Highlight
Spartina pectinata (prairie cordgrass) has superior rhizome freezing tolerance, spring leaf frost and freezing tolerance, and greater first year establishment compared to Miscanthus × giganteus at a cool temperate site.
Keywords: Establishment success, leaf frost tolerance, Miscanthus × giganteus, perennial C4 grasses, prairie cordgrass (Spartina pectinata), rhizome freezing tolerance.
Abstract
Miscanthus × giganteus grown in cool temperate regions of North America and Europe can exhibit severe mortality in the year after planting, and poor frost tolerance of leaves. Spartina pectinata (prairie cordgrass), a productive C4 perennial grass native to North America, has been suggested as an alternative biofuel feedstock for colder regions; however, its cold tolerance relative to M. × giganteus is uncertain. Here, we compare the cold tolerance thresholds for winter-dormant rhizomes and spring/summer leaves of M. × giganteus and three accessions of S. pectinata. All genotypes were planted at a field site in Ontario, Canada. In November and February, the temperatures corresponding to 50% rhizome mortality (LT50) were near −24°C for S. pectinata and −4°C for M. × giganteus. In late April, the LT50 of rhizomes rose to −10°C for S. pectinata but remained near −4°C for M. × giganteus. Twenty percent of the M. × giganteus rhizomes collected in late April were dead while S. pectinata rhizomes showed no signs of winter injury. Photosynthesis and electrolyte leakage measurements in spring and summer demonstrate that S. pectinata leaves have greater frost tolerance in the field. For example, S. pectinata leaves remained viable above −9°C while the mortality threshold was near −5°C for M. × giganteus. These results indicate M. × giganteus will be unsuitable for production in continental interiors of cool-temperate climate zones unless freezing and frost tolerance are improved. By contrast, S. pectinata has the freezing and frost tolerance required for a higher-latitude bioenergy crop.
Introduction
Miscanthus × giganteus Greef & Deuter ex Hodkinson & Renvoize is one of the most productive C4 perennial grasses in temperate zone climates, and is therefore a leading candidate for bioenergy production at higher latitudes (Lewandowski et al., 2000; Clifton-Brown et al., 2001b; Heaton et al., 2008). The high productivity of M. × giganteus in temperate regions, however, depends on its ability to establish and successfully survive in climates where low winter and spring temperatures can damage or kill maladapted plants (Christian and Haase, 2001). Because Miscanthus cultivars are derived from material originally collected from eastern Asia (Heaton et al., 2010; Clifton-Brown et al., 2013; Sacks et al., 2013), it is not certain that they will survive low temperatures common in northern North America and Europe where winter and spring cold may be more intense than in their native range. Field trials in Germany, Denmark, and southern Ontario, Canada, often find high mortality of M. × giganteus plants in the first winter after planting (Clifton-Brown et al., 2001a; Jørgensen et al., 2003; Deen et al., 2011). Poor overwinter survival of M. × giganteus may be explained by a modest thermal tolerance threshold for rhizomes near −4°C (Clifton-Brown and Lewandowski, 2000). Selected varieties of M. × giganteus parent species have slightly colder tolerance thresholds for rhizomes, with the lowest being near −6°C for M. sinensis (Clifton-Brown and Lewandowski, 2000). Although cultivation practices can mitigate winterkill, this limited degree of rhizome cold tolerance may restrict the use of Miscanthus to regions with mild winters and a low chance of hard spring frosts, for example, in the southern and central USA. Much of the northern USA, Canada and northern Eurasia may be unsuitable for Miscanthus cultivation if cold tolerance thresholds are universally above −6°C within the Miscanthus gene pool (Clifton-Brown and Lewandowski, 2000; Christian and Haase, 2001). This could be a problem for the bioenergy sector, as the high productive potential represented by Miscanthus would be unavailable where there is both an abundance of land and a high demand for heat. If so, other C4 grass species may be needed to replace Miscanthus as the leading C4 biofuel feedstocks at higher latitudes. One promising candidate is Spartina pectinata Link (prairie cordgrass).
Spartina is a genus of perennial C4 grasses that may be ‘a New World parallel to Miscanthus’ as it contains productive species whose distributions extend to high latitudes (Long and Spence, 2013). Spartina pectinata in particular has attracted interest as it grows up to 61°N in its native range in North America; however, its cold tolerance limits remain uncertain. Field trials in eastern South Dakota, USA, and near Essex, UK, show S. pectinata can yield an average of 12 t ha-1 yr-1 biomass above ground within 6–10 years of establishment (Potter et al., 1995; Boe et al., 2009). This is up to 50% greater than the most productive C3 perennial grasses at similar latitudes such as reed canary grass (Phalaris arundinacea L.) (Lewandowski et al., 2003). On drier, marginal uplands it achieves yields comparable to switchgrass (Panicum virgatum L.), but unlike switchgrass and M. × giganteus it also grows well on low-lying waterlogged soils and riparian land in eastern Canada and the American upper midwest (Madakadze et al., 1998; Montemayor et al., 2008; Boe et al., 2009; Gonzalez-Hernandez et al., 2009; Pyter et al., 2009). It is found throughout much of continental North America from Texas, USA, up to the Northwest Territories, Canada, which represents the most northerly distribution of any candidate C4 biomass species (Porsild and Cody, 1980; Schwarz and Redmann, 1988). The closely related S. gracilis Trin. (alkali cordgrass) has a similar northern distribution as S. pectinata and shows ≥ 70% survival down to −29°C (Baumel et al., 2002; Schwarz and Reaney, 1989). Another related C4 species, Zoysia japonica Steud., shows temperatures corresponding to 50% rhizome mortality (LT50) of −12°C to −18°C in the winter acclimated state, with significant differences between geographically disparate genotypes (Dunn et al., 1999; Rogers et al., 1975). These patterns indicate S. pectinata could have superior cold tolerance of overwintering rhizomes relative to Miscanthus genotypes, yet still maintain the high productive potential expected of candidate bioenergy feedstocks (Boe et al., 2013).
In regions with periodic spring frosts, cold tolerance of leaves is also critical for establishment and productivity of C4 perennial grass crops. If such tolerance is lacking, episodic chilling or frost events during the early part of the growing season could kill developing canopies, as has been observed in Miscanthus plantations in southern Canada (Friesen et al., 2014). In Ireland, Miscanthus × giganteus trials failed to re-sprout after late spring frosts killed the young canopy (Christian and Haase, 2001). Despite its superior tolerance and growth potential under chilling temperatures (7.5°C to 12°C), M. × giganteus leaves show weak leaf freezing tolerance compared to other varieties of Miscanthus (Clifton-Brown and Jones, 1997; Farrell et al., 2006; Friesen et al., 2014; Zub et al., 2012). Even if cultivars have cold tolerance, delay in the onset of canopy development, or alternatively, improper senescence, could hinder yields, for example, by preventing the exploitation of the long photoperiods of mid-to-late spring. An important requirement for the success of a bioenergy crop is the ability to convert as much sunshine as possible into biomass, but this will require tolerance of early season frost and chilling episodes that are inevitable in northern climates. In the case of S. pectinata, freezing tolerance of leaves is probably well developed as dormant shoots are present above the soil surface from late autumn until growth begins in early-to-mid spring (Boe et al., 2009).
Because of its success in northern latitudes, we hypothesize that rhizomes and leaves of S. pectinata have greater cold tolerance compared to M. × giganteus during the autumn, winter, and spring following planting. Here, we evaluate the rhizome LT50 and degree of winterkill in rhizomes of M. × giganteus and S. pectinata grown outdoors in a common garden during the first year following planting. We evaluate whether differences in rhizome freezing tolerance are present between three populations of S. pectinata from midwestern and maritime North America. To evaluate cold tolerance of rhizomes collected in the autumn, winter, and spring, we used the electrolyte leakage method and re-growth assays following controlled exposure to a range of subzero temperatures. These tests assessed seasonal changes in cold hardiness as well as maximum hardiness levels. We also evaluated cold tolerance of field-grown leaves in the early spring by measuring photosynthesis and chlorophyll fluorescence the day after a mild frost event. Freezing tolerance of leaves was also evaluated with electrolyte leakage after controlled exposure to subzero temperatures. Finally, we evaluated shoot emergence dates and canopy height during the spring to compare early season growth of M. × giganteus and the three populations of S. pectinata.
