Abstract
Substantial evidence has established the value of high levels of physical activity (PA), exercise training (ET), and overall cardiorespiratory fitness (CRF) in the prevention and treatment of cardiovascular diseases (CVD). This paper reviews some basics of exercise physiology and the acute and chronic responses of ET, as well as the impact of PA and CRF on CVD. This review also surveys data from epidemiologic and ET studies in the primary and secondary prevention of CVD, particularly coronary heart disease (CHD) and heart failure (HF). These data strongly support the routine prescription of ET to all patients and referrals for patients with CVD, especially CHD and HF, to specific cardiac rehabilitation and ET programs.
Keywords: Exercise, Fitness, Cardiovascular Disease, Cardiac Rehabilitation
Despite the fact that the American Heart Association (AHA) has established sedentary lifestyle as a major modifiable risk factor for cardiovascular (CV) diseases (CVD), a sizable percentage of the United States population has very low levels of physical activity (PA).1–3 Many organizations, including the AHA and the American College of Sports Medicine, have recommended increasing PA or aerobic exercise training (ET) to increase levels of cardiorespiratory fitness (CRF) in the general population, including individuals with CVD.1–3
In this manuscript, we review the physiology of ET and the acute and chronic adaptation, including the, interaction of PA, ET and CRF on overall CVD risk, Additionally we discuss the relative value of PA versus CRF, as well as the importance of CRF in obesity in the “fitness vs fatness” debate. We also review the role of formal cardiac rehabilitation and ET (CRET) programs on coronary heart disease (CHD) risk factors and morbidity and mortality in patients with CVD, including CHD and heart failure (HF). Guidance for EET dosing, as well as the potential toxicity of extremely high doses of ET is reviewed. Finally, we provide recommendations for the routine ET prescription.
Cardiac Exercise Physiology: The Acute Response and Chronic Adaptations to Aerobic Exertion
An appropriate response to an acute aerobic exercise stimulus requires robust and integrated physiologic augmentation from the pulmonary, respiratory, skeletal muscle and CV systems. Age, sex and genetic predispositions influence the physiological response and therefore performance during aerobic exertion. For example, with respect to maximal aerobic capacity, genetic components have been estimated to account for 20–40% of the variability, age causes a progressive decline, and female values are on average 25% lower than males.4 However, in apparently healthy individuals, irrespective of non-modifiable factors, chronic, repetitive bouts of aerobic ET lead to significant improvements in physiologic function and therefore performance. While all systems (i.e., pulmonary, respiratory, skeletal muscle and CV) involved in orchestrating an appropriate response to aerobic exercise are important, the CV system, in particular cardiac systolic and diastolic function, may be thought of as the central hub. This section will provide a concise review on CV exercise physiology as it relates to both an acute response, focusing on the central response, and chronic adaptations, addressing both central and peripheral responses to aerobic ET. The healthy adult model across the lifespan will be highlighted with some discussion pertaining to the impact of CV dysfunction/CVD. Lastly, our emerging understanding of the potential adverse consequences of chronic aerobic ET at high volumes and intensities will be addressed.
Cardiac Response to Acute Aerobic Exercise: Focus on Augmenting Cardiac Output
Augmentation of cardiac output (CO) is the central determinant of maximal oxygen consumption (VO2), as defined by the Fick equation: VO2 = CO * a-vO2diff; where a-vO2 difference is the arteriovenous oxygen difference. At rest, CO is homogenous at ~5 L/min. However, at maximal exercise, CO varies greatly, from ~20 L/min in apparently healthy untrained individuals to ~40 L/min in elite aerobic athletes.4,5 This wide variability in CO in part explains the wide range in maximal VO2, with normal values ranging from ~35 to 85 mlO2•kg−1•min−1.6 CO is the product of stroke volume (SV) and heart rate (HR), and both significantly increase during aerobic exercise. Left ventricular (LV) SV, commonly the heart chamber focal point of discussion with respect to CV exercise physiology, is augmented during aerobic exertion by a synergistic increase in end diastolic volume (i.e., preload) and myocardial contractility.7 While resting SV is ~50 ml, the increases in filling volume and contractility raise SV several fold during exercise, with large variability that is influenced by age, sex, genetics and ET status. For example, the SV at maximal exercise for two 20 year old males, both with a maximal HR of 200 bpm, with maximal CO of 20 and 35 L/min, respectively, will have maximal SVs of 100 ml and 175 ml, respectively. The increase in SV during exercise plateaus at ≈50% of maximal VO2.4,8 Once SV plateaus at ≈50% of maximal VO2, it is the continued linear rise in HR that drives the further increases CO. During a bout of progressive aerobic exercise to maximal capacity, HR rises in a linear fashion at a rate of ≈10 bpm per 3.5 mlO2•kg−1•min−1 increase in oxygen demand.6 Maximal HR during an aerobic exercise test is still commonly estimated using the 220-age equation, although considerable variability in this estimation exists (i.e., standard deviation of ±12 bpm).6 At maximal exercise, a high HR has the potential to decrease LV ventricular filling time possibly resulting in a reduced CO.
Cardiac Adaptations to Chronic Aerobic ET: Mechanisms for Increased CO
Participation in a chronic aerobic ET program produces a host of positive morphologic and physiologic CV adaptations in apparently healthy individuals, irrespective of age and sex.8–13 Commonly reported morphologic adaptations associated with chronic aerobic ET is LV dilation (i.e., increased end-diastolic diameter) and hypertrophy (i.e., increased wall thickness), referred to as ET-induced cardiac remodeling. These morphologic LV adaptations parallel enhanced physiologic function during exercise through: 1) Increased early-diastolic filling secondary to a combination of increased preload and increased myocardial relaxation;10 and 2) Increased contractile strength as captured by advanced imaging techniques, such as tissue Doppler and speckle-tracking imaging.9 While much focus has been directed toward the LV, it is important to note that morphologic adaptations also occur in the right ventricle (RV) that appear to mirror LV adaptations.10 The magnitude of ET-induced cardiac adaptations in apparently healthy individuals is influenced by the interplay of several factors, including age, sex, genetics, prior training status, mode of ET and ET volume. As such, an accurate prediction of the degree of cardiac adaptations expected with a given aerobic ET program for a given individual is not feasible. Suffice to say, aerobic ET, performed within general ET prescription parameters,6 positively alters cardiac morphology and physiologic performance. These adaptations lead to increased CO during exercise, facilitating a significantly higher maximal VO2 post-training. Moreover, the declines in cardiac function and, therefore, aerobic performance associated with aging are significantly attenuated by participation in an ET program across the lifespan.14 However, morphologic changes are less pronounced in patients with CVD, which is an important distinction between young, healthy individuals, who adapt readily in terms of central adaptation, and those who have existing CVD and the elderly.
The Impact of Aerobic ET on the Vasculature
Repetitive bouts of aerobic ET results in a number of favorable vascular adaptations as well, significantly attenuating deleterious adaptations precipitated by the aging process.15 Measures of arterial stiffness are significantly lower in individuals with a higher aerobic capacity (i.e., cross-sectional analysis) 15,16 as well as individuals who have recently completed an aerobic ET program (i.e., longitudinal analysis). 17 Protection against systemic oxidative stress and inflammation induced by chronic aerobic ET are posited to be primary mechanisms for the observed reductions in arterial stiffness.17 Enhanced endothelium-dependent vasodilation through increased production of nitric oxide (NO) is also a clear aerobic ET benefit,18–20 including in the coronary circulation. 21 When aerobic ET involves large muscle groups (e.g., treadmill training or lower extremity ergometry), systemic vascular benefits are realized. Aerobic ET also improves endothelium-dependent vasodilation in the coronary microcirculation, again through increased production of NO.22,23
Cardiac Disease/Dysfunction: Altered Cardiac Physiology = Diminished Aerobic Performance
Given the clear and central role normal cardiac function plays in defining maximal aerobic capacity, disease or dysfunction that detrimentally impacts CO will also compromise maximal VO2.5, 6 In fact, while aerobic ET is clearly safe and effective in improving functional capacity in a number of patient populations diagnosed with cardiac conditions,6 as discussed in more detail in this review, maximal aerobic capacity is unlikely to normalize if impaired cardiac physiology persists. This is readily apparent in training studies in patients diagnosed with HF, where post-aerobic ET maximal VO2 values, although significantly improved compared to pre-ET, commonly do not greatly exceed 20 mlO2•kg−1•min−1, which is well below age- and sex-predicted normative values.24 This is not to suggest that ET is not highly beneficial in patient populations, such as HF, in fact the converse is true and CRET is considered a standard of care for individuals with HF. 25 However, without normalization of cardiac physiology and, therefore, CO, true normalization of maximal aerobic capacity is not possible.
Impact of PA and CVD
Data from numerous epidemiological studies demonstrate that low levels of PA are associated with higher prevalence of most CVD risk factors, including hypertension (HTN), obesity, dyslipidemia, metabolic syndrome (MetS), depression, and type 2 diabetes (T2D).3,26–29 Additionally, substantial data demonstrate a strong inverse relationship between PA levels and all-cause and CVD mortality.3,30–33 Several studies, mostly from Finnish cohorts, suggest that low levels of occupational PA may have an independent contribution to overall CVD.3,32,34,35 High levels of PA have also been demonstrated to reduce CVD mortality risk in high risk populations, including those with T2D and the elderly.3,32 In obesity, the consensus among studies is that high levels of PA attenuate, but do not completely eliminate, the increased CV mortality risk associated with obesity.3,31,36,37 Additionally, increases in PA levels over time have been associated with reduced CHD and CVD mortality risk.3,38–40
CRF and CVD Risk
As discussed above for PA, a low level of CRF is a well-recognized risk factor for CHD and CVD mortality,1–3,41,42 and although PA is probably the most important factor determining CRF along with non-PA inherited factors,43 most studies demonstrate that CRF is a more potent predictor of prognosis than is PA, at least as determined by self-report questionnaires.3,44,45 The potential benefits of CRF are numerous and are summarized in Table 1. Typically, CRF is expressed in metabolic equivalents or METs, which are typically estimated from workload on submaximal or maximal treadmill exercise stress tests (based on speed and incline), and this can be more precisely assessed by using cardiopulmonary exercise testing (CPX) and assessing peak VO2 as well as a host of other parameters (e.g., anaerobic or lactate threshold).46
Table 1.
