Inflammation plays a critical role in a broad range of human diseases. The immune system must create sufficient inflammation to protect the host from infectious agents without causing hypercytokinemia and chronic inflammation that increases the risk for developing diseases ranging from autoimmunity to cancer. Many factors influence the strength and duration of inflammatory responses including genetics, environmental factors, and the microbiome1. Recruitment of circulating innate immune cells is a key step in initiating local inflammation, and the work of Liu and colleagues in this issue provides novel insights into the role of bone marrow-derived inflammatory cells in the pathogenesis of abdominal aortic aneurysms (AAA)2.
The pathogenesis of human AAA occurs over many years and involves decreased elastin deposition, increased vascular smooth muscle cell death, immune cell infiltration, atherosclerosis, and thrombi. In contrast, mouse models of AAA utilize an acute injury to generate chronic aortic inflammation, leading to aneurysm formation in the following weeks3. The elastase and CaCl2 models induce pathologies that resemble human AAA and are the preferred models to address the role of inflammatory cells and study specific gene deletions. The angiotensin-II model is restricted to Apoe −/− mice but exhibits robust thrombus and atherosclerosis pathology that is lacking from other models. Liu et al. identify an important role for bone marrow-derived monocytes and thrombospondin-1 in each of these models and in human AAA tissues.
The authors had several reasons to focus on the secreted matricellular protein thrombospondin-1. Thrombospondin-1 plays known roles in other diseases of large vessels including atherosclerosis and pulmonary arterial hypertension4, and signaling through thrombospondin-1 receptors on vascular cells controls signaling pathways that regulate vascular physiology5. Thrombospondin-1 is also known to regulate inflammatory responses in infectious diseases, cardiovascular disease, reperfusion injuries, and cancer6–8. Moreover, a recent study found that a peptide antagonist of thrombospondin-1-mediated activation of latent TGFβ1-promoted AAA progression in angiotensin-II-treated Apoe−/− mice9. Liu et al. also examined TGFβ activation but concluded that the primary role of thrombospondin-1 originates from the bone marrow.
The authors found increased thrombospondin-1 expression in the adventitia of human AAA. Thrombospondin-1 co-localized with macrophages infiltrating AAA in mice induced with elastase or calcium phosphate. Thrombospondin-1 expression was similarly induced in aneurysms of Apoe−/− mice treated with angiotensin II, establishing that increased thrombospondin-1 expression in AAA is not species- or model-dependent. They therefore employed mice lacking thrombospondin-1 (Thbs1−/−) to examine its role in development of AAA. Thbs1−/− and WT aortas showed no gross differences basally. However, Thbs1−/− mice were more resistant to developing aneurysms in two AAA models. Those Thbs1−/− mice that developed aneurysms exhibited less aortic expansion and inflammation than aneurysms in WT mice.
Aortas in the Thbs1−/− mice had fewer infiltrating macrophages after AAA induction. This phenotype is consistent with previous evidence that thrombospondin-1 promotes macrophage recruitment in mice subjected to various challenges. Thbs1−/− mice showed decreased macrophage recruitment into an excisional skin wound10. Similarly, decreased macrophage recruitment was reported in Thbs1−/− mice challenged with λ-carrageenan in an air pouch inflammation model7. Conversely, over-expression of thrombospondin-1 in tumor xenografts was associated with increased macrophage recruitment11.
Although the macrophage deficit in excisional skin wounds was associated with a deficit in production of the macrophage chemotactic factor MCP110, the present authors established that the lack of macrophages after AAA induction in Thbs1−/− mice results from a defect in macrophage migration rather than a decrease in proinflammatory cytokines or chemokines. Although MCP1 levels were decreased in the adventitia of challenged Thbs1−/− mice, this was attributed to deficit in macrophages that produce this chemokine because MCP1 levels in the tunica media did not differ. Likewise, TGFβ levels and activity assessed by Smad3 phosphorylation did not differ in aortas of treated Thbs1−/− and WT mice. This excluded thrombospondin-1-mediated activation of latent TGFβ as the primary cause for the diminished macrophage recruitment in Thbs1−/− mice.