Materials and methods
Plant material and field plots
Miscanthus × giganteus (M161) rhizomes were extracted from a one-year-old unfertilized plot adjacent to our field site on 1 May 2013. This variety of M. × giganteus is the research standard at the University of Illinois and is an accession from the Chicago Botanical Gardens (Heaton et al., 2010). Spartina pectinata ‘Red River’ is an octaploid cultivar originating from an open pollination cross among populations collected from east-central Minnesota, northeastern South Dakota, and east-central North Dakota (Boe et al., 2009; Kim et al., 2010). All plantlets originated from plants grown by Millborn Seeds Inc. (Brookings, South Dakota, USA <http://www.millbornseeds.com/>) and were donated by Professor D.K. Lee (Department of Crop Sciences, University of Illinois at Urbana-Champaign). Spartina pectinata ‘IL-102’ is a tetraploid cultivar collected from a natural population near Savoy, Illinois, USA (Kim et al., 2010). Spartina pectinata ‘Summerford’ was collected by the authors near Summerford, Newfoundland, Canada (49°28’3.3”N, 54°44’50.8”W). This is near the northern edge of the S. pectinata range in eastern North America (Supplementary Fig. S1). Plantlets were established as plugs that were then planted into a field plot at the University of Guelph Agricultural Research Station near Elora, Ontario, Canada (43.64°N, 80.40°W) on 21 June 2013 (see Friesen 2015 for details of the field plantation). Plots consisted of four blocks with 12 plants per genotype in each block. Plants of each genotype were planted in parallel rows within a block at 1 m intervals and 3 m spacing between blocks (Supplementary Fig. S2). Soil temperature at 2cm and 8cm depth in the plots was monitored using thermistors and Hobo Data Loggers (Onset Computer Corporation, http://www.onsetcomp.com) (see Supplementary Fig. S2 for location). Air temperature, snow depth, wind speed, and relative humidity were measured at an Environment Canada (2015) weather station 350 m from the plot.
Rhizome harvesting and freezing treatments
Twelve plants of each genotype (three per block) were extracted from the soil for rhizome cold treatments on 21 November 2013 (the autumn trial), 2 February 2014 (the winter trial), and 28 April 2014 (the spring trial). Pairs of rhizomes (including erect tillers for S. pectinata) from each plant were sorted into trays (S. pectinata) or 0.5 l plug containers (M. × giganteus), one for each temperature treatment. The soil in the trays and plugs was either soil from the field site (autumn trial), or a mixture of 40% triple mix (topsoil, sand, and compost blend), 40% coarse sand, and 20% ProMix (Premier Tech, Quebec, Canada) (winter and spring trials).
Two Thermotron S-16–8200 temperature test chambers (Thermotron Industries, http://www.thermotron.com) provided the treatments. For the autumn and spring, rhizomes were first cooled to 0.5°C from the control temperature (2°C for autumn and 4°C spring) for 2h, then cooled to the treatment temperatures; for the winter trials, rhizomes were first placed in the chambers at 0°C. Rhizomes were cooled at 1°C h-1 to the treatment temperature, held there for four hours, and then warmed to 0°C at 1°C h-1. The cooling rate of 1°C h-1 is a recommended cooling rate for determining the LT50 of rhizomes, as it is slow enough to allow for tissue adjustments during ice crystal formation, and also to allow for thermal equilibrium between the chamber and rhizomes (Schimming and Messersmith, 1988; Peixoto et al., 2015 this issue). Rhizome, soil, and air temperature during the trials were monitored with thermocouples attached to Veriteq dataloggers (Vaisala Inc., http://www.vaisala.com). Temperature treatments were −2.5°C, −6°C, −14°C, −19°C, and −29°C for all genotypes for the autumn trial, with controls held at 2°C. For the winter trial, treatment temperatures were −2.5°C, −6°C, and −14°C for M. × giganteus and −14°C, −19°C, −24°C, −29°C, −34°C, and −39°C for S. pectinata, with controls held at 0 to -1°C. Cold treatments for the spring were −2.5°C and −6°C for M. × giganteus and −6°C, −14°C, −19°C, −24°C, and −29°C for S. pectinata, with controls held at 4°C. For the winter trial, colder temperatures were selected for S. pectinata to explore the possibility that it could acclimate and survive below −30°C. The order of freezing treatments was randomized to minimize the effects of time. All rhizomes were stored with the controls prior to treatment for each trial. Rhizomes were stored in temperature-controlled plant growth chambers (Percival Scientific, http://www.percival-scientific.com/; Conviron, http://www.conviron.com) in the dark at −1° to 4°C, sorted in trays/plugs in moist soil (see online supplement Supplementary Appendix S1 for specifics). For the autumn trial, all freezing treatments were completed nine days after harvest. In the winter, treatments were completed 11 days after harvest, and for the spring, eight days after harvest.
After each cold treatment, one rhizome from each pair was removed from the soil and prepared for electrolyte leakage assays by removing leaf bud scales around the rhizome tip. The rhizome was then cut 1.5cm below the tip and the piece briefly rinsed in dH2O before being immersed in 10ml ddH2O (S. pectinata) or 15ml ddH2O (M. × giganteus) at room temperature (20°C). Initial electrolyte leakage (I EL) was measured as the electrical conductivity of the solution after rhizome pieces were allowed to infuse for 24h without agitation. Agitation did not alter the electrolyte leakage pattern (Supplementary Fig. S3). Before sampling for electrical conductivity measurements, vials were vigorously shaken by hand. Electrical conductivity was measured with a conductivity meter (Ultrameter II 4P, Myron L Company, http://www.myronl.com). Total electrolyte leakage (T EL) was measured as the electrical conductivity after vials were boiled for 2h and cooled to room temperature. Percent relative conductivity (%RC) from freezing treatments was calculated as:
As a second assessment of rhizome viability, the second rhizome of the treated pair was allowed to sprout in a greenhouse at 25–27°C day/ 17–19°C night under supplemental lights which provided at least 200 µmol photons m-2 s-1 to the rhizome trays (see Friesen, 2015 for detailed methods). Rhizomes were assessed for sprouting 14–16 d after being moved into the greenhouse. Sprouting was recorded as an actively growing shoot or root from the rhizome. Rhizomes that failed to sprout typically showed signs of deterioration such as discoloration and rot, allowing us to confidently score them as dead.
To assess mortality of rhizomes at the end of the winter season, all of the unused rhizomes from spring-harvested plants on 28 April were inspected for signs of injury and death. All S. pectinata rhizomes were healthy, while numerous M. × giganteus rhizomes were discoloured and showed signs of rot. To assess the viability of the M. × giganteus rhizomes, the entire set was assessed for re-growth in the greenhouse as described above.
Cold tolerance of leaves
On 29 May and 25 June 2014, leaves were harvested from the remaining plants in the field to determine their chilling and subzero cold tolerance. The youngest fully expanded leaves were sampled, and stored in moist paper towel at 4°C until assay. Leaves were stored for 3–18h before assay. For assay, 8cm segments were placed into closed petri plates lined with wet filter paper and treated in either an S-16–8200 or S-1.2C-B Thermotron test chamber. Leaves were first brought to 0.5°C, cooled at 1.5°C h-1 to the treatment temperature, held there for 2h, and then warmed to 0°C at 1.5°C h-1. This cooling rate mimics observed air cooling rates at the field site in the days preceding the harvests (Environment Canada, 2015). Treatment leaf temperatures were 0°C, −1°C, −4°C, −7°C, −10°C and −14°C. After treatment, 1cm2 of leaf was incubated for 24h at 20°C in 5ml ddH2O to allow for electrolyte leakage. Percent relative conductivity (%RC) was then measured as described above for rhizomes with T EL determined after boiling for 45min, followed by cooling of the extract to room temperature.
Canopy measurements
At each rhizome harvest date, samples of senesced leaves were collected from the middle of upper canopy prior to extracting rhizomes from the soil. The leaf samples were dried at 60°C for four days, pulverized and the resulting powder analysed for total % nitrogen and % carbon with a Costech elemental analyser (model ECS 4010; Costech Analytical Technologies Inc., Valencia, CA, USA) at the University of Toronto Mississauga.
Canopy height was measured on plants in the field plots on 17, 29 May and 25 June 2014. The height of each plant was measured from the ground surface to the top of the average culm.
Effects of a mild overnight frost, 16–17 May 2014
Photosynthesis rates and pulse-modulated fluorescence were measured on plants in the field on the morning of 17 May after a mild overnight frost. Upon arrival at the field site at 5:40 am, leaf temperature was immediately measured with a type-T thermocouple and LI-1000 datalogger (Licor Biosciences, http://www.licor.com) just before sunrise. From 9:30 am to 3:30 pm, net CO2 assimilation rate (A, µmol CO2 m-2 s-1) of young, fully expanded leaves was measured at ambient CO2 (400 µmol mol-1) at light levels from 0–1800 µmol m-2 s-1 photons (photosynthetic photon flux density, PPFD). Gas exchange and chlorophyll a fluorescence measurements were made with the LI-6400XT photosynthesis system and LI-6400 Leaf Chamber Fluorometer (Licor Biosciences). The maximum quantum yield of linear electron transport through photosystem II (F v /F m) and the realized yield under light (ΦP) were measured using a multiphase flash in the dark and at each light intensity as the ratio of variable to maximal fluorescence [F v /F m=F m−F o/F m or ΦP=F m′−F s/F m′ (Genty et al., 1989)]. To determine incident ΦCO2max, A was measured at 0, 10, 20, 40, 60 and 80 PPFD. Next, A was measured at 450, 750, 1000, 1250, 1500 and 1800 PPFD to complete the light response curve. The leaf to air vapour pressure difference (vpdL) was held at 0.7–0.9 kPa, and leaf temperature remained close to 11°C, near the daytime high of 10.5°C. All measurements followed the Licor LI-6400XT manual (http://www.licor.com/env/support/index.html?n=6400).
