.1038/oby.2010.57. [DOI] [PubMed] [Google Scholar]
- 20.Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44(3):457–65. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 21.Jiang A, Kennedy DN, Baker JR, Weisskoff RM, Tootell RB, Woods RP, et al. Motion detection and correction in functional MR imaging. Hum Brain Mapp. 1995;3:224–35. [Google Scholar]
- 22.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 23.Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, et al. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high- motion data points. Hum Brain Mapp. 2014;35(5):1981–96. doi: 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, Nievelstein RA, van der Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr. 2006;83(6):1297–305. doi: 10.1093/ajcn/83.6.1297. [DOI] [PubMed] [Google Scholar]
- 26.Neto LL, Oliveira E, Correia F, Ferreira AG. The human nucleus accumbens: where is it? A stereotactic, anatomical and magnetic resonance imaging study. Neuromodulation : journal of the International Neuromodulation Society. 2008;11(1):13–22. doi: 10.1111/j.1525-1403.2007.00138.x. [DOI] [PubMed] [Google Scholar]
- 27.Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, et al. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage. 2010;50(1):267–76. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kareken DA, Dzemidzic M, Oberlin BG, Eiler WJ., 2nd A preliminary study of the human brain response to oral sucrose and its association with recent drinking. Alcohol Clin Exp Res. 2013;37(12):2058–65. doi: 10.1111/acer.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leggio L, Schwandt ML, Oot EN, Dias AA, Ramchandani VA. Fasting-induced increase in plasma ghrelin is blunted by intravenous alcohol administration: a within-subject placebo- controlled study. Psychoneuroendocrinology. 2013;38(12):3085–91. doi: 10.1016/j.psyneuen.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calissendorff J, Danielsson O, Brismar K, Rojdmark S. Inhibitory effect of alcohol on ghrelin secretion in normal man. European journal of endocrinology / European Federation of Endocrine Societies. 2005;152(5):743–7. doi: 10.1530/eje.1.01905. [DOI] [PubMed] [Google Scholar]
- 31.Calissendorff J, Danielsson O, Brismar K, Rojdmark S. Alcohol ingestion does not affect serum levels of peptide YY but decreases both total and octanoylated ghrelin levels in healthy subjects. Metabolism: clinical and experimental. 2006;55(12):1625–9. doi: 10.1016/j.metabol.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Rojdmark S, Calissendorff J, Brismar K. Alcohol ingestion decreases both diurnal and nocturnal secretion of leptin in healthy individuals. Clinical endocrinology. 2001;55(5):639–47. doi: 10.1046/j.1365-2265.2001.01401.x. [DOI] [PubMed] [Google Scholar]
- 33.Chari ST, Harder H, Teyssen S, Knodel C, Riepl RL, Singer MV. Effect of beer, yeast- fermented glucose, and ethanol on pancreatic enzyme secretion in healthy human subjects. Digestive diseases and sciences. 1996;41(6):1216–24. doi: 10.1007/BF02088240. [DOI] [PubMed] [Google Scholar]
- 34.Rojdmark S, Rydvald Y, Aquilonius A, Brismar K. Insulin-like growth factor (IGF)-1 and IGF-binding protein-1 concentrations in serum of normal subjects after alcohol ingestion: evidence for decreased IGF-1 bioavailability. Clinical endocrinology. 2000;52(3):313–8. doi: 10.1046/j.1365-2265.2000.00908.x. [DOI] [PubMed] [Google Scholar]
- 35.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annual review of psychology. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 36.Barson JR, Morganstern I, Leibowitz SF. Complementary roles of orexin and melanin- concentrating hormone in feeding behavior. International journal of endocrinology. 2013;2013:983964. doi: 10.1155/2013/983964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcohol Clin Exp Res. 2010;34(5):886–96. doi: 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morganstern I, Chang GQ, Chen YW, Barson JR, Zhiyu Y, Hoebel BG, et al. Role of melanin-concentrating hormone in the control of ethanol consumption: Region-specific effects revealed by expression and injection studies. Physiol Behav. 2010;101(4):428–37. doi: 10.1016/j.physbeh.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marxen M, Gan G, Schwarz D, Mennigen E, Pilhatsch M, Zimmermann US, et al. Acute effects of alcohol on brain perfusion monitored with arterial spin labeling magnetic resonance imaging in young adults. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(3):472–9. doi: 10.1038/jcbfm.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seifritz E, Bilecen D, Hanggi D, Haselhorst R, Radu EW, Wetzel S, et al. Effect of ethanol on BOLD response to acoustic stimulation: implications for neuropharmacological fMRI. Psychiatry research. 2000;99(1):1–13. doi: 10.1016/s0925-4927(00)00054-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.