Potential Benefits of Cardiorespiratory Fitness on Prognosis.
| Physiological Benefits | |
| Reduced blood pressure | Improved insulin sensitivity |
| Improved heart rate variability | Decreased myocardial oxygen demands |
| Increased myocardial infarction | Maintain lean mass |
| Improved endothelial function | Reduced visceral adiposity |
| Reduced blood and plasma viscosity | Increased capillary density |
| Increased mitochondrial density | Improved mood and psychological stress |
| Reduced systemic inflammation | Improved sleep |
| Reduced Risk of Developing: | |
| Hypertension | Osteoporosis |
| Depression | Osteoarthritis |
| Metabolic Syndrome | Dementia and Alzheimer’s Disease |
| Diabetes Mellitus | Breast, colon, and other cancers |
High levels of CRF, like higher PA, are associated with reduced prevalence of many CHD and CVD risk factors, including HTN, obesity, MetS, and T2D.3,44,45 More importantly, considerable data has demonstrated the powerful prognostic impact of CRF, including in the general population, patients at high risk of CVD, as well as in CVD populations, such as CHD and HF.1,3,41–45
A recent high-profile meta-analysis by Kodama et al47 observed that a 1 MET increase in CRF was associated with 13% and 15% reductions, respectively, in all-cause and CHD/CVD mortality. Additionally, this meta-analysis defined age- and gender-specific normal levels of CRF associated with lower event rates in both men (40 years: 9 METs; 50 years: 8 METs; at 60 years: 7 METs) and women (40 years: 7 METs; 50 years: 6 METs; 60 years: 5 METs).
Even in high-risk individuals with MetS, pre-diabetes or T2D, high levels of CRF are associated with good prognosis, typically better than the prognosis in unfit individuals without these conditions.3,47 Berry and colleagues48 have demonstrated the importance of high CRF to protect against lifetime CVD risk, as these authors found that those with a high burden of traditional CVD risk factors but a high level of CRF had lifetime CVD mortality rates that were similar or lower than those with a low burden of traditional CVD risk factors, suggesting the importance of CRF in those with otherwise high CVD risk.
Several studies have also focused on changes in CRF over time and the impact on CVD morbidity and mortality.3 Blair and colleagues,49 using data from the Aerobics Center Longitudinal Study (ACLS; n=9,777), reported that men classified as unfit (i.e., bottom 20th percentile of CRF based on age and gender of the entire ACLS population) at their first examination but fit at their second examination several years later had a 52% reduction in CVD mortality compared to men classified as unfit on both examinations. Lee et al,50 also using the ACLS data (n=14,345), evaluated the long-term (mean follow-up 11.4 years) effects of changes in CRF on CVD mortality and observed significant reductions in CVD mortality of 27% and 42%, respectively, in those who had either no change or improvements in CRF at their second examination on average 6.3 years later. For every 1 MET increase in CRF over time, all-cause and CVD-mortality were reduced by 15% and 19%, respectively. Moreover, in the fitness vs. fatness debate, discussed below, these improvements persisted after adjusting for changes in body mass index (BMI). Others have also reported favorable impact of CRF changes over time and subsequent mortality.3,51
CRF vs BMI
Several authors of this review and others have evaluated the independent effects of CRF and adiposity on subsequent CVD mortality, and considerable evidence suggest that high levels of CRF eliminate or significantly attenuate the CVD mortality risk in overweight and obese individuals, which has been demonstrated in the general population, in those with dyslipidemia, and in T2D.3, 41, 42, 44, 52 Therefore, CRF appears to markedly alter the relationship between adiposity and subsequent prognosis.
Barry and colleagues42 recently performed a meta-analysis of 10 major studies and quantified the combined impact of CRF and obesity on mortality. They demonstrated that compared to normal weight and fit individuals, unfit individuals had double the mortality regardless of BMI, whereas an obese but fit individual had similar survival compared with normal weight individuals. In a study from the ACLS of 3,148 healthy adults, changes over time in both body fatness and CRF predicted the development of HTN, MetS, and dyslipidemia, but changes in CRF were superior to increases in body fatness for predicting future risk of these disorders.53 As reviewed earlier, after adjustment for changes in CRF, BMI changes over time no longer were associated with CVD or all-cause mortality.50 Therefore, the constellation of these data indicate that CRF is more important than obesity regarding long-term prognosis.
We have also addressed the impact of CRF to alter the relationship between obesity status and subsequent prognosis in the obesity paradox that has been described in patients with CVD,52 particularly CHD,54 and HF.55 Although obesity adversely impacts most CVD risk factors and increases the risk of most CVD, considerable evidence during the past 15 years has indicated an obesity paradox, where the overweight and obese with most CVD seem to have a better outcome than do their leaner counterparts with the same CVD, as has been reviewed in detail elsewhere.52, 54, 55 In a study of 9,563 patients with CHD, only those in the bottom tertile of age- and gender-related CRF demonstrated an obesity paradox, with leaner patients by BMI, body fat percentage, and even waist circumference or central obesity had a higher all-cause and CVD mortality than did heavier patients who were unfit.54 On the other hand, those CHD patients who were more fit had a favorable prognosis, regardless of their level of adiposity. Likewise, in 2,066 patients with systolic HF who had CRF assessed by CPX, the HF patients with low CRF (i.e., peak VO2 < 14 mlO2•kg−1•min−1) had a poor prognosis and survival was related with BMI, showing a typical “obesity paradox” where the best survival occurred in obese with BMI ≥ 30 kg/m2, worst survival with BMI 18.5–24.9 kg/m2, and intermediate survival in the overweight BMI.55 As demonstrated in patients with CHD, HF patients with more preserved CRF (i.e, peak VO2 > mlO2•kg−1•min−1) had a good survival, regardless of BMI, and no obesity paradox was evident. Therefore, these data indicate that CRF also markedly impacts the obesity paradox.
Impact of ET on CRF and CVD Risk Factors
Although genetic hereditability is a determinant of CRF,43 the most important contribution to CRF is PA and ET. Many studies indicate significant improvements in CRF associated with moderate aerobic ET, but more vigorous ET seems to confer equal or enhanced health and CVD benefits, as well as greater improvements in CRF.3,56
Additionally, ET impacts many of the standard CVD risk factors, including plasma lipids, especially high-density lipoprotein cholesterol (HDL-C),57–59 adposity,60,61 fasting glucose levels and T2D control,62,63 and blood pressure lowering and HTN control;64 however, for all of these parameters, the effect of ET may be statistically significant but the impact is overall quite modest, often < 3–5%.
Benefits of ET and Secondary CVD Prevention
Several observational studies, as well as randomized control trials (RCTs) have established the benefits of PA and ET in cohorts with CVD, including CHD and HF.3 Perhaps the most impressive evidence of the benefits of ET is in formal CRET programs of patients following major CHD events (Table 2).3
Table 2.
Benefits of Formal Cardiac Rehabilitation and Exercise Training Programs
Improvement in exercise capacity
|
Improvement in lipid profiles
|
| Reduction in inflammation (hs-CRP −40%) |
Reduction in indices of obesity
|
Improvements in behavioral characteristics
|
| Improvements in quality of life and components |
Improvement in autonomic tone
|
| H. Improvements in blood rheology |
| I. Reduction in hospitalization costs |
| J. Reduction in major morbidity and mortality |
BMI = body mass index; hs-CRP = high sensitivity C-reactive protein; HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol.
Adapted from Swift DL et al. 3
Impact of CRET on Obesity
Considering the high prevalence of overweight and obesity in society and in particular in patients with CHD, there are potential benefits of formal CRET in the promotion of weight loss and weight maintenance.61 Despite the extensive data on the obesity paradox in CVD, including CHD, briefly discussed above,55 support still exists for the benefits of at least purposeful (as opposed to non-purposeful) weight loss in patients with CHD.65
In patients with CHD, CRET has produced an impressive 37% reduction in the prevalence of MetS.66 Moreover, in patients who successfully lost weight in CRET programs (e.g., > 5% or mean 10%), statistically greater improvements in CRF and plasma lipids were noted compared with those who did not lose weight.67 In a study of 377 patients from the Mayo Clinic, weight loss was associated with reductions in total mortality plus major CVD events, even among CHD patients with BMI < 25 kg/m2, as well as in those with higher BMI.68 Ades and colleagues61,69 demonstrated that greater weight loss with CRET occurred utilizing a high-calorie-expenditure program, in the range of 3,000–3,500 kcal/wk, which was associated with reductions in insulin resistance, improvements in HDL-C, and triglycerides (TGs), as well as reduced blood pressure and plasminogen activator inhibitor I. In a recent large meta-analysis in patients with CHD, weight loss was associated with a 30% increase in major CVD endpoints; however, this was due to observational weight loss in 10 cohorts who had a 62% increase in major events, compared with presumed intentional weight loss in 4 cohorts, who had a 33% reduction in major events.65 Therefore, the magnitude of data still suggest benefits of purposeful weight loss, ideally through the synergistic implementation of ET and a healthy, calorically appropriate, diet, during CRET.