Statistical analyses
To estimate the exposure temperature causing 50% mortality (LT50) and the %RC that corresponds to 50% rhizome death (=lethal electrolyte leakage causing 50% mortality, or LEL50), data were fitted to generalized linear models (glm) with a binomial distribution (Quinn and Keough, 2002). First, to test for spatial heterogeneity across the plot that could affect LT50 or LEL50 values, differences between blocks were tested with model intercept contrasts under the following model: re-growth ~ genotype + block + season + treatment temperature or %RC. No significant effects from any block were found and the entire data set was pooled for subsequent analyses. To test for differences between genotypes in their LT50 and LEL50 response across all sampling dates, and for differences between dates across all genotypes, glm models of the form: re-growth ~ genotype + season + treatment temperature or %RC were performed. Different genotypes and seasons were set as the model intercept to contrast each genotype and season. To estimate the LT50 and LEL50 for rhizomes, fitted values from the glm models were regressed with best-fit sigmoidal curves, and the x-value (temperature or %RC) at 50% survivability (y=0.5) was calculated. To calculate the temperature corresponding to 50% RC (TEL50) for leaves, the raw data were fitted with a best-fit sigmoidal curve, and the temperature (x-value) at 50% RC (y=0.5) was calculated. The TEL50 was chosen to compare leaf freezing tolerance between genotypes as it is reported by other authors for grasses (Rowley et al., 1975; Bykova and Sage, 2012), but may not necessarily represent the lethal temperature threshold for leaves. All generalized linear models were performed in R statistical software (R-Core-Team, 2013) and all logistic regressions were performed with SigmaPlot version 12 (http://www.systat.com/).
For the photosynthesis data, one-way ANOVAs found no significant differences between S. pectinata ecotypes, and data was pooled to compare against M. × giganteus. Differences in incident ΦCO2max were tested between S. pectinata and M. × giganteus by comparing slopes of linear regressions of A and PPFD up to 80 PPFD following Zar (1996). Above this light intensity, A and ΦP were compared with t-tests. Intergenotypic differences in canopy height and leaf nitrogen were tested across all three accessions of S. pectinata and M. × giganteus with Holm-Sidak post hoc tests following significant one-way ANOVAs. All ANOVAs and t-tests were performed in SPSS Statistics version 20 (http://www-01.ibm.com/software/analytics/spss/). For more information on the calculation of LT50, LEL50, and TEL50 values and statistical treatment of photosynthesis and canopy height data see Supplementary Appendix S2.
Results
Air and soil temperature
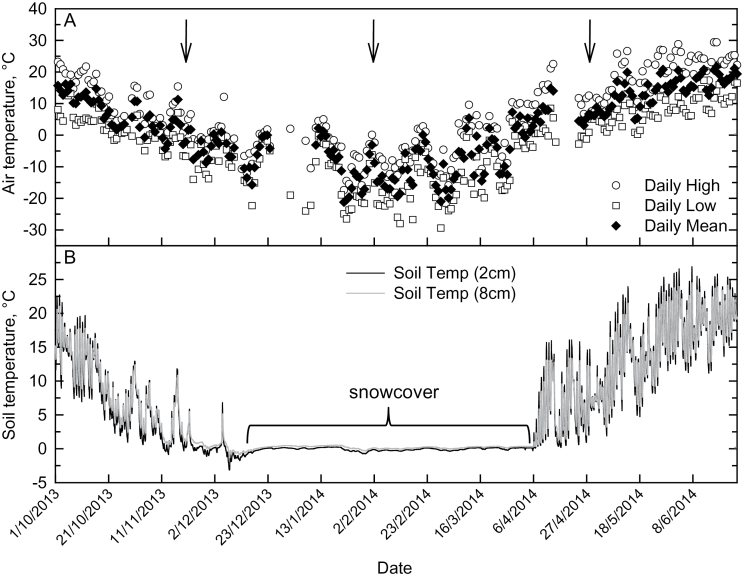
Minimum daily air temperature at the field site fell below 0°C by the end of October 2013 and did not consistently rise above 0°C until the middle of April 2014 (Fig. 1A). Minimum air temperature was below −20°C for much of January and February 2014, and fell to seasonal minimums near −30°C on four dates in later January to February (Fig. 1A). Soil temperature followed the decline in air temperature from the beginning of September until 11 November, when temperatures at 2cm depth first fell below 0°C (Fig. 1). Following a warm front on 5 December, a cold front reduced soil temperatures to −3°C at 2cm depth and −1°C at 8cm depth. On 7 December, strong winds up to 41 km h-1 reduced soil temperatures to their coldest point of the autumn/winter season such that on the morning of 8 December, soil temperatures across the plot ranged from −0.5°C to −6.0°C at 2cm depth to −0.1°C to −3.4°C at 8cm depth. Snow accumulated shortly after this time, and stayed until approximately 4 April, with mid-winter accumulations of over 40cm (Jordan Forsyth, Elora Weather Station Manager, personal communication). Despite periodic air temperatures that fell below −25°C, soil temperatures remained near zero throughout the period of snow cover and only warmed above 0°C following complete snow melt after 6 April (Fig. 1).
Fig. 1.
Air and soil temperatures at Elora, Ontario field site, 1 October 2013–25 June 2014. (A) Mean, maximum and minimum air temperature and (B) mean soil temperature. Soil temperatures are an average of thermistors at 2cm and 8cm depth below soil surface across the field plot (n=7 for each depth). Arrows indicate harvest dates for rhizomes and senesced leaves (21 November 2013, 2 February 2014 and 28 April 2014). Air temperature is from the Environment Canada National Climate Data and Information Archive (Environment Canada 2015).
Rhizome freezing tolerance and overwintering capacity
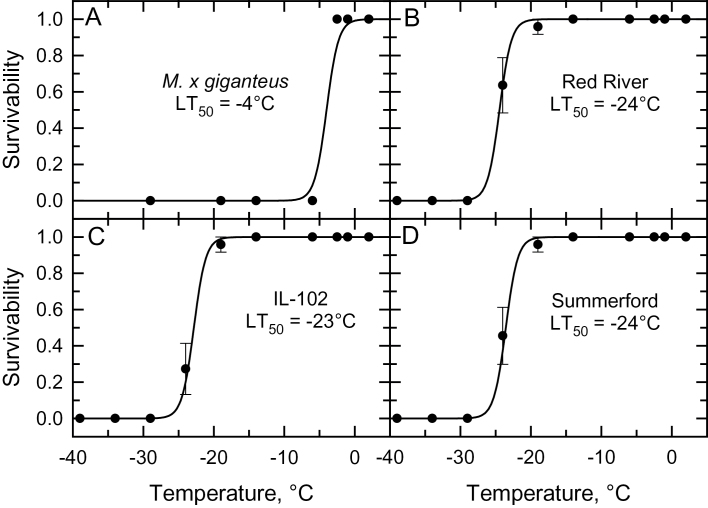
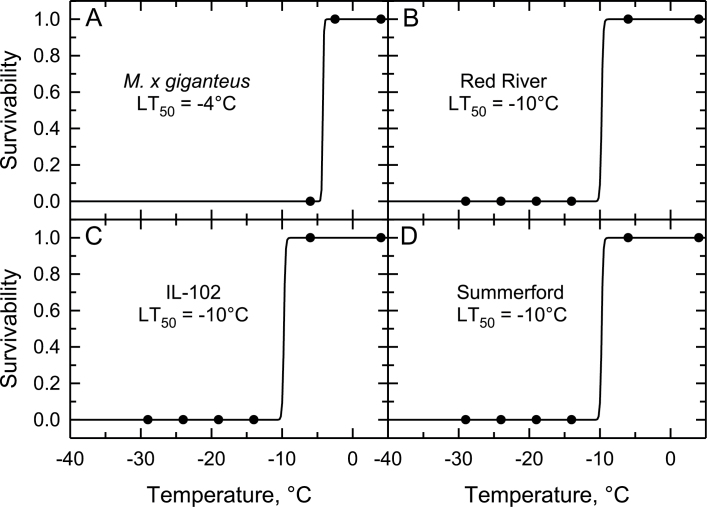
Within each genotype, we observed no difference in the LT50 or LEL50 of rhizomes between the November (autumn) and February (winter) trials, allowing us to pool the results for these sampling periods (Table 1, Supplementary Table S1). All accessions of S. pectinata showed a lower LT50 compared to M. × giganteus (Table 2). The LT50 of rhizomes from the autumn/winter harvest of the three S. pectinata genotypes was −23°C to −24°C versus −4°C for M. × giganteus (Table 2, Fig. 2). The LT50 for the 28 April spring harvest of the S. pectinata genotypes was −10°C, while it remained at −4°C for M. × giganteus (Table 2, Fig. 3). When harvested on 28 April, 20% of all rhizomes from M. × giganteus were dead; most of these were close to the soil surface in the rhizome cluster. There was no rhizome mortality in any of the three S. pectinata genotypes.