An important insight provided by Liu and colleagues is that the effects of thrombospondin-1 on monocyte recruitment are cell autonomous. A deficit in macrophage recruitment following thioglycollate injection into Thbs1−/− mice was reproduced when Thbs1−/− macrophages were transferred into WT mice. Correspondingly, transplantation of Thbs1−/− bone marrow mononuclear cells into WT mice was sufficient to decrease AAA induction and macrophage accumulation. Conversely, adoptive transfer of WT bone marrow mononuclear cells into the Thbs1−/− mice restored aneurysm induction and macrophage recruitment close to WT levels. These striking results indicate that macrophages are a major source of thrombospondin-1 in AAA and that thrombospondin-1 cell-autonomously promotes macrophage recruitment.
Because thrombospondin-1-mediated TGFβ activation and cytokine induction had been excluded, the authors considered additional pathways through which thrombospondin-1 could support macrophage recruitment. Chemotaxis and cell adhesion to the extracellular matrix are critical for macrophage migration and were used as endpoints for in vitro experiments. Thrombospondin-1 directly promotes chemotaxis and haptotaxis of peripheral blood monocytes12. Thrombospondin-1 can promote leukocyte adhesion directly by engaging cell surface integrins and indirectly by engaging its receptor CD478. Consistent with their in vivo data, Thbs1−/− macrophages displayed defects in migration and adhesion. Acute knockdown of thrombospondin-1 in WT cells using siRNA decreased chemotaxis towards platelet-derived growth factor and MCP1 and decreased macrophage adhesion on fibronectin and MCP1-induced phosphorylation of focal adhesion kinase. Conversely, addition of recombinant thrombospondin-1 restored Thbs1−/− macrophage migration in a MCP1 gradient and adhesion to immobilized fibronectin.
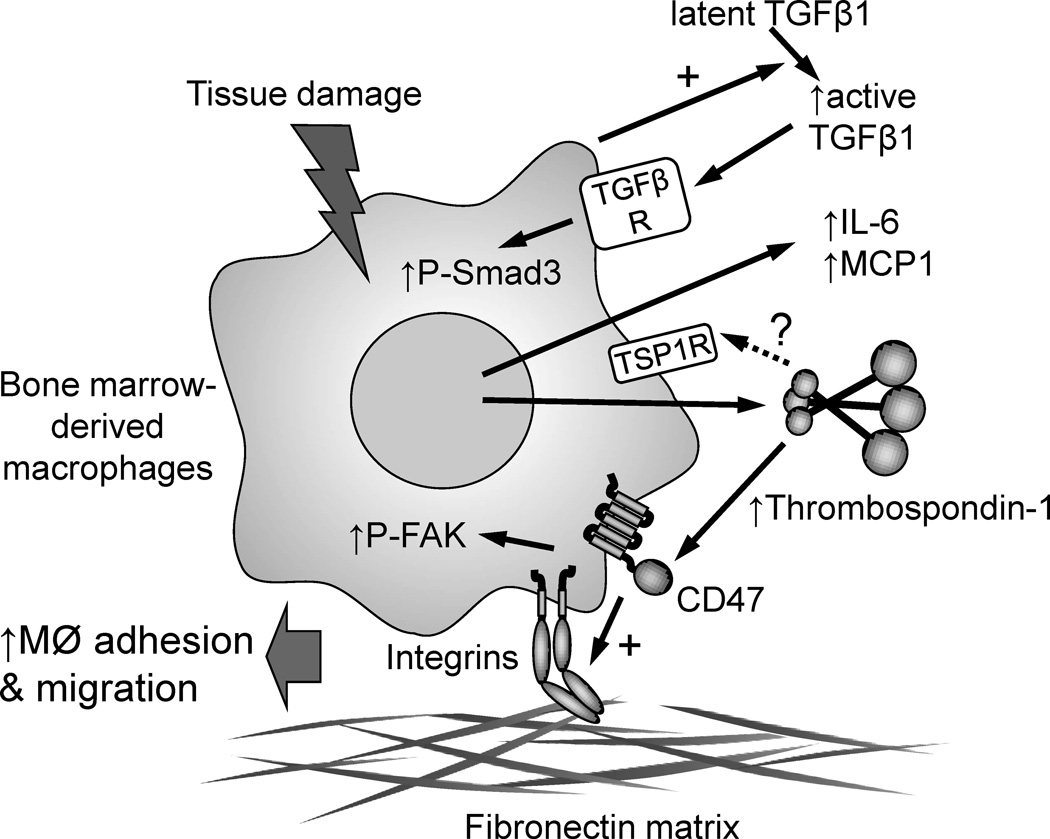
Several truncated recombinant forms of thrombospondin-1 were tested to map the functional domain. Of these, only a trimeric C-terminal construct (DelN) fully restored adhesion. A slightly shorter monomeric construct showed partial activity, whereas a trimeric N-terminal construct was inactive. Thus, direct integrin engagement by the N-module of thrombospondin-1 can be excluded, but DelN still contains binding sites for several thrombospondin-1 receptors. The authors focused their attention on CD47. CD47-binding peptides have been identified in the C-terminal domain, and a lysine-modified derivative (4N1K) of the thrombospondin-1 sequence 1016RFYVVMWK1024 was tested along with a control peptide in which the valines were replaced by glycines (4NGG). The 4N1K peptide at 50 μM restored adhesion but did not restore chemotaxis, whereas the control peptide 4NGG was inactive. Thus, the authors propose that macrophages release thrombospondin-1 into the inflammatory milieu of an aortic injury, which acts as an adhesive and motility factor by engaging CD47 to further stimulate macrophage recruitment and inflammation (Fig. 1).