Impact of CRET on Lipids and Inflammation
Although improvements in low-density lipoprotein cholesterol with CRET are minimal, improvements in HDL-C and TGs are more substantial (mean changes ≈+6% and −15%, respectively), with relatively greater improvements in those with remarkably abnormal baseline values.3,70,71
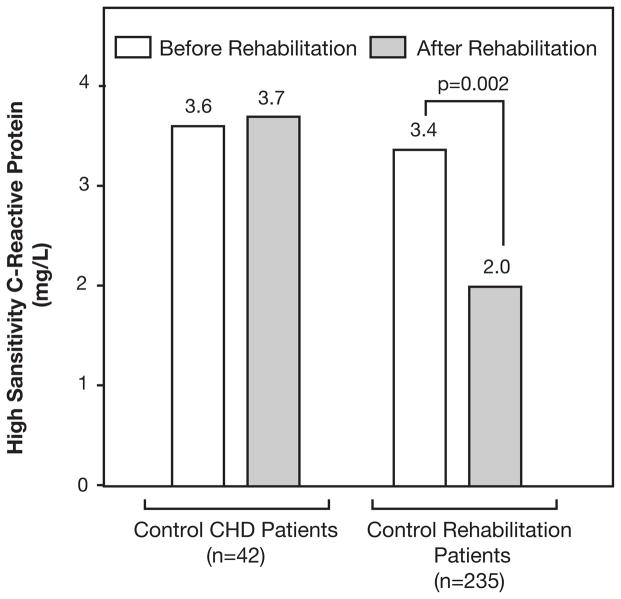
We have recently reviewed the potential of PA and ET to improve levels of high-sensitivity C-reactive protein (hs-CRP).72 In patients with CHD, hs-CRP fell by approximately 40% in those who completed CRET, with no improvement noted in control CHD patients who did not attend CRET (Figure 1).73 Patients with MetS had nearly two-fold higher levels of hs-CRP compared to those without MetS, and both groups received substantial improvements in hs-CRP following CRET.66 Whereas lean CHD patients as well as obese CHD patients who did not lose weight had only minor improvements in hs-CRP following CRET, obese patients with weight loss had marked reductions in hs-CRP.74
Figure 1.
Median changes in high-sensitivity C-reactive protein (CRP) in control patients with CHD and cardiac rehabilitation patients (data adapted from Milani RV et al, J Am Coll Cardiol 2004.73
Effects of CRET on Psychological Risk Factors
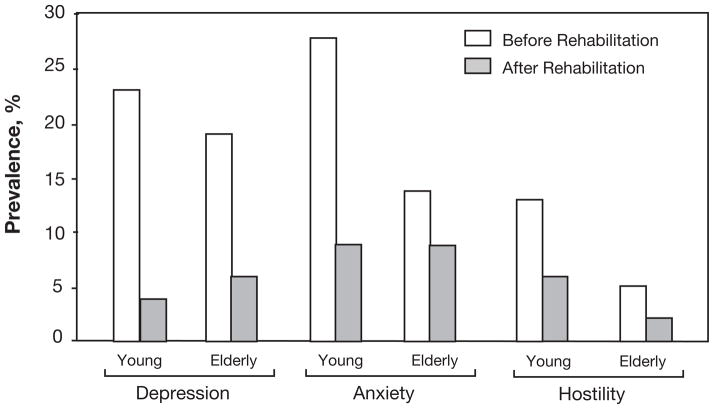
One of the most important effects of CRET may be in the area of psychological stress, including levels of depression, anxiety, hostility, and total PS.75 Patients with CHD have a high prevalence of psychological stress, with marked benefits following formal CRET (Figure 2).76 Additionally, CHD patients with depression who completed CRET had 70% reductions in three-year mortality (8% vs 30%; p<0.0001) compared to a control group of depressed CHD patients who did not attend CRET.77 Since CRET involves other aspects of therapeutic lifestyle changes besides ET, we divided patients into those who did not improve peak VO2, those who had mild improvements in peak VO2 (≤10%), and in those with more marked improvements (>10%) and demonstrated that improvements in depression and depression-related increased mortality only occurred in those who improved CRF, although the improvements were similar in those who had mild and more marked improvements in peak VO2.77,78
Figure 2.
Impact of formal cardiac rehabilitation and exercise training programs on prevalence of adverse psychological stress parameters (depression, anxiety, and hostility) in younger and older patients with CHD (data adapted from Lavie CJ et al, Arch Intern Med 2006.76
Impact of CRET on CRF
Probably the most important improvement in CRET is on CRF, which has recently been reviewed in detail.1 In a review of over 18,000 CHD patients in 9 studies, where CRF was assessed by several methods (estimated METs, peak VO2, walking distance, and 6-minute walk test), improvements in CRF were strongly associated with reductions in all-cause and CVD mortality.1 For patients with stable CHD, every 1 MET increase in CRF was associated with an 8%–35% (median 16%) reduction in mortality. Considering the typical 15% improvement in peak VO2 and 35% increase in estimated METs following CRET, these benefits translate into substantial reductions in subsequent mortality.
Effects of CRET on Morbidity and Mortality
One of the first major meta-analyses of CRET programs by O’Connor and colleagues79 in 1989 included 22 RCTs in 4,551 CHD patients who were post-myocardial infarction (MI) and demonstrated reductions in total and CVD mortality of 20% and 25%, respectively, at 3-year follow up after CRET. A more recent meta-analysis of 8,440 participants in 32 RCTs demonstrated a 31% reduction in CVD mortality following CRET.80 Although most of the benefits from CRET in CHD have been described in post-MI patients, a recent study from Olmsted County Registry in 2,395 post-percutaneous coronary intervention patients demonstrated a 45% reduction in all-cause mortality during a 6-year follow up in those attending CRET.81 In another study from the Olmsted County Registry that included 846 patients who underwent coronary artery bypass grafting, all-cause mortality was reduced by 46% during 10-year follow up in those who attended CRET.82 The benefits of CRET on mortality have also recently been noted in a large cohort of elderly Medicare beneficiaries.83,84
Benefits of ET in HF
The potential benefits of PA, ET, and increased CRF in the prevention and treatment of HF has recently been reviewed.85,86 Berry et al87 showed that although higher CRF is associated with a 10% lower risk of MI in men (and only non-significant 3% lower risk in women), each 1 MET increase in CRF was associated with a 20% reduction in HF risk. Pandey et al88 recently assessed changes in midlife CRF over time, demonstrating that every 1 MET improvement in midlife CRF was associated with a 17% lower risk of developing HF later in life.
In addition, substantial evidence indicates that PA, ET, and CRF markedly impact the prognosis of patients with established HF.85 Most ET studies in HF have demonstrated 15%–17% improvements in peak VO2, which have translated into reductions in hospitalization and mortality of 28%–35%.85,89–91 Probably the most well-known study of ET in HF is the recent HF- ACTION Trial, which assessed 2,333 patients with Class II-IV systolic HF.92 Although this trial hypothesized a 10%–15% improvement in peak VO2 with ET, only a 4% average improvement was noted, reflective of the relatively low adherence of ET in the intervention group, which unfortunately was due to the fact that only 30% exercised to their target training level minutes per week. After a median 30-month follow up, a non-significant 7% reduction was noted in the combined end-point of hospitalization and all-cause mortality; however, after adjustment for pre-described mortality predictors in HF, the primary end-point was significantly lowered in the ET group by 11%. Additionally, there was a very close relationship between ET volume and clinical prognosis, with a 30% reduction in the primary end-point among subgroups who achieved their ET prescription.85,86,93
Based on the considerable body of evidence, the recent American College of Cardiology Foundation/AHA Guidelines for HF recognized ET at a Class I level94 and the Center of Medicare and Medicaid Service recently approved formal CRET programs for patients with systolic HF.85,86
ET in HFpEF
Although the majority of the early ET studies in HF concentrated on patients with significant systolic dysfunction, approximately 50% of HF patients have HFpEF, which is particularly common in older patients with HF and in women.95–97 Recently, Edelmann and colleagues98 assessed the impact of structured ET, including supervised endurance ET/resistance programs, on exercise capacity (peak VO2), LV diastolic function and quality of life (QoL) in 64 patients with HFpEF compared with 44 patients who received usual care, demonstrating similar improvements with ET in HFpEF as noted in most of the smaller ET studies in systolic HF patients, with improvements in peak VO2 of over 16% following ET. Additionally, ET resulted in improvements in diastolic function, as determined by E/e′ and LA volume indices, and improvements in the physical dimensions QoL component.