Table 1.
Summary of contrast tests of inter-seasonal differences in the temperatures (LT50) and percent electrolyte leakage (LEL50) that correspond to 50% rhizome mortality in Miscanthus × giganteus and three genotypes of Spartina pectinataSeasons (as model coefficients) were set as the model intercept and contrasted against each of the other seasons. ** indicates significance at P<0.01 and *** at P<0.001
| Seasonal contrast | LT 50 | LEL 50 |
|---|---|---|
| Autumn–winter | ns | ns |
| Autumn–spring | *** | ** |
| Winter–spring | *** | ** |
Table 2.
The temperatures (LT50) and percent electrolyte leakage (LEL50) corresponding to 50% rhizome mortality in Miscanthus × giganteus and three genotypes of Spartina pectinata harvested in the autumn/winter, and spring trialsDifferent letters indicate significant contrast differences between genotypes (P<0.01). Genotypes (as model coefficients) were set as the model intercept and contrasted against each of the other genotypes.
| Genotype | Autumn/Winter | Spring | ||
|---|---|---|---|---|
| LT 50 | LEL 50 | LT 50 | LEL 50 | |
| M. × giganteus (M161) | −4°CA | 48%A | -4°CA | 47%A |
| S. pectinata (Red River) | −24°CB | 29%B | -10°CB | 23%B |
| S. pectinata (IL-102) | −23°CB | 34%C | -10°CB | 27%C |
| S. pectinata (Summerford) | −24°CB | 28%B | -10°CB | 29%B |
Fig. 2.
The relationship between exposure temperature and the % of rhizome survival for the autumn and winter sampling dates. (A) Results from Miscanthus × giganteus. (B–D) Results from the ‘Red River’, ‘IL-102’ and ‘Summerford’ accessions of Spartina pectinata. Each symbol is the pooled mean ±SE of the available rhizomes from plants harvested on 21 November 2013 (autumn harvest) and 2 February 2014 (winter harvest). n=11–24 rhizomes per treatment temperature. The estimated temperatures corresponding to 50% rhizome mortality (LT50) are shown in each panel. The trend line is the predicted relationship using a generalized linear model fitted to the data. See online Supplementary Tables S1 and S2 for means of autumn and winter data.
Fig. 3.
The relationship between exposure temperature and the % of rhizome survival for the spring harvest on 28 April 2014. (A) Results from Miscanthus × giganteus. (B–D) Results from the ‘Red River’, ‘IL-102’ and ‘Summerford’ accessions of Spartina pectinata. Mean ±SE, n=12 rhizomes per treatment temperature. The estimated temperatures corresponding to 50% rhizome mortality (LT50) are shown in each panel. The trend line is the predicted relationship using a generalized linear model fitted to the data. See online Supplementary Table S3 for means.
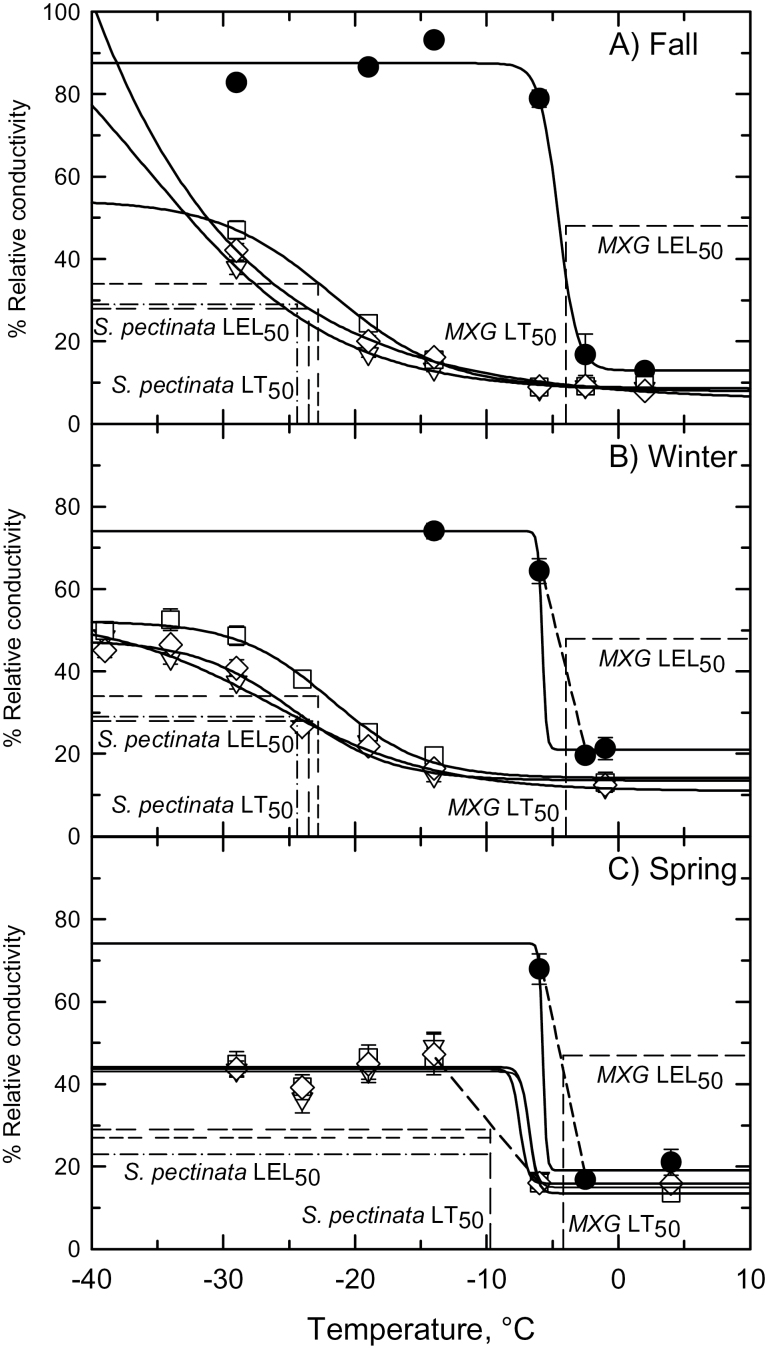
The response of rhizome electrolyte leakage to treatment temperature was markedly different between S. pectinata and M. × giganteus. In M. × giganteus, %RC rose sharply as treatment temperatures fell below −3°C, while in the three S. pectinata genotypes, %RC showed a gradual increase below −10°C during the autumn and winter, and below −6°C at the time of the spring harvest (Fig. 4). Using our LT50 values, we estimated the %RC value that corresponded to 50% mortality (LEL50), and observed it was near 30% in all S. pectinata genotypes and near 47% for M. × giganteus in both the autumn/winter and spring trials (Supplementary Figs S4, S5). We then estimated the temperature that corresponded to these LEL50 values to obtain an independent estimate of the lethal cold threshold for S. pectinata and M. × giganteus rhizomes. In the autumn for both species, and winter for S. pectinata, best-fit sigmoidal regressions corresponded well to the intersection of the LEL50 values and the LT50 values (Fig. 4A, B). This indicates that the sigmoidal fit was a good approximation of the threshold response. However, the intersect of the LEL50 and LT50 values did not correspond well to the sigmoidal fit for the winter response of M. × giganteus and the spring responses for both species (Fig. 4B, C). In these three cases, a straight line connecting the two data points bracketing the threshold portion of the response provided better correspondence with the intersect of the LEL50 and LT50 values. The range of temperatures corresponding to the LEL50 estimated using best-fit regressions or straight lines were near −23°C for S. pectinata harvested in November and February, and between −7°C and −10°C for S. pectinata harvested in April. For M. × giganteus, the range of temperatures corresponding to the LEL50 estimated using best-fit regressions or straight lines was −4°C to −6°C for M. × giganteus at all sample dates (Fig. 4B, C).
Fig. 4.
The relationship between % relative conductivity and treatment temperature for rhizomes of Miscanthus × giganteus and three genotypes of Spartina pectinata harvested on (A) 21 November 2013 (the autumn harvest), (B) 2 February2014 (the winter harvest) and (C) 28 April 2014 (the spring harvest). Mean ±SE, n=10–12 rhizomes per treatment temperature, except for M. × giganteus in the winter (n=1–11). Miscanthus × giganteus (●); S. pectinata accessions: ‘Red River’ (∆), ‘IL-102’ (□), ‘Summerford’ (◊). Solid curves represent best-fit sigmoidal responses. Dashed lines show intersection of LEL50 and LT50 values for each sampling time. Miscanthus × giganteus (single-dash); S. pectinata genotypes: ‘Red River’ (dash-dot), ‘IL-102’ (triple-dash), ‘Summerford’ (double-dash). In (C), where the intersection of the LEL50 and LT50 did not closely correspond to a solid regression curve, the two points that bracket the sharp transition in the %RC versus temperature response are connected with a dashed line.