Fig. 1.
Proposed role of thrombospondin-1 in promoting abdominal aortic aneurysms. Bone marrow-derived macrophages release thrombospondin-1, inflammatory cytokines, and activate latent TGFβ when exposed to tissue damage in an early aneurysm. Liu et al. show that the increased TGFβ activity and inflammatory cytokine responses are independent of thrombospondin-1, but thrombospondin-1 expression is necessary for macrophage adhesion and macrophage chemotaxis promoted by MCP1. This may be mediated by signaling through the thrombospondin-1 receptor CD47, which activates integrins to mediate macrophage adhesion to extracellular matrix and phosphorylation of focal adhesion kinase (FAK). Additional undefined thrombospondin-1 receptors may also be involved.
Although the data presented clearly document a role for macrophage-derived thrombospondin-1 in AAA, the evidence supporting a role for CD47 remains preliminary. Three independent groups have established that the peptide 4N1K has activities that are independent of CD478. Furthermore, the control peptide 4NGG lacks some documented CD47-independent activities of 4N1K and so is not an informative control. In the context of the adhesion data, 4N1K can activate integrins in a CD47-independent manner8. Therefore, additional studies are needed to confirm or exclude a role for CD47. In addition, thrombospondin-1 binds directly to fibronectin, and some fibronectin binding sites are present in DelN, so additional adhesive proteins should be tested to determine whether thrombospondin-1 can enhance macrophage adhesion on integrin substrates that are not thrombospondin-1 ligands.
These interesting results raise a number of additional questions. Transfer of WT bone marrow into Thbs1−/− mice did not completely correct the AAA phenotype. Thus, the effects of thrombospondin-1 on development of AAA may involve both the macrophage-autonomous mechanism and indirect functions of thrombospondin-1. Could thrombospondin-1 released by BM-derived monocytes alter the severity of aneurysms by altering blood pressure? Thbs1−/− mice have a reduced pulse pressure13. Therefore, any challenge that induces an aneurysm might yield a milder injury because the hydrodynamic stress on the aorta is less in the Thbs1−/− mice. Extrapolating to the bone marrow transplantation models, could thrombospondin-1 in circulation or produced in the aorta wall by bone marrow-derived monocytes alter central blood pressure or local contractility of the aorta wall? This could be assessed using telemetry in the chimeric mice and would establish whether bone marrow-derived cells are a major source of thrombospondin-1 in the arterial wall that regulates vessel tone.