Although there is no data on ET on mortality in patients with HFpEF, the study by Edelmann and colleagues98 and others97 has established proof-of-concept for the potential benefits of ET not only for HF or systolic dysfunction but also for the full spectrum of HF, including those with HFpEF. Considering the cost of HF on society, particularly the impact of HFpEF in old HF patients and in women,95–97 future large studies are needed to assess the impact of ET in various ET modalities (including resistance training and high-intensity interval training or (HIIT) on cost, QoL, and major HF morbidity and mortality.95–97,99,100
Mechanisms of ET Benefits in HF
In patients with HFpEF, as well as in patients with systolic cardiac dysfunction, the degree of exercise intolerance is not directly related to the degree of cardiac weakness but somewhat surprisingly, the symptoms of dyspnea and fatigue in HF or often directly related to abnormalities of skeletal musculature in HF,101 which has been reviewed in detail elsewhere.102
Clearly, patients with chronic HF have decreased muscle bulk compared with healthy subjects, and HF patients have a shift in muscle fiber type, from slow twitch, oxidative type I fibers to fast twitch, glycolytic type IIb fibers, which have been correlated with reduced exercise capacity, such as peak VO2.97,102 Additionally, there is evidence of a systemic inflammatory response in HF, which involves the skeletal musculature and contributes importantly to the skeletal myopathy in HF. There is also decreased capillary numbers per muscle fiber, rapid depletion of high energy phosphates and rapid decrease in muscle pH during ET, with decreased mitochondria density and oxidative enzyme content. Each of these features has been correlated with reduced exercise capacity in patients with chronic HF.102 Additionally, sympathetic nerve activation typifies many chronic diseases, such as renal failure, lung disease, as well as HF, which are all characterized by systemic inflammation and skeletal myopathy.102
Certainly, deconditioning contributes to the skeletal myopathy of HF; PA and ET reverses many of the features of skeletal myopathy, particularly the elevated sympathetic nerve activation and increase level of inflammation.95,102 Clearly, skeletal muscle functions somewhat as an endocrine organ, as skeletal muscle has been shown to produce and release cytokines (myokines), with interleukin (IL)-6 being the prototype and the first cytokine present in the circulation during ET.103 A number of studies indicate anti-inflammatory effects of ET and IL-6, which may be particularly applicable to the benefits of ET in chronic diseases, such as HF.95,102,103 Nevertheless, deconditioning is not the only mechanism for the skeletal muscle dysfunction in HF, so ET would not be expected to completely reverse the adverse effects. However, there are numerous potential effects of ET that benefit patients with HF, in addition to producing improvements in skeletal muscle function, including those with systolic dysfunction as well as HFpEF (Table 3).95 Although most of the studies of ET in HF have focused on aerobic ET, considering the skeletal muscle deficiency in quantity and function in HF, resistance ET, which improves CV risk factors and prognosis, may be particularly applicable for patients with HF.104
Table 3.
Potential Benefits of Exercise Training on Heart Failure
|
Potential Role of HIIT
Moderate-intensity continuous ET (MICT) has become part of the standard care for most patients with CVD, including for CHD and HF. Recently, however, evidence has emerged demonstrating that HIIT may be performed safely and results in improvements in functional capacity, including peak VO2 and QoL, leading some to intimate that HIIT, as opposed to the more traditional MICT, should be the preferred clinical approach to ET in patients with CVD.99,100 In fact, in studies of patients with CHD and HF, as well as in cohorts with obesity and MetS, HIIT has been typically superior to MICT for improving CRF, determined by peak VO2, and for more positive adaptations in cardiac structure and function, including hemodynamics, cardiac biomarkers, and various echocardiographic parameters.99,100 This has led some to call for a paradigm shift, which may be controversial, particularly considering the theoretical increases in adverse CVD events associated with ET at higher intensities.
We recently reviewed data in over 100 HF patients that assessed the efficacy and safety of HIIT for patients with HF.99 While the initial evidence demonstrates the benefits of HIIT in patients with CVD, including CHD and HF, is compelling, we feel that currently there is still insufficient evidence to supplant a MICT approach with HIIT. This recommendation is not based on the findings of any one study, which have generally all been positive up to this point in time, but rather the relatively small body of collective evidence demonstrating the efficacy of HIIT that is currently available. For example, in our current analysis of HIIT and HF, we were only able to analyze just over 100 subjects with HF in the HIIT arms, and there is relatively little information on long-term safety, training compliance, and no data on long-term clinical events.
Therefore, despite the theoretical benefits of HIIT in patients with CVD, including HF, we believe that further studies of long-term efficacy, safety, compliance and clinical event data is needed before this supplants MICT as the ET modality of first-choice in the prevention and treatment of CVD.99,100
Importance of ET in Muscular Fitness (MF)
Although this review mostly emphasizes the importance of aerobic ET to improve CRF and CVD prognosis, MF and muscle strength are also important, as MF has been shown to have substantial impact on CVD risk factors and prognosis.104 Moreover, MF also is a major determinant of frailty and cachexia, which is particularly important for patients with advanced HF and in the elderly.105 Frailty is defined as a biological syndrome characterized by declining overall function and loss of resistance to stresses, and this is known to be associated with increased morbidity, mortality, and healthcare utilization, especially in elderly and patients with advanced HF. In those with advanced HF, cachexia and wasting seem to be independent predictors of increased mortality. On the other hand, we and others have demonstrated an obesity paradox in many groups of patients with CVD,52,53 including CHD54 and HF,55 demonstrating the importance of maintaining higher levels of lean muscle mass,106 which are associated with greater MF and muscle strength.104 In fact, an obesity paradox has been noted with better prognosis with higher body fat in CHD54,106–109 and HF,110 possibly due to the fact that patients with higher body fat also generally have higher MF and muscle strength.111 Although generally ET increases both CRF and MF, specifically resistance training may be especially helpful to improve MF and maintain lean muscle mass in elderly patients and those with advanced HF.104
Exercise Dosing
The PA Federal Guidelines call for a minimum of 150 min/wk of moderate aerobic PA or 75 min/wk of vigorous aerobic PA; the Institute of Medicine suggests 60 minutes daily of some aerobic PA.1–3,56,112 However, recent evidence suggest that more than half of American adults still don’t meet these minimal requirements based on self-report, and only 10% of American adults meet these minimal guidelines based on objective accelerometry.1–3,113,114 Additionally, recent evidence suggest that substantial benefits are obtained with ET doses much lower than these guidelines.115–117 Clearly, efforts to have individuals who lead a completely sedentary lifestyle engage in regular PA, even if not meeting the target levels described above, is of paramount importance.
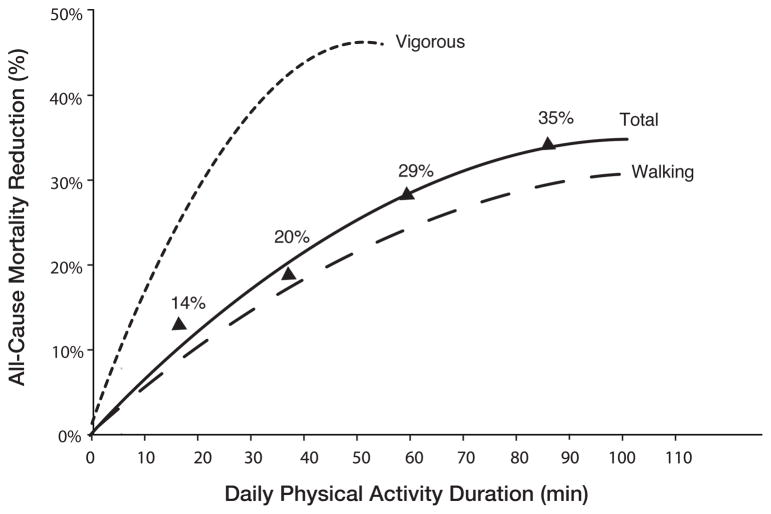
For example, in a study 416,175 individuals from Taiwan, a dose response relationship between aerobic PA and mortality is noted, with progressive reductions in mortality noted up to 90 daily minutes of moderate PA and up to approximately 40 daily minutes of vigorous PA (defined as 6.5–8.5 METs; Figure 3).115 Even those who did only 15 minutes of ET daily had a 14% reduction in all-cause mortality and a 3-year longer life expectancy.
Figure 3.
Daily physical activity duration and all-cause mortality reduction (reproduced from Wen CP et al, Lancet 2011).115
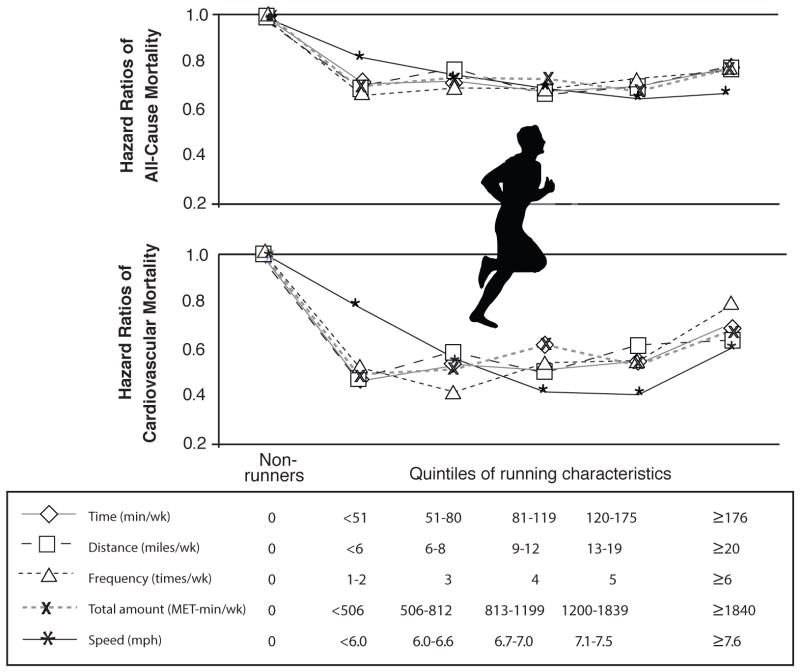
In a recent study of 55,000 people from the ACLS, including 13,000 runners and 42,000 non-runners followed on average for 15 years, runners had impressive reductions in all-cause and CVD mortality of 30% and 45%, respectively, with an average increase in life expectancy of 3 years.116 Persistent runners had the greatest reduction in risk, whereas those who began running but stopped or vice versa received about half of the benefit. However, when dividing runners into quintiles (Q) of exercise volumes (i.e., miles/wk, times/wk, and min/wk), Q 1 (<6 mile/wk, 1–2 times/wk, and <51 min/wk) had similar all-cause and CVD mortality risk reductions as Q 2–4 and a slight trend towards greater benefit than Q5 (Figure 4).116 These results suggest that with running, as a common and convenient method of ET, maximal benefit on all-cause and CVD mortality occur at very low doses, including ET doses well below the current major PA Guidelines. 112
Figure 4.