Leaf cold tolerance, emergence and canopy growth
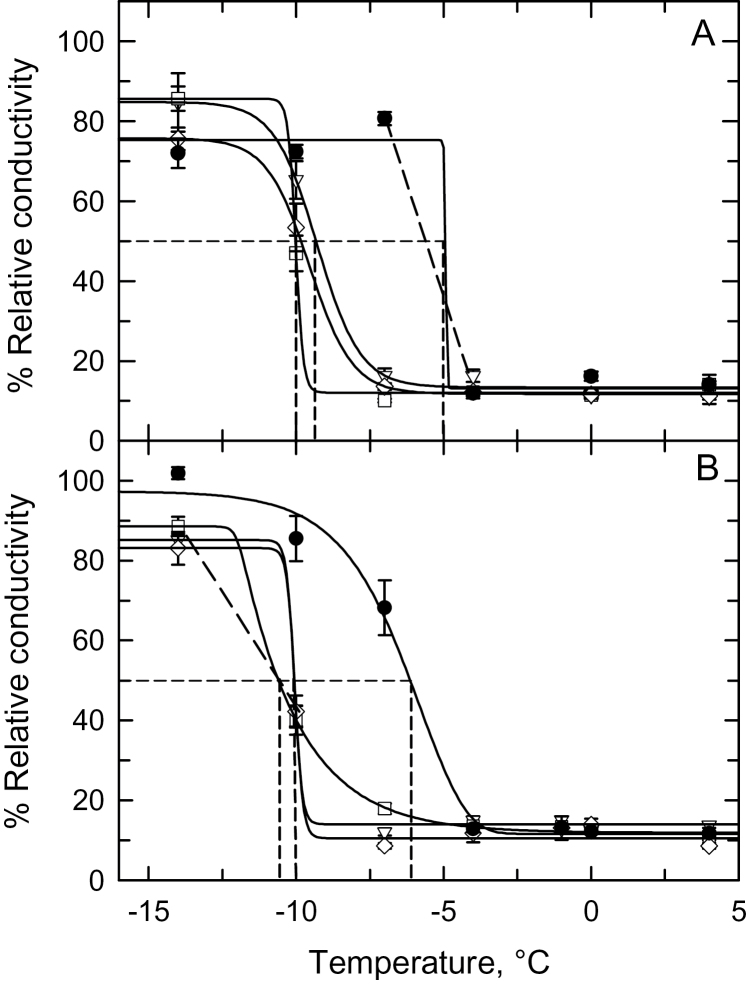
Cold tolerance thresholds of leaves showed the same general pattern for both spring harvest dates. For the 29 May harvest, the temperature corresponding to 50% electrolyte leakage (TEL50) was −5°C in M. × giganteus and −9°C to −10°C in the three S. pectinata genotypes (Fig. 5A). For the 25 June harvest the TEL50 was −6°C in M. × giganteus and −10°C to −11°C in the three S. pectinata genotypes (Fig. 5B).
Fig. 5.
The relationship between % relative conductivity and treatment temperature for leaves of Miscanthus × giganteus and the three genotypes of Spartina pectinata on (A) 29 May 2014 harvest and (B) 25 June 2014. Miscanthus × giganteus (●); S. pectinata accessions: ‘Red River’ (∆), ‘IL-102’ (□), ‘Summerford’ (◊). Mean ±SE, n=9–12 per treatment temperature. Solid curves show the corresponding best-fit logistic regressions. Temperatures corresponding to 50% electrolyte leakage (=50% RC and the TEL50) are indicated by the dashed lines.
Growth phenology, emergence dates, and canopy height
On 8 September 2013 both S. pectinata ‘Summerford’ and ‘Red River’ had fully flowered and were maturing seed, however S. pectinata ‘IL-102’ and M. × giganteus had still not flowered and showed no flag leaves. On 4 November, a hard overnight frost (−5°C overnight low) killed leaves of M. × giganteus while still green. In contrast, green leaves of S. pectinata ‘IL-102’ were still viable and healthy (Henk Wichers, University of Guelph, personal observation). Leaves of S. pectinata ‘Summerford’ and ‘Red River’ had already fully senesced by this time.
Senesced leaves of M. × giganteus had greater leaf nitrogen content compared to all genotypes of S. pectinata for both the autumn (21 November 2013) and winter (2 February 2014) harvests. Autumn leaf nitrogen content ranged from 0.96% in S. pectinata ‘Red River’ to 2.02% in M. × giganteus (Table 3). Spartina pectinata ‘Summerford’ showed significantly greater leaf nitrogen content than S. pectinata ‘Red River’ but S. pectinata ‘IL-102’ was not significantly different from either genotype (Table 3). Nitrogen content of senesced leaves on 28 April 2014 ranged from 0.96% in S. pectinata ‘IL-102’ to 1.43% in M. × giganteus (Table 3).
Table 3.
Mean nitrogen content (±SE) of senesced upper canopy leaves harvested on 21 November 2013 (autumn), 2 February 2014 (winter), or 28 April 2014 (spring)Values are percent nitrogen (g N g-1×100%). Different letters indicate significant differences between genotypes from Hold-Sidak post hoc tests following one way ANOVAs (P<0.05). n=9–12
| Genotype | Autumn | Winter | Spring | |||
|---|---|---|---|---|---|---|
| M. × giganteus (M161) | 2.02 | (0.1)a | 1.99 | (0.1)a | 1.43 | (0.1)a |
| S. pectinata (Red River) | 0.96 | (0.1)c,d | 1.04 | (0.2)b,c,d | 1.16 | (0.1)a,c |
| S. pectinata (IL-102) | 1.19 | (0.1)b,c,d | 0.88 | (0.1)c,d | 0.96 | (0.1)b,c |
| S. pectinata (Summerford) | 1.47 | (0.1)b | 1.36 | (0.1)b | 1.34 | (0.1)a |
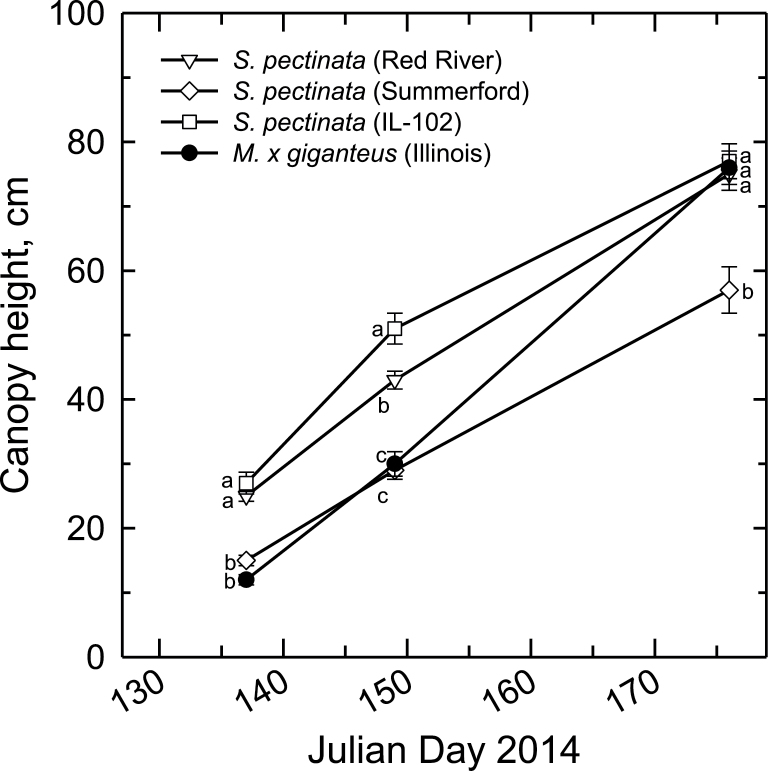
At the autumn harvest (21 November), all genotypes of S. pectinata had senesced their leaf canopies from the previous summer and produced spikes of scale-like leaves that emerged above the soil surface before entering dormancy. These spikes appeared dormant on 19 April of the following year (2014) but by 28 April, some were opening to reveal new green leaves. Shoots of M. × giganteus had not emerged on 28 April, and had just recently emerged by 12 May (Henk Wichers, personal communication). A linear regression of M. × giganteus canopy heights from 17 May and 29 May estimated 9 May to be the emergence date (not shown). Canopy heights were significantly greater for S. pectinata ‘IL-102’ and ‘Red River’ compared to S. pectinata ‘Summerford’ and M. × giganteus throughout May (Fig. 6). By 25 June, canopy heights of S. pectinata ‘IL-102’, ‘Red River’ and M. × giganteus were all close to 80cm with only S. pectinata ‘Summerford’ being significantly lower (Fig. 6). On 25 June, S. pectinata ‘Summerford’ had visible or emerging flower spikes, however none of the other genotypes showed signs of flowering. By 6 October, S. pectinata ‘Red River’ had finished maturing seed, whereas ‘IL-102’ and M. × giganteus showed flag leaves but no visible flowers.