These results could have clinical implications for prevention or treatment of AAA. The present study and that of Krishna et al9 suggest that agents targeting thrombospondin-1 could improve the pathophysiology of AAA. In this context it is important to note that thrombospondin-1 expression is elevated by major risk factors for AAA including diabetes, obesity, and hypertension4, 14, 15. Potential therapeutics could either inhibit the expression of thrombospondin-1 in these patients or block its interactions with CD47 or other required receptors.
Supplementary Material
Acknowledgments
Sources of funding
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- 1.Bornigen D, Morgan XC, Franzosa EA, Ren B, Xavier RJ, Garrett WS, Huttenhower C. Functional profiling of the gut microbiome in disease-associated inflammation. Genome Med. 2013;5:65. doi: 10.1186/gm469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Z, Morgan S, Ren J, Wang Q, Annis DS, Mosher DF, Zhang J, Sorenson CM, Sheibani N, Liu B. Thrombospondin-1 (TSP1) contributes to the development of vascular inflammation by regulating monocytic cell motility in mouse models of abdominal aortic aneurysm. Circ Res. 2015;xx:xxx. doi: 10.1161/CIRCRESAHA.117.305262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaer RA, DeRubertis BG, Hynecek R, Kent KC, Faries PL. Models of abdominal aortic aneurysm: characterization and clinical applications. Vascular. 2006;14:343–352. doi: 10.2310/6670.2006.00059. [DOI] [PubMed] [Google Scholar]
- 4.Rogers NM, Sharifi-Sanjani M, Csanyi G, Pagano PJ, Isenberg JS. Thrombospondin-1 and CD47 regulation of cardiac, pulmonary and vascular responses in health and disease. Matrix Biol. 2014;37:92–101. doi: 10.1016/j.matbio.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMaken S, Exline MC, Mehta P, Piper M, Wang Y, Fischer SN, Newland CA, Schrader CA, Balser SR, Sarkar A, Baran CP, Marsh CB, Cook CH, Phillips GS, Ali NA. Thrombospondin-1 contributes to mortality in murine sepsis through effects on innate immunity. PLoS One. 2011;6:e19654. doi: 10.1371/journal.pone.0019654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Manso G, Navarathna DH, Galli S, Soto-Pantoja DR, Kuznetsova SA, Tsokos M, Roberts DD. Endogenous thrombospondin-1 regulates leukocyte recruitment and activation and accelerates death from systemic candidiasis. PLoS One. 2012;7:e48775. doi: 10.1371/journal.pone.0048775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soto-Pantoja DR, Kaur S, Roberts DD. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol. 2015:1–19. doi: 10.3109/10409238.2015.1014024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishna SM, Seto SW, Jose RJ, Biros E, Moran CS, Wang Y, Clancy P, Golledge J. A peptide antagonist of thrombospondin-1 promotes abdominal aortic aneurysm progression in the angiotensin II-infused apolipoprotein-E-deficient mouse. Arterioscler Thromb Vasc Biol. 2015;35:389–398. doi: 10.1161/ATVBAHA.114.304732. [DOI] [PubMed] [Google Scholar]
- 10.Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161:831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin-Manso G, Galli S, Ridnour LA, Tsokos M, Wink DA, Roberts DD. Thrombospondin-1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity by differentiated U937 cells. Cancer Res. 2008;68:7090–7099. doi: 10.1158/0008-5472.CAN-08-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansfield PJ, Suchard SJ. Thrombospondin promotes chemotaxis and haptotaxis of human peripheral blood monocytes. J Immunol. 1994;153:4219–4229. [PubMed] [Google Scholar]
- 13.Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009;28:110–119. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Tong X, Rumala C, Clemons K, Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS One. 2011;6:e26656. doi: 10.1371/journal.pone.0026656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong P, Cavalera M, Frangogiannis NG. The role of thrombospondin (TSP)-1 in obesity and diabetes. Adipocyte. 2014;3:81–84. doi: 10.4161/adip.26990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.