Central illustration: Hazard ratios (HRs) of all-cause and cardiovascular mortality by running characteristic (weekly running time, distance, frequency, total amount, and speed). Participants were classified into 6 groups: nonrunners (reference group) and 5 quintiles of each running characteristic. All HRs were adjusted for baseline age (years), sex, examination year, smoking status (never, former, or current), alcohol consumption (heavy drinker or not), other physical activities except running (0, 1 to 499, or ≥500 metabolic equivalent-minutes/week), and parental history of cardiovascular disease (yes or no). All p values for HRs across running characteristics were <0.05 for all-cause and cardiovascular mortality except for running frequency of ≥6 times/week (p = 0.11) and speed of <6.0 miles/h (p = 0.10) for cardiovascular mortality (reproduced with permission from Lee DC et al, J Am Coll Cardiol).116
Dangers of Excessive Endurance ET (EEE)
As Hippocrates said centuries ago “Everything in excess is opposed to nature.” 118 We and others have reviewed the potential adverse effects of very high levels of exercise,119,120 although these dangers have also been disputed.121
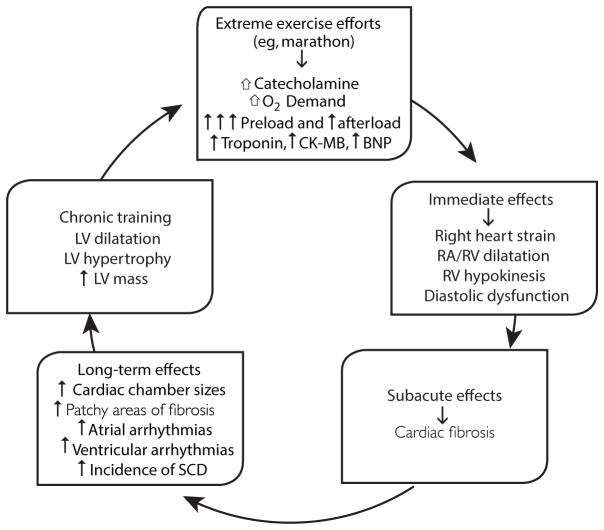
There are many potential adverse effects of EEE on cardiac structure and function (Figure 5).119 Acutely, EEE increases markers of myocardial injury, such as cardiac troponin and B-type natriuretic peptide, as well dilation of cardiac chambers, especially the atrium and the RV, and reduction in RV function.119–121 Chronically, there is concern that these levels of EEE can lead to detrimental cardiac remodeling and fibrosis, as well as non-lethal arrhythmias, particularly increased risk of atrial fibrillation, and potentially more lethal ventricular arrhythmias, which have been especially noted with very vigorous EEE in animals, with some suggestion of the same finding in humans.119,120,122,123 Recent studies have also suggested that longer distance runners, despite having more favorable overall CHD risk profiles, may have increased levels of atherosclerosis and CHD.124,125
Figure 5.
Proposed pathogenesis of cardiomyopathy in endurance athletes. BNP = B-type natriuretic peptide; CK-MB = creatine kinase MB; LV = left ventricle; RA = right atrium; RV = right ventricle; SCD = sudden cardiac death (reproduced with permission from O’Keefe et al, Mayo Clin Proc).119
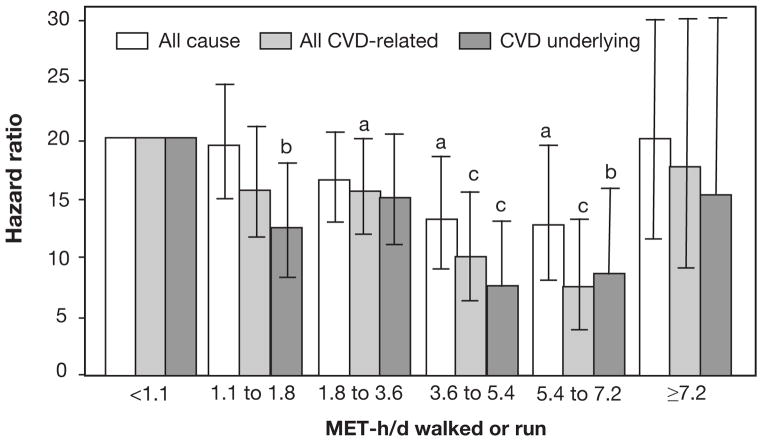
In a very recent study of 24,000 patients with CHD with personal history of MI, those doing more ET had progressive reductions in CVD mortality, up to a point (Figures 6 and 7).126 However, at running doses of >30 mile/wk or walking >46 mile/wk, there appears to be substantial loss of the ET benefit on CVD mortality.
Figure 6.
Categorical model. Cox proportional survival analyses of the risk of CVD-related mortality vs MET-h/d run or walked. Relative risk is calculated for 1.07 to 1.8, 1.8 to 3.6, 3.6 to 5.4, 5.4 to 7.2, and 7.2 MET-h/d or more relative to the inadequate exercisers (<1.07 MET-h/d). “All CVD-related” mortality includes both “CVD as an underlying cause” and “CVD as a contributing cause for some other underlying cause.” Significance levels are coded as follows: aP≤.05; bP≤.01; cP≤.001. The significance levels for 7.2 MET-h/d or more vs less than 1.07 MET-h/d were all nonsignificant, that is, P=.99 for all-cause mortality, P=.68 for all CVD-related mortality, and P=.46 for CVD as the underlying cause of death. CVD = cardiovascular disease; MET-h/d = metabolic equivalent of task-h/d (reproduced with permission from Williams P et al, Mayo Clin Proc).126
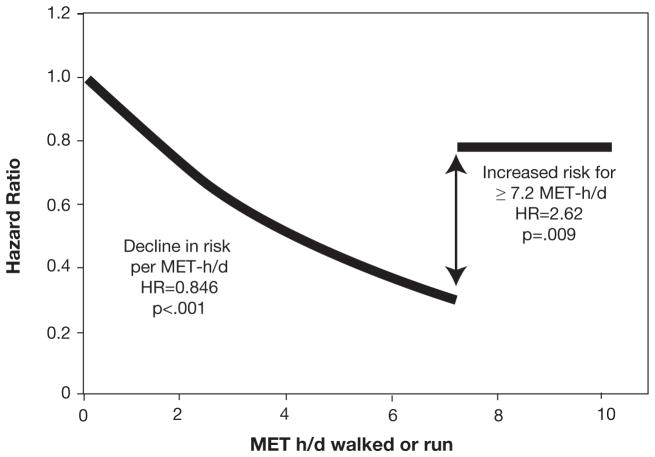
Figure 7.
Continuous model. Cox proportional survival analyses of the risk of CVD-related mortality vs MET-h/d run or walked. In the model “αMET-h/d Trimmed(MET-h/d if MET-h/d≤7.2, 7.2 otherwise) + βIndicator function (1=MET-h/d≥7.2, 0 otherwise) + covariates,” the hypothesis β=0 tests whether the hazard ratio is increased significantly above 7.2 MET-h/d relative to the hazard ratio at 7.2. Shown is the 15.4% average decrease in the risk for CVD-related mortality per MET-h/d between 0 and 7.2 MET-h/d (95% CI, 8.9%–21.5%; P<.001) and a 2.62-fold risk increase above 7.2 MET-h/d relative to the risk at 7.2 MET-h/d (95% CI, 1.29- to 5.06-fold; P=.009). CVD = cardiovascular disease; HR = hazard ratio; MET-h/d = metabolic equivalent of task-h/d. Reproduced with permission from Williams P et al, Mayo Clin Proc).126
Although clearly from a population perspective, lack of PA is much more prevalent than is EEE, with the potential for much greater adverse effects on overall and CVD health at the societal level.1,119,121,127 However, these studies also point to the fact that more does not appear to be better, and even low doses of ET, particularly for running, seem to be beneficial for conferring long-term CVD health and longevity.1,127
Exercise Prescription
Based on a constellation of data, the current recommendation of 150 min/wk of moderate aerobic PA or 75 min/wk of vigorous aerobic PA based on the Federal PA Guidelines appears reasonable,112 realizing that substantial benefits occur at levels of PA well below this, indicating that some PA is always better than no PA.115–117,128 Additionally, as reviewed above, with some more vigorous PA (e.g., running), maximal benefits seem to occur at quite low levels.116,117,128 Although resistance ET was not reviewed in detail in this report, exercises such as weight lifting will improve muscular strength, which is an important predictor of CVD risk factors and prognosis. 104 Resistance ET will also help to improve insulin insensitivity, and will also prevent or reverse sarcopenia—a pernicious and progressive problem that common affects individuals as they age., Therefore, including resistance ET for at least 15–20 minutes twice weekly, including frequent repetition exercises of the large muscle groups, combined with aerobic PA/ET, would be ideal,3,104 particularly to maintain MF and muscle strength in elderly and in patients with advanced HF who are at risk of frailty and cachexia.
Future Consideration
Although numerous aspects of ET in the prevention and treatment of CVD still require further study, large issues of controversy, including the impact of ET on major clinical events in patients with HFpEF, the efficacy and safety of HIIT, particularly on “hard” clinical events in many subgroups of patients, and the relative pros and cons of very high levels of ET (EEE) still require further data. Additionally, data regarding doses of ET, various types of ET, including doses of ET well below Federal Guidelines, still require further validation regarding overall clinical benefits.