Fig. 6.
Mean canopy heights (±SE) for the spring of 2014 for Miscanthus × giganteus and the three indicated accessions of Spartina pectinata. n=4–10 plants for each date. Different letters indicate significant differences between genotypes (p<0.05) from Holm-Sidak post hoc tests following one-way ANOVAs.
Photosynthesis after a mild overnight frost
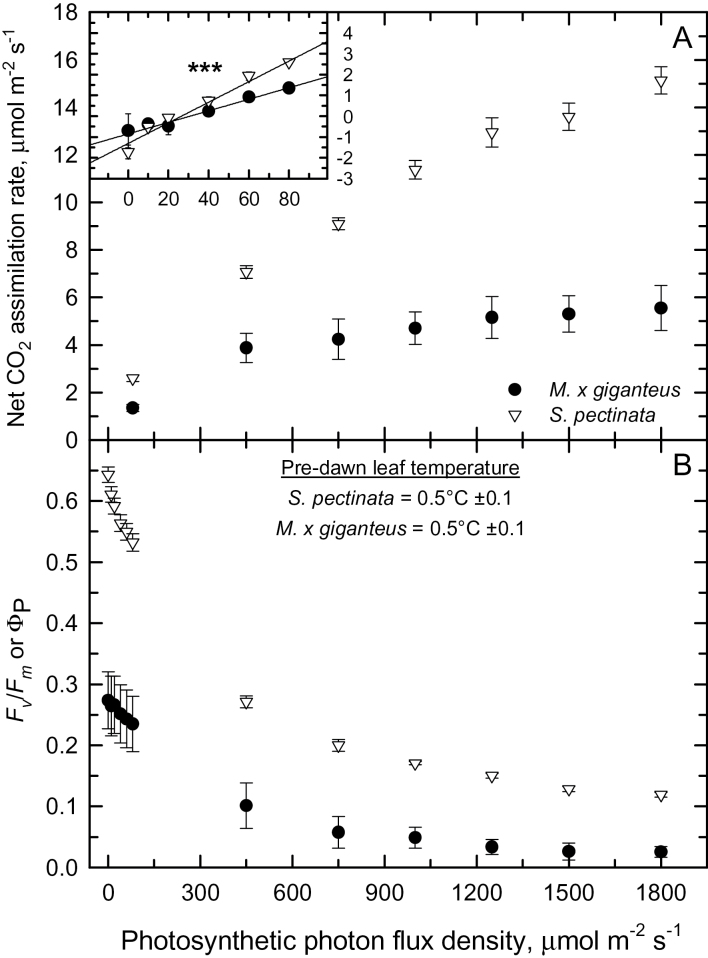
Air temperature fell to 0.3°C early in the morning of 17 May, following 24h of air temperatures <10°C (Fig. 1A). Just before dawn on 17 May, frost was visible on leaves of both S. pectinata and M. × giganteus with leaf temperatures between 0°C and 1°C. Leaves of M. × giganteus were visibly yellow and chlorotic compared to S. pectinata, whose leaves looked healthy and similar in appearance to those later in the spring. At leaf temperatures between 9°C and 12°C, S. pectinata leaves had a greater incident ΦCO2max of 0.051 compared to 0.026 for M. × giganteus on 17 May (Fig. 7A, inset). At every light intensity above 40 µmol m-2 s-1, S. pectinata had a significantly higher A compared to M. × giganteus and was almost three times higher at 1800 µmol m-2 s-1 (Fig. 7A). Photosystem II operating efficiency (ΦP) was also significantly higher in S. pectinata compared to M. × giganteus at every light intensity, being over two times higher at low light and over four times higher at the highest light intensities (Fig. 7B).
Fig. 7.
Photosynthesis after a mild frost in Miscanthus × giganteus and three Spartina pectinata accessions ‘Red River’, ‘IL-102’, and ‘Summerford’ (pooled means). (A) The light response of the net CO2 assimilation rate and (B) F v /F m and ΦP. Measurements were conducted on 17 May 2014 after a mild frost event earlier that day. Means ±SE, n=5–12 for net CO2 assimilation rate, 3–5 for F v /F m, and 2–5 for ΦP. Measurements were made at ambient CO2 concentrations of 400 µmol mol-1 and leaf temperatures of 10.7°C (±0.4) for M. × giganteus and 10.9°C (±0.3) for S. pectinata. T-tests show both parameters are significantly different between the species at each light intensity (p<0.01). (Inset) The light response of net CO2 assimilation rate and light intensity between 0 and 80 µmol photons m-2 s-1 for Miscanthus × giganteus and pooled measurements of the three Spartina pectinata genotypes. Means ±SE, n=3–5 at 0 PPFD and 5–8 for all other light intensities. The maximum incident quantum yield of CO2 assimilation (ΦCO2max) was calculated as the slope of the linear regression shown for each species. Asterisks indicate significantly different slopes (p<0.001).
Discussion
Miscanthus has been promoted as a bioenergy crop due to high productivity in temperate climates, which is due in part to the ability of hybrid lines to maintain carbon gain under cool conditions (Beale et al., 1996; Naidu et al., 2003; Wang et al., 2008). However, to exploit northern temperate and boreal regions, a perennial feedstock such as Miscanthus must endure severe winter cold, episodic frost, and periodic chilling that can extend into summer. The emerging picture from this and other studies is that Miscanthus genotypes generally lack the necessary cold tolerance to guarantee survival in cool temperate to boreal climates (Clifton-Brown et al., 2001a; Jørgensen et al., 2003; Heaton et al., 2008; Deen et al., 2011; Rosser, 2012; Peixoto et al., 2015). Overwintering rhizomes of allopolyploids such as M. × giganteus harvested from the field show >50% mortality and lethal electrolyte leakage at −4°C to −6°C, even when cooled using staging procedures designed to maximize cold acclimation (Table 2; Clifton-Brown et al., 2001a; Clifton-Brown et al., 2011; Peixoto et al., 2015). We also observed that following a winter where the upper soil zone briefly chilled to an average of −3°C in December, 20% of the M. × giganteus rhizomes failed to survive. These rhizomes were mainly along the upper part of the rhizome mass where the cold approached the mean LT50 of −4°C recorded for M. × giganteus rhizomes harvested on 21 November and 2 February. In turn, following emergence, young shoots of many Miscanthus lines are generally intolerant of spring frost, as observed here and by Friesen et al. (2014). Allopolyploid lines were universally killed in a May 2010 frost event (Friesen et al., 2014), and M. × giganteus showed substantial physiological impairment following a mild 17 May 2014 frost. Frost does not generally destroy Miscanthus stands because the rhizomes are protected belowground [although see Christian and Haase (2001), who describe loss of first year plantlets due to frost]. It does however, set back canopy development by killing new growth and thus delaying the onset of canopy closure that is essential for exploiting the long days of late spring.
From these results, we hypothesize that the Elora field site is near the northern range limit of where M. × giganteus plantations would be viable in southern Ontario. In most years, we predict that frost injury would impair southern Ontario Miscanthus canopies, but the overwintering rhizomes would be protected by soil and snow insulation; however, in times of low snow cover, the overwintering rhizomes would periodically encounter intense cold that could kill a substantial fraction of the plantation. In the 2013/2014 winter, the December cold that lowered upper soil temperatures to −3°C was relatively mild, and rhizomes were protected from more intense cold later in the season by a robust snowpack. However, if the intense cold of January to February had occurred when there was no snow and under windy conditions, then a deeper penetration of the cold front would occur, causing widespread rhizome mortality and potential loss of the stand. Given that Miscanthus is a perennial that requires two to three years to reach harvestable yields and is expensive to establish (Heaton et al., 2010), periodic stand loss would be catastrophic from a grower’s perspective. In most of Canada, soil temperatures often fall to −6°C or below at 5cm depth (Environment Canada, 2015; Peixoto et al., 2015), supporting our hypothesis that Elora is on the fringe of viable climates for biomass production of currently available Miscanthus lines. We therefore conclude that expansion of Miscanthus production into climates colder than southern Ontario will require new lines with greater cold tolerance, or non-Miscanthus crops such as switchgrass and Spartina. As demonstrated here, S. pectinata is suitable for colder climates because it shows superior tolerance of mid-winter cold and mild frosts, and initiates canopy development two weeks before M. × giganteus.
Spartina pectinata shows much greater rhizome and leaf freezing tolerance than M. × giganteus, with a midwinter LT50 near −24°C, and a spring leaf TEL50 near −10°C. These are among the coldest viability thresholds reported for C4 grasses and comparable to cold acclimated C3 grasses such as winter oats (Avena sativa) (Rowley et al., 1975; Rowley, 1976; Webb et al., 1994) and its sister species S. gracilis, which remains viable to −27°C in the winter-hardened state (Schwarz and Reaney, 1989). Leaves of S. pectinata exhibited no physiological signs of stress following a mild frost on 17 May, in contrast to M. × giganteus where photosynthesis and quantum yields were well below peak values. In Canada, there are few recorded soil temperatures cold enough to kill any of our S. pectinata accessions in their winter-hardened state outside the Arctic (Environment Canada, 2015). The ability of S. pectinata to acclimate to cold autumn temperatures is also superior to cold tolerant switchgrass varieties, which exhibit LT50 values of −19°C and −22°C at the end of November (Hope and McElroy, 1990).
Little variation was observed in the cold tolerance thresholds of the three accessions of S. pectinata used in this study, indicating little interpopulation variation in this species. With the procedures used here, it would be possible to test this hypothesis by rapidly screening the cold tolerance thresholds of many genotypes using the electrolyte leakage method, assuming a conservative LEL50 of ~30% as reported here. Numerous authors note an LEL50 near 30% is a suitable threshold that corresponds to the 50% mortality threshold in cold tolerance studies (Palta et al., 1993; Coiner, 2012; Peixoto et al., 2015). We note, however, that the cold tolerance of S. pectinata rhizomes is already strong enough to allow for cultivation in most arable lands of higher latitudes, such that crop improvement efforts can focus on other priorities, notably yield enhancement.
By maintaining an active canopy late into the growing season, frost tolerance in the autumn can contribute to seasonal productivity as it does in the spring, but is also important for complete senescence of leaves and culms, with the result that sugars and nutrients can be fully translocated back to rhizomes. In Miscanthus, failure to completely senesce is evident by frequent frost kill of leaves while still green, trapping the nutrients inside the dead leaves (Patrick Friesen, personal observation). Loss of nutrients in senesced leaves complicates Miscanthus cultivation by contributing to leaching loss of nutrients from the stand, which increases fertilizer requirements and could reduce regional water quality (Mann and Tolbert, 2000; Heaton et al., 2009). Miscanthus × giganteus leaves had almost twice the leaf nitrogen content of S. pectinata leaves when senesced. By contrast, the 1.1% to 1.5% nitrogen concentrations of S. pectinata leaves were more typical of autumn senesced leaves in a wide range of species (Aerts, 1996; Bausenwein et al., 2001; Lee et al., 2003).
Spartina has been called ‘a New World parallel to Miscanthus’ (Long and Spence, 2013), a conclusion we support, but add that in many ways, S. pectinata is superior to Miscanthus. In addition to showing superior tolerance of deep winter cold and spring frost relative to Miscanthus, S. pectinata is able to begin canopy development two weeks earlier than Miscanthus, and maintains higher photosynthetic capacity and quantum yield during cold fronts in May. This allows for superior harvesting of photons during the long photoperiods of mid-to-late spring, which are crucial if maximum yields are to be achieved at higher latitude sites. At higher latitudes, the long days of spring and summer partially compensate for the longer growing seasons of lower latitudes. While S. pectinata can achieve respectable biomass yields of 12 t ha-1 yr-1 (Potter et al., 1995; Boe et al., 2009), Miscanthus is clearly superior in terms of overall growth potential, with typical yields that exceed 20 t ha-1 yr-1 (Heaton et al., 2008). This growth differential of S. pectinata and Miscanthus was observed here. The canopy heights of M. × giganteus and S. pectinata were equivalent on 25 June 2014, and by July M. × giganteus had a larger canopy. S. pectinata, however, is unimproved, and reported yields are likely well below peak yields that may arise with genetic improvement (Boe et al., 2013). If greater growth could be achieved, the earlier canopy development of S. pectinata would be a clear benefit that would add to its value as a bioenergy feedstock. Given the lack of differences in cold tolerance between genotypes and the greater growth of ‘Red River’ and ‘IL-102’ that we observed relative to ‘Summerford’, there does not appear to be a tradeoff between cold tolerance and growth capacity in S. pectinata. Such a trade-off is an important consideration during breeding, and efforts to improve S. pectinata growth will need to consider this possibility. The procedures used here would allow for rapid screening of cold tolerance in new varieties, thus ensuring that cold-tolerant lines of S. pectinata are fully capable of exploiting the superior productivity enabled by the C4 photosynthetic pathway.
Supplementary material
Supplementary Appendix S1. Description of rhizome harvest procedure.
Supplementary Appendix S2. Calculation of LT50, LEL50, and TEL50 values and statistical analyses of photosynthesis, canopy height, and leaf nitrogen data.
Supplementary Table S1. Re-growth of rhizomes (%) for autumn harvest.
Supplementary Table S2. Re-growth of rhizomes (%) for winter harvest.
Supplementary Table S3. Re-growth of rhizomes (%) for spring harvest.
Supplementary Fig. S1. Spartina pectinata ‘Summerford’ collection information.
Supplementary Fig. S2. Field site plot map.
Supplementary Fig. S3. Agitation test for rhizome electrolyte leakage.
Supplementary Fig. S4. Electrolyte leakage corresponding to 50% rhizome mortality (LEL50) for autumn/winter harvest.
Supplementary Fig. S5. Electrolyte leakage corresponding to 50% rhizome mortality (LEL50) for spring harvest.
Acknowledgements
We thank Professor Bill Deen and Henk Wichers for assistance with plot access and management, and Kate Withers for donating M. × giganteus rhizomes. We thank Corey Stinson for help with planting as well as gas exchange and leaf temperature measurements, and Jeff Harsant, Jack Radford, Rayyan Abd El Hai, Nick Mirotchnick, Matt Stata, and Stefanie Sultmanis for help with sample preparation. This work was funded by an NSERC Discovery grant to RFS and an Ontario Forage Council grant to Bill Dean and RFS.
References
- Aerts R. 1996. Nutrient resorption from senescing leaves of perennials: are there general patterns? Journal of Ecology 84, 597–608. [Google Scholar]
- Baumel A, Ainouche ML, Bayer RJ, Ainouche AK, Misset MT. 2002. Molecular phylogeny of hybridizing species from the genus Spartina Schreb. (Poaceae). Molecular Phylogenetics and Evolution 22, 303–314. [DOI] [PubMed] [Google Scholar]
- Bausenwein U, Millard P, Raven JA. 2001. Remobilized old-leaf nitrogen predominates for spring growth in two temperate grasses. New Phytologist 152, 283–290. [Google Scholar]
- Beale CV, Bint DA, Long SP. 1996. Leaf photosynthesis in the C4 grass Miscanthus × giganteus, growing in the cool temperate climate of southern England. Journal of Experimental Botany 47, 267–273. [Google Scholar]
- Boe A, Owens V, Gonzalez-Hernandez J, Stein J, Lee DK, Koo BC. 2009. Morphology and biomass production of prairie cordgrass on marginal lands. Global Change Biology Bioenergy 1, 240–250. [Google Scholar]
- Boe A, Springer T, Lee DK, Rayburn AL, Gonzalez-Hernandez J. 2013. Underutilized grasses. In: Saha M, Bhandari HS, Bouton JH, eds. Bioenergy feedstocks: breeding and genetics. John Wiley & Sons, Inc, 173–205. [Google Scholar]
- Bykova O, Sage RF. 2012. Winter cold tolerance and the geographic range separation of Bromus tectorum and Bromus rubens, two severe invasive species in North America. Global Change Biology 18, 3654–3663. [Google Scholar]
- Christian DG, Haase E. 2001. Agronomy of Miscanthus . In: Jones MB, Walsh M, eds. Miscanthus for energy and fibre. London: James & James, 21–45. [Google Scholar]
- Clifton-Brown J, Renvoize S, Chiang YC, et al. 2011. Developing Miscanthus for bioenergy. In: Halford NG, Karp A, eds. Energy crops. Cambridge: Royal Society of Chemistry (Great Britain), 301–321. [Google Scholar]
- Clifton-Brown J, Robson P, Davey C, et al. 2013. Breeding Miscanthus for bioenergy. In: Saha M, Bhandari HS, Bouton JH, eds. Bioenergy feedstocks: breeding and genetics. John Wiley & Sons, Inc, 67–81. [Google Scholar]