Conclusions
Substantial evidence has established the value of high levels of PA, ET, and overall CRF in the prevention and treatment of CVD, especially CHD and HF. Although there may be some risk of EEE, which was briefly reviewed, the major threat to health in the 21st century is clearly inadequate levels of PA. The constellation of data reviewed in this manuscript support the marked efficacy of ET for all patients and the routine referral of eligible patients with CVD, especially CHD and HF (particularly systolic HF but also HfpEF), to formal CRET programs.
Acknowledgments
Sources of funding:
None
Nonstandard Abbreviations and Acronyms
- CHD
Coronary heart disease
- CO
Cardiac output
- CPX
Cardiopulmonary stress testing
- CRET
Cardiac rehabilitation and exercise training
- CRF
Cardiorespiratory fitness
- CV
Cardiovascular
- CVD
Cardiovascular disease
- EEE
Extreme endurance exercise training
- ET
Exercise training
- HDL-C
High-density lipoprotein cholesterol
- HF
Heart failure
- HFpEF
Heart failure preserved ejection fraction
- HIIT
High intensity interval training
- HR
Heart rate
- HTN
Hypertension
- LV
Left ventricular
- MetS
Metabolic syndrome
- METs
Metabolic equivalents
- MF
Muscular fitness
- MI
Myocardial infarction
- MICT
Moderate intensity continuous training
- NO
Nitric oxide
- PA
Physical activity
- RV
Right ventricle
- SV
Stroke volume
- T2D
Type-2 diabetes
- TGs
Triglycerides
- VO2
Oxygen consumption
Footnotes
Financial Disclosure: Dr. Lavie has served as a consultant and speaker on fitness/obesity for the Coca-Cola Company; and has published a book on the obesity paradox with potential royalties. Drs. Church and Blair have served as consultants for weight loss and fitness companies and for the Coca-Cola Company, which has also provided them un-restricted research grants. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Franklin BA, Lavie CJ, Squires RW, Milani RV. Exercise-based cardiac rehabilitation and improvements in cardiorespiratory fitness: implications regarding patient benefit. Mayo Clin Proc. 2013;88:431–437. doi: 10.1016/j.mayocp.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446–1461. doi: 10.1016/j.mayocp.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Swift DL, Lavie CJ, Johannsen NM, Arena R, Earnest CP, O’Keefe JH, Milani RV, Blair SN, Church TS. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ J. 2013;77:281–292. doi: 10.1253/circj.cj-13-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera-Brown AM, Frontera WR. Principles of Exercise Physiology: Responses to Acute Exercise and Long-term Adaptations to Training. PM&R. 2012;4:797–804. doi: 10.1016/j.pmrj.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Arena R, Myers J, Guazzi M. The clinical significance of aerobic exercise testing and prescription: from apparently healthy to confirmed cardiovascular disease. Am J Lifestyle Med. 2008;2:519–536. [Google Scholar]
- 6.Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 7.Myers J, Froelicher VF. Basic Exercise Physiology. In: Piolo SF, editor. Exercise and the Heart. 5. Philadelphia: Saunders Elsevier; 2006. pp. 1–10. [Google Scholar]
- 8.Seals DR, Hagberg JM, Spina RJ, Rogers MA, Schechtman KB, Ehsani AA. Enhanced left ventricular performance in endurance trained older men. Circulation. 1994;89:198–205. doi: 10.1161/01.cir.89.1.198. [DOI] [PubMed] [Google Scholar]
- 9.Baggish AL, Yared K, Wang F, Weiner RB, Hutter AM, Jr, Picard MH, Wood MJ. The impact of endurance exercise training on left ventricular systolic mechanics. Am J Physiol Heart Circ Physiol. 2008;295:H1109–H1116. doi: 10.1152/ajpheart.00395.2008. [DOI] [PubMed] [Google Scholar]
- 10.Weiner RB, Baggish AL. Exercise-induced cardiac remodeling. Prog Cardiovasc Dis. 2012;54:380–386. doi: 10.1016/j.pcad.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arbab-Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams-Huet B1, Haykowsky MJ, Levine BD. Cardiac remodeling in response to 1 year of intensive endurance training. Circulation. 2014;130:2152–2161. doi: 10.1161/CIRCULATIONAHA.114.010775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen LJ, Hansen PR, Sogaard P, Madsen JK, Bech J, Krustrup P. Improvement of systolic and diastolic heart function after physical training in sedentary women. Scand J Med Sci Sports. 2010;20 (Suppl 1):50–57. doi: 10.1111/j.1600-0838.2009.01088.x. [DOI] [PubMed] [Google Scholar]
- 14.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 15.Santos-Parker JR, LaRocca TJ, Seals DR. Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Adv Physiol Educ. 2014;38:296–307. doi: 10.1152/advan.00088.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 17.Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9:e110034. doi: 10.1371/journal.pone.0110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson EA, Green DJ, Cable NT, Thijssen DH. Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol (1985) 2013;115:1589–1598. doi: 10.1152/japplphysiol.00450.2013. [DOI] [PubMed] [Google Scholar]
- 19.Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108:530–535. doi: 10.1161/01.CIR.0000080893.55729.28. [DOI] [PubMed] [Google Scholar]
- 20.Maiorana A, O’Driscoll G, Taylor R, Green D. Exercise and the nitric oxide vasodilator system. Sports Med. 2003;33:1013–1035. doi: 10.2165/00007256-200333140-00001. [DOI] [PubMed] [Google Scholar]
- 21.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 22.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 23.Laughlin MH, Oltman CL, Bowles DK. Exercise training-induced adaptations in the coronary circulation. Med Sci Sports Exerc. 1998;30:352–360. doi: 10.1097/00005768-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Williams MA, Pozehl B. Reasonable expectations: how much aerobic capacity, muscle strength, and quality of life can improve with exercise training in heart failure. Heart Fail Clin. 2015;11:37–57. doi: 10.1016/j.hfc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Arena RA. Functional capacity and exercise training have earned a primary role in the assessment and treatment of patients with heart failure. Heart Fail Clin. 2015;11:xv–xvii. doi: 10.1016/j.hfc.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Hu G, Barengo NC, Tuomilehto J, Lakka TA, Nissinen A, Jousilahti P. Relationship of physical activity and body mass index to the risk of hypertension: a prospective study in Finland. Hypertension. 2004;43:25–30. doi: 10.1161/01.HYP.0000107400.72456.19. [DOI] [PubMed] [Google Scholar]
- 27.Lahti-Koski M, Pietinen P, Heliövaara M, Vartiainen E. Associations of body mass index and obesity with physical activity, food choices, alcohol intake, and smoking in the 1982–1997 FINRISK studies. Am J Clin Nutr. 2002;75:809–817. doi: 10.1093/ajcn/75.5.809. [DOI] [PubMed] [Google Scholar]
- 28.Kriska AM, Saremi A, Hanson RL, Bennett PH, Kobes S, Williams DE, Knowler WC. Physical activity, obesity, and the incidence of type 2 diabetes in a high-risk population. Am J Epidemiol. 2003;158:669–675. doi: 10.1093/aje/kwg191. [DOI] [PubMed] [Google Scholar]
- 29.Rennie K, McCarthy N, Yazdgerdi S, Marmot M, Brunner E. Association of the metabolic syndrome with both vigorous and moderate physical activity. Int J Epidemiol. 2003;32:600–606. doi: 10.1093/ije/dyg179. [DOI] [PubMed] [Google Scholar]
- 30.Barengo NC, Hu G, Lakka TA, Pekkarinen H, Nissinen A, Tuomilehto J. Low physical activity as a predictor for total and cardiovascular disease mortality in middle-aged men and women in Finland. Eur Heart J. 2004;25:2204–2211. doi: 10.1016/j.ehj.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 32.Hu G, Eriksson J, Barengo NC, Lakka TA, Valle TT, Nissinen A, Jousilahti P, Tuomilehto J. Occupational, commuting, and leisure-time physical activity in relation to total and cardiovascular mortality among Finnish subjects with type 2 diabetes. Circulation. 2004;110:666–673. doi: 10.1161/01.CIR.0000138102.23783.94. [DOI] [PubMed] [Google Scholar]
- 33.Paffenbarger RS, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 34.Hu G, Jousilahti P, Borodulin K, Barengo NC, Lakka TA, Nissinen A, Tuomilehto J. Occupational, commuting and leisure-time physical activity in relation to coronary heart disease among middle-aged Finnish men and women. Atherosclerosis. 2007;194:490–497. doi: 10.1016/j.atherosclerosis.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 35.Slattery ML, Jacobs DR, Jr, Nichaman MZ. Leisure time physical activity and coronary heart disease death: the US Railroad Study. Circulation. 1989;79:304–311. doi: 10.1161/01.cir.79.2.304. [DOI] [PubMed] [Google Scholar]
- 36.Fang J, Wylie-Rosett J, Cohen HW, Kaplan RC, Alderman MH. Exercise, body mass index, caloric intake, and cardiovascular mortality. Am J Prev Med. 2003;25:283–289. doi: 10.1016/s0749-3797(03)00207-1. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein AR, Sesso HD, Lee IM, Rexrode KM, Cook NR, Manson JE, Buring JE, Gaziano JM. The joint effects of physical activity and body mass index on coronary heart disease risk in women. Arch Intern Med. 2008;168:884–890. doi: 10.1001/archinte.168.8.884. [DOI] [PubMed] [Google Scholar]
- 38.Paffenbarger RS, Hyde RT, Wing AL, Lee I-M, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 39.Gregg EW, Cauley JA, Stone K, Thompson TJ, Bauer DC, Cummings SR, Ensrud KE Study of Osteoporotic Fractures Research Group. Relationship of changes in physical activity and mortality among older women. JAMA. 2003;289:2379–2386. doi: 10.1001/jama.289.18.2379. [DOI] [PubMed] [Google Scholar]
- 40.Wannamethee SG, Shaper AG, Walker M. Changes in physical activity, mortality, and incidence of coronary heart disease in older men. Lancet. 1998;351:1603–1608. doi: 10.1016/S0140-6736(97)12355-8. [DOI] [PubMed] [Google Scholar]
- 41.Kaminsky LA, Arena R, Beckie TM, Brubaker PH, Church TS, Forman DE, Franklin BA, Gulati M, Lavie CJ, Myers J, Patel MJ, Piña IL, Weintraub WS, Williams MA American Heart Association Advocacy Coordinating Committee, Council on Clinical Cardiology, and Council on Nutrition, Physical Activity and Metabolism. The importance of cardiorespiratory fitness in the United States: the need for a national registry: A policy statement from the American Heart Association. Circulation. 2013;127:652–662. doi: 10.1161/CIR.0b013e31827ee100. [DOI] [PubMed] [Google Scholar]
- 42.Barry VW, Baruth M, Beets MW, Durstine JL, Liu J, Blair SN. Fitness vs. fatness on all-cause mortality: A meta-analysis. Prog Cardiovasc Dis. 2014;56:382–390. doi: 10.1016/j.pcad.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Fagard R, Bielen E, Amery A. Heritability of aerobic power and anaerobic energy generation during exercise. J Appl Phsiol. 1991;70:357–362. doi: 10.1152/jappl.1991.70.1.357. [DOI] [PubMed] [Google Scholar]
- 44.Myers J, McAuley P, Lavie CJ, Despres JP, Arena R, Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis. 2015;57:306–314. doi: 10.1016/j.pcad.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 45.DeFina LF, Haskell WL, Willis BL, Barlow CE, Finley CE, Levine BD, Cooper KH. Physical activity versus cardiorespiratory fitness: two (partly) distinct components of cardiovascular health? Prog Cardiovasc Dis. 2015:324–329. doi: 10.1016/j.pcad.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Balady GJ, Arena R, Sietsema K, et al. American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research. . Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 47.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 48.Berry JD, Willis B, Gupta S, Barlow CE, Lakoski SG, Khera A, Rohatgi A, de Lemos JA, Haskell W, Lloyd-Jones DM. Lifetime risks for cardiovascular disease mortality by cardiorespiratory fitness levels measured at ages 45, 55, and 65 years in Menthe Cooper Center Longitudinal Study. J Am Coll Cardiol. 2011;57:1604–1610. doi: 10.1016/j.jacc.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blair SN, Kohl HW, III, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality: A prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 50.Lee D-c, Sui X, Artero EG, Lee IM, Church TS, McAuley PA, Stanford FC, Kohl HW, 3rd, Blair SN. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: The Aerobics Center Longitudinal Study. Circulation. 2011;124:2483–2490. doi: 10.1161/CIRCULATIONAHA.111.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erikssen G, Liestol K, Bjornholt J, Thaulow E, Sandvik L, Erikssen J. Changes in physical fitness and changes in mortality. Lancet. 1998;352:759–762. doi: 10.1016/S0140-6736(98)02268-5. [DOI] [PubMed] [Google Scholar]
- 52.Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63:1346–1354. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 53.Lee D-c, Sui X, Church TS, Lavie CJ, Jackson AS, Blair SN. Changes in fitness and fatness on the development of cardiovascular disease risk factors: hypertension, metabolic syndrome, and hypercholesterolemia. J Am Coll Cardiol. 2012;59:665–672. doi: 10.1016/j.jacc.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McAuley PA, Artero EG, Sui X, Lee DC, Church TS, Lavie CJ, Myers JN, España-Romero V, Blair SN. The obesity paradox, cardiorespiratory fitness, and coronary heart disease. Mayo Clin Proc. 2012;87:443–451. doi: 10.1016/j.mayocp.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavie CJ, Cahalin LP, Chase P, Myers J, Bensimhon D, Peberdy MA, Ashley E, West E, Forman DE, Guazzi M, Arena R. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc. 2013;88:251–258. doi: 10.1016/j.mayocp.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 57.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 58.Tambalis K, Panagiotakos DB, Kavouras SA, Sidossis LS. Responses of blood lipids to aerobic, resistance, and combined aerobic with resistance exercise training: a systematic review of current evidence. Angiology. 2009;60:614–632. doi: 10.1177/0003319708324927. [DOI] [PubMed] [Google Scholar]
- 59.Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, Suzuki E, Shimano H, Yamamoto S, Kondo K, Ohashi Y, Yamada N, Sone H. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med. 2007;167:999–1008. doi: 10.1001/archinte.167.10.999. [DOI] [PubMed] [Google Scholar]
- 60.Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441–447. doi: 10.1016/j.pcad.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ades PA, Savage PD. Potential benefits of weight loss in coronary heart disease. Prog Cardiovasc Dis. 2014;56:448–456. doi: 10.1016/j.pcad.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud’homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P, Jennings A, Jaffey J. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 63.Bacchi E, Negri C, Zanolin ME, Milanese C, Faccioli N, Trombetta M, Zoppini G, Cevese A, Bonadonna RC, Schena F, Bonora E, Lanza M, Moghetti P. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study) Diabetes Care. 2012;35:676–682. doi: 10.2337/dc11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA American College of Sports Medicine. American College of Sports Medicine position stand: exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 65.Pack QR, Rodriguez-Escudero JP, Thomas RJ, Ades PA, West CP, Somers VK, Lopez-Jimenez F. The prognostic importance of weight loss in coronary artery disease: a systematic review and meta-analysis. Mayo Clin Proc. 2014;89:1368–1377. doi: 10.1016/j.mayocp.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milani RV, Lavie CJ. Prevalence and profile of metabolic syndrome in patients following acute coronary events and effects of therapeutic lifestyle change with cardiac rehabilitation. Am J Cardiol. 2003;92:50–54. doi: 10.1016/s0002-9149(03)00464-8. [DOI] [PubMed] [Google Scholar]
- 67.Lavie CJ, Milani RV. Effects of cardiac rehabilitation, exercise training, and weight reduction on exercise capacity, coronary risk factors, behavioral characteristics, and quality of life in obese coronary patients. Am J Cardiol. 1997;79:397–401. doi: 10.1016/s0002-9149(97)89239-9. [DOI] [PubMed] [Google Scholar]
- 68.Sierra-Johnson J, Romero-Corral A, Somers VK, Lopez-Jimenez F, Thomas RJ, Squires RW, Allison TG. Prognostic importance of weight loss in patients with coronary heart disease regardless of initial body mass index. Eur J Cardiovasc Prev Rehabil. 2008;15:336–340. doi: 10.1097/HJR.0b013e3282f48348. [DOI] [PubMed] [Google Scholar]
- 69.Ades PA, Savage PD, Toth MJ, Harvey-Berino J, Schneider DJ, Bunn JY, Audelin MC, Ludlow M. High-calorie-expenditure exercise: a new approach to cardiac rehabilitation for overweight coronary patients. Circulation. 2009;119:2671–2678. doi: 10.1161/CIRCULATIONAHA.108.834184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lavie CJ, Milani RV. Effects of nonpharmacologic therapy with cardiac rehabilitation and exercise training in patients with low levels of high-density lipoprotein cholesterol. Am J Cardiol. 1996;78:1286–1289. doi: 10.1016/s0002-9149(96)00614-5. [DOI] [PubMed] [Google Scholar]
- 71.Lavie CJ, Milani RV. Effects of cardiac rehabilitation and exercise training on low density lipoprotein cholesterol in patients with hypertriglyceridemia and coronary artery disease. Am J Cardiol. 1994;74:1192–1195. doi: 10.1016/0002-9149(94)90546-0. [DOI] [PubMed] [Google Scholar]
- 72.Lavie CJ, Church TS, Milani RV, Earnest CP. Impact of physical activity, cardiorespiratory fitness, and exercise training on markers of inflammation. J Cardiopulm Rehabil Prev. 2011;31:137–145. doi: 10.1097/HCR.0b013e3182122827. [DOI] [PubMed] [Google Scholar]
- 73.Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43:1056–1061. doi: 10.1016/j.jacc.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 74.Lavie CJ, Milani RV, Artham SM, Patel DA, Ventura HO. The obesity paradox, weight loss, and coronary disease. Am J Med. 2009;122:1106–1114. doi: 10.1016/j.amjmed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Lavie CJ, Milani RV, O’Keefe JH, Lavie TJ. Impact of exercise training on psychological risk factors. Prog Cardiovasc Dis. 2011;53:464–470. doi: 10.1016/j.pcad.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 76.Lavie CJ, Milani RV. Adverse psychological and coronary risk profiles in young patients with coronary artery disease and benefits of formal cardiac rehabilitation. Arch Intern Med. 2006;166:1878–1883. doi: 10.1001/archinte.166.17.1878. [DOI] [PubMed] [Google Scholar]
- 77.Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med. 2007;120:799–806. doi: 10.1016/j.amjmed.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 78.Milani RV, Lavie CJ, Mehra MR, Ventura HO. Impact of exercise training and depression on survival in heart failure due to coronary heart disease. Am J Cardiol. 2011;107:64–68. doi: 10.1016/j.amjcard.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 79.O’Connor GT, Buring JE, Yusuf S, Goldhaber SZ, Olmstead EM, Paffenbarger RS, Jr, Hennekens CH. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80:234–244. doi: 10.1161/01.cir.80.2.234. [DOI] [PubMed] [Google Scholar]
- 80.Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2001;(1):CD001800. doi: 10.1002/14651858.CD001800. [DOI] [PubMed] [Google Scholar]
- 81.Goel K, Lennon RJ, Tilbury T, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation. 2011;123:2344–2352. doi: 10.1161/CIRCULATIONAHA.110.983536. [DOI] [PubMed] [Google Scholar]
- 82.Pack QR, Dudycha KJ, Roschen KP, Thomas RJ, Squires RW. Safety of early enrollment into outpatient cardiac rehabilitation after open heart surgery. Am J Cardiol. 2015;115:548–552. doi: 10.1016/j.amjcard.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 83.Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009;54:25–33. doi: 10.1016/j.jacc.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 84.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121:63–70. doi: 10.1161/CIRCULATIONAHA.109.876383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lavie CJ, Berra K, Arena R. Formal cardiac rehabilitation and exercise training in heart failure: evidence for substantial clinical benefits. J Cardiopulm Rehabil Prev. 2013;33:209–211. doi: 10.1097/HCR.0b013e31829f95c9. [DOI] [PubMed] [Google Scholar]
- 86.Lavie CJ, Ventura HO, Milani RV, Arena R. Critical impact of fitness in the prevention and treatment of heart failure. Am Heart J. 2015;169:194–196. doi: 10.1016/j.ahj.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 87.Berry JD, Pandey A, Gao A Leonard D, Farzaneh-Far R, Ayers C, DeFina L, Willis B. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail. 2013;6:627–634. doi: 10.1161/CIRCHEARTFAILURE.112.000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pandey A, Patgel M, Gao A, Willis BL, Das SR, Leonard D, Drazner MH, de Lemos JA, DeFina L, Berry JD. Changes in mid-life fitness predicts heart failure risk at a later age independent of interval development of cardiac and noncardiac risk factors: The Cooper Center Longitudinal Study. Am Heart J. 2015;169:290–297. doi: 10.1016/j.ahj.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keteyan SJ. Exercise training in congestive heart failure: risks and benefits. Prog Cardiovasc Dis. 2011;53:119–128. doi: 10.1016/j.pcad.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 90.Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116:693–706. doi: 10.1016/j.amjmed.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 91.Piepoli MF, Davos C, Francis DP, Coats AM ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328:189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Connor CM, Whellan DJ, Lee KL, et al. HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keteyian SJ, Leifer ES, Houston-Miller N, Kraus WE, Brawner CA, O’Connor CM, Whellan DJ, Cooper LS, Fleg JL, Kitzman DW, Cohen-Solal A, Blumenthal JA, Rendall DS, Piña IL HF-ACTION Investigators. Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2012;601:1899–1905. doi: 10.1016/j.jacc.2012.08.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yancy CW, Jessup M, Bozkurt B, et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AGA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:1495–1539. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 95.Downing J, Balady GJ. The role of exercise training in heart failure. J Am Coll Cardiol. 2011;58:561–569. doi: 10.1016/j.jacc.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 96.Kitzman DW. Exercise training in heart failure with preserved ejection fraction: beyond proof-of-concept. J Am Coll Cardiol. 2011;58:1792–1794. doi: 10.1016/j.jacc.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 97.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–H1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Edelmann F, Gelbrich G, Düngen HD, Fröhling S, Wachter R, Stahrenberg R, Binder L, Töpper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Löffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Exertional chest discomfort-DHF (Exercise training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 99.Arena R, Myers J, Forman DE, Lavie CJ, Guazzi M. Should high-intensity-aerobic interval training become the clinical standard in heart failure? Heart Fail Rev. 2013;18:95–105. doi: 10.1007/s10741-012-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lavie CJ, Arena R, Earnest CP. High-intensity interval training in patients with cardiovascular diseases and heart transplantation. J Heart and Lung Transplant. 2013;32:1056–1058. doi: 10.1016/j.healun.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 101.Forman DE, Daniels KM, Cahalin LP, Zavin A, Allsup K, Cao P, Santhanam M, Joseph J, Arena R, Lazzari A, Schulze PC, Lecker SH. Analysis of skeletal muscle gene expression patterns and the impact of functional capacity in patients with systolic heart failure. J Cardiac F. 2014;20:422–430. doi: 10.1016/j.cardfail.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail. 2010;3:537–546. doi: 10.1161/CIRCHEARTFAILURE.109.903773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pedersen BK, Edward F. Adolph Distinguished Lecture: muscle as an endocrine organ: IL-6 and other myokines. J Appl Physiol. 2009;107:1006–1014. doi: 10.1152/japplphysiol.00734.2009. [DOI] [PubMed] [Google Scholar]
- 104.Artero EG, Lee D-c, Lavie CJ, et al. Effects of muscular strength on cardiovascular risk factors and prognosis. J Cardiopulm Rehabil Prev. 2012;32:351–358. doi: 10.1097/HCR.0b013e3182642688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lavie CJ, De Schutter A, Alpert MA, Mehra MR, Milani RV, Ventura HO. Obesity paradox, cachexia, frailty, and heart failure. Heart Failure Clin. 2014;10:319–326. doi: 10.1016/j.hfc.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 106.Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV. Body composition and survival in stable coronary heart disease: impact of lean mass index and body fat in the “obesity paradox. J Am Coll Cardiol. 2012;60:1374–1380. doi: 10.1016/j.jacc.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 107.Lavie CJ, De Schutter A, Patel D, Artham SM, Milani RV. Body composition and coronary heart disease mortality: an obesity or a lean paradox? Mayo Clin Proc. 2011;86:857–864. doi: 10.4065/mcp.2011.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Schutter A, Lavie CJ, Patel DA, Artham SM, Milani RV. Relation of body fat categories by Gallagher classification and by continuous variables to mortality in patients with coronary heart disease. Am J Cardiol. 2013;111:657–660. doi: 10.1016/j.amjcard.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 109.De Schutter A, Lavie CJ, Milani RV. The impact of obesity on risk factors and prevalence of coronary heart disease: the obesity paradox. Prog Cardiovasc Dis. 2014;56:401–408. doi: 10.1016/j.pcad.2013.08.003. C. [DOI] [PubMed] [Google Scholar]
- 110.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91:891–894. doi: 10.1016/s0002-9149(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 111.Zavin A, Daniels K, Arena R, Allsup K, Lazzari A, Joseph J, Schulze PC, Lecker SH, Forman DE. Adiposity facilitates increased strength capacity in heart failure patients with reduced ejection fraction. Int J Cardiol. 2013;167:2468–2471. doi: 10.1016/j.ijcard.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.U.S. Department of Health and Human Services. 2008 physical activity guidelines for Americans. The Department; 2008. [Google Scholar]
- 113.Carlson SA, Fulton JE, Schoenborn CA, Loustalot F. Trend and prevalence estimates based on the 2008 Physical Activity Guidelines for Americans. Am J Prev Med. 2010;39:305–313. doi: 10.1016/j.amepre.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 114.Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S: adults compliance with the Physical Activity Guidelines for Americans. Am J Prev Med. 2011;40:454–461. doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 115.Wen CP, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 116.Lee D-c, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol. 2014;64:472–481. doi: 10.1016/j.jacc.2014.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wen CP, Wai JP, Tsai MK, Chen CH. Minimal amount of exercise to prolong life: to walk, to run, or just mix it up? J Am Coll Cardiol. 2014;64:482–484. doi: 10.1016/j.jacc.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 118.Everything in excess is opposed to nature. [Accessed June 23, 2014];Hippocrates. 2014 http://www.brainyquote.com/quotes/quotes/h/hippocrate118541.html.
- 119.O’Keefe JH, Patil HR, Lavie CJ, et al. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin Proc. 2012;87:587–595. doi: 10.1016/j.mayocp.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.O’Keefe JH, Lavie CJ, Guazzi M. Part 1: potential dangers of extreme endurance exercise: how much is too much? Part 2: screening of school-age athletes. Prog Cardiovasc Dis. 2015;57:396–405. doi: 10.1016/j.pcad.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 121.Levine BD. Can intensive exercise harm the heart? The benefits of competitive endurance training for cardiovascular structure and function. Circulation. 2014;130(12):987–991. doi: 10.1161/CIRCULATIONAHA.114.008142. [DOI] [PubMed] [Google Scholar]
- 122.La Gerche A, Heidbuchel H. Can intensive exercise harm the heart? You can get too much of a good thing. Circulation. 2014;130:992–1002. doi: 10.1161/CIRCULATIONAHA.114.008141. [DOI] [PubMed] [Google Scholar]
- 123.Menezes AR, Lavie CJ, DiNicolantonio JJ, et al. Atrial fibrillation in the 21st century: a current understanding of risk factors and primary prevention strategies. Mayo Clin Proc. 2013;88:394–409. doi: 10.1016/j.mayocp.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 124.Schwartz RS, Kraus SM, JGS, et al. Increased coronary artery plaque volume among male marathon runners. Mo Med. 2014;111:85–90. [PMC free article] [PubMed] [Google Scholar]
- 125.McCullough PA, Lavie CJ. Coronary artery plaque and cardiotoxicity as a result of extreme endurance exercise. Mo Med. 2014;111:91–94. [PMC free article] [PubMed] [Google Scholar]
- 126.Williams P, Thompson P. Increased cardiovascular disease mortality from excessive exercise in heart attack survivors. Mayo Clin Proc. 2014;89(9):1187–1194. doi: 10.1016/j.mayocp.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 127.O’Keefe JH, Franklin B, Lavie CJ. Exercising for health and longevity vs peak performance: different regimens for different goals. Mayo Clin Proc. 2014;89:1171–1175. doi: 10.1016/j.mayocp.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 128.Lee D-c, Lavie CJ, Vedanthan R. Optimal dose of running for longevity: is more better or worse? J Am Coll Cardiol. 2015 doi: 10.1016/j.jacc.2014.11.022. [DOI] [PubMed] [Google Scholar]