- Clifton-Brown JC, Jones MB. 1997. The thermal response of leaf extension rate in genotypes of the C4 grass Miscanthus: an important factor in determining the potential productivity of different genotypes. Journal of Experimental Botany 48, 1573–1581. [Google Scholar]
- Clifton-Brown JC, Lewandowski I. 2000. Overwintering problems of newly established Miscanthus plantations can be overcome by identifying genotypes with improved rhizome cold tolerance. New Phytologist 148, 287–294. [Google Scholar]
- Clifton-Brown JC, Lewandowski I, Andersson B, Basch G, Christian DG, Kjeldsen JB, Jørgensen U, Mortensen JV, Riche AB, Schwarz KU, Tayebi K, Teixeira F. 2001. a Performance of 15 Miscanthus genotypes at five sites in Europe. Agronomy Journal 93, 1013–1019. [Google Scholar]
- Clifton-Brown JC, Long SP, Jørgensen U. 2001. b Miscanthus productivity. In: Jones MB, Walsh M, eds. Miscanthus for energy and fibre. London: James & James, 46–67. [Google Scholar]
- Coiner HA. 2012. The role of low temperatures in determining the northern range limit of Kudzu (Pueraria montana var lobata), an invasive vine in North America. PhD thesis, Department of Ecology and Evolutionary Biology, University of Toronto. [Google Scholar]
- Deen B, Young D, Rowsell J, Tubeileh A, Engbers H, Rosser B. 2011. A comparative study assessing variety and management effects on C4 perennial grasses in a northern climate. Aspects of Applied Biology ,112, 205–211. [Google Scholar]
- Dunn JH, Bughrara SS, Warmund MR, Fresenburg BF. 1999. Low temperature tolerance of zoysia grasses. Hortscience 34, 96–99. [Google Scholar]
- Environment Canada. 2015. Elora RCS Ontario. Government of Canada. weather.gc.ca/canada_e.html; http://climate.weather.gc.ca/climateData/dailydata_e.html?timeframe=2&Prov=ONT&StationID=41983&dlyRange=1840-01-01%7C2015-2-22&cmdB1=Go&Year=2013&Month=10&cmdB1=Go#
- Farrell AD, Clifton-Brown JC, Lewandowski I, Jones MB. 2006. Genotypic variation in cold tolerance influences the yield of Miscanthus . Annals of Applied Biology 149, 337–345. [Google Scholar]
- Friesen PC. 2015. Exploiting the productivity of C4 photosynthesis in cool temperate climates: mechanisms and thresholds of cold tolerance in Miscanthus, Saccharum, and Spartina pectinata . PhD thesis. Department of Ecology and Evolutionary Biology, University of Toronto. [Google Scholar]
- Friesen PC, Peixoto MM, Busch FA, Johnson DC, Sage RF. 2014. Chilling and frost tolerance in Miscanthus and Saccharum genotypes bred for cool temperate climates. Journal of Experimental Botany 65, 3749–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990, 87–92. [Google Scholar]
- Gonzalez-Hernandez JL, Sarath G, Stein JM, Owens V, Gedye K, Boe A. 2009. A multiple species approach to biomass production from native herbaceous perennial feedstocks. In Vitro Cellular & Developmental Biology-Plant 45, 267–281. [Google Scholar]
- Heaton EA, Dohleman FG, Long SP. 2008. Meeting US biofuel goals with less land: the potential of Miscanthus . Global Change Biology 14, 2000–2014. [Google Scholar]
- Heaton EA, Dohleman FG, Long SP. 2009. Seasonal nitrogen dynamics of Miscanthus × giganteus and Panicum virgatum . Global Change Biology Bioenergy 1, 297–307. [Google Scholar]
- Heaton EA, Dohleman FG, Miguez AF, Juvik JA, Lozovaya V, Widholm J, Zabotina OA, McIsaac GF, David MB, Voigt TB, Boersma NN, Long SP. 2010. Miscanthus: A promising biomass crop. In: Jean-Claude K, Michel D, eds. Advances in botanical research , Vol. 56: Academic Press, 75–137. [Google Scholar]
- Hope HJ, Mcelroy A. 1990. Low-temperature tolerance of switchgrass (Panicum virgatum L). Canadian Journal of Plant Science 70, 1091–1096. [Google Scholar]
- Jørgensen U, Mortensen J, Kjeldsen JB, Schwarz KU. 2003. Establishment, development and yield quality of fifteen Miscanthus genotypes over three years in Denmark. Acta Agriculturae Scandinavica Section B-Soil and Plant Science 53, 190–199. [Google Scholar]
- Lee DW, O’Keefe J, Holbrook NM, Feild TS. 2003. Pigment dynamics and autumn leaf senescence in a New England deciduous forest, eastern USA. Ecological Research 18, 677–694. [Google Scholar]
- Lewandowski I, Clifton-Brown JC, Scurlock JMO, Huisman W. 2000. Miscanthus: European experience with a novel energy crop. Biomass & Bioenergy 19, 209–227. [Google Scholar]
- Lewandowski I, Scurlock JMO, Lindvall E, Christou M. 2003. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass & Bioenergy 25, 335–361. [Google Scholar]
- Long SP, Spence AK. 2013. Toward cool C4 crops. Annual Review of Plant Biology 64, 701–722. [DOI] [PubMed] [Google Scholar]
- Madakadze IC, Coulman BE, Mcelroy AR, Stewart KA, Smith DL. 1998. Evaluation of selected warm-season grasses for biomass production in areas with a short growing season. Bioresource Technology 65, 1–12. [Google Scholar]
- Mann L, Tolbert V. 2000. Soil sustainability in renewable biomass plantings. Ambio 29, 492–498. [Google Scholar]
- Montemayor MB, Price JS, Rochefort L, Boudreau S. 2008. Temporal variations and spatial patterns in saline and waterlogged peat fields. Environmental and Experimental Botany 62, 333–342. [Google Scholar]
- Naidu SL, Moose SP, Al-Shoaibi AK, Raines CA, Long SP. 2003. Cold tolerance of C4 photosynthesis in Miscanthus × giganteus: adaptation in amounts and sequence of C4 photosynthetic enzymes. Plant Physiology 132, 1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palta JP, Whitaker BD, Weiss LS. 1993. Plasma-membrane lipids associated with genetic-variability in freezing tolerance and cold-acclimation of Solanum species. Plant Physiology 103, 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto MM, Friesen PC, Sage RF. 2015. Winter cold-tolerance thresholds in field-grown Miscanthus hybrid rhizomes. Journal of Experimental Botany 66, 4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsild AE, Cody W. 1980. Vascular plants of continental Northwest Territories, Canada. Ottawa: National Museum of Natural Sciences, National Museums of Canada. [Google Scholar]
- Potter L, Bingham MJ, Baker MG, Long SP. 1995. The potential of two perennial C4 grasses and a perennial C4 sedge as ligno-cellulosic fuel crops in NW Europe. Crop establishment and yields in E England. Annals of Botany 76, 513–520. [Google Scholar]
- Pyter R, Heaton E, Dohleman F, Voigt T, Long S. 2009. Agronomic experiences with Miscanthus × giganteus in Illinois, USA. Methods in Molecular Biology 581, 41–52. [DOI] [PubMed] [Google Scholar]
- Quinn GP, Keough MJ. 2002. Experimental design and data analysis for biologists. Cambridge: Cambridge University Press. [Google Scholar]
- R-Core-Team. 2013. R: a language and environment for statistical computing.
- Rogers RA, Dunn JH, Nelson CJ. 1975. Cold hardening and carbohydrate composition of Meyer zoysia. Agronomy Journal 67, 836–838. [Google Scholar]
- Rosser B. 2012. Evaluation of Miscanthus winter hardiness and yield potential in Ontario. Masters thesis, Department of Plant Agriculture, University of Guelph. [Google Scholar]
- Rowley J. 1976. Development of freezing tolerance in leaves of C4 grasses. Functional Plant Biology 3, 597–603. [Google Scholar]
- Rowley JA, Tunnicliffe CG, Taylor AO. 1975. Freezing sensitivity of leaf tissue of C4 grasses. Functional Plant Biology 2, 447–451. [Google Scholar]
- Sacks EJ, Juvik JA, Lin Q, Stewart R, Yamada T. 2013. The gene pool of Miscanthus species and its improvement. In: Paterson AH, ed. Genomics of the Saccharinae. New York: Springer, 73–101. [Google Scholar]
- Schimming WK, Messersmith CG. 1988. Freezing resistance of overwintering buds of 4 perennial weeds. Weed Science 36, 568–573. [Google Scholar]
- Schwarz AG, Reaney MJT. 1989. Perennating structures and freezing tolerance of northern and southern populations of C4 grasses. Botanical Gazette 150, 239–246. [Google Scholar]
- Schwarz AG, Redmann RE. 1988. C4 grasses from the boreal forest region of northwestern Canada. Canadian Journal of Botany-Revue Canadienne de Botanique 66, 2424–2430. [Google Scholar]
- Wang DF, Portis AR, Moose SP, Long SP. 2008. Cool C4 photosynthesis: pyruvate pi dikinase expression and activity corresponds to the exceptional cold tolerance of carbon assimilation in Miscanthus × giganteus . Plant Physiology 148, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb MS, Uemura M, Steponkus PL. 1994. A comparison of freezing injury in oat and rye: two cereals at the extremes of freezing tolerance. Plant Physiology 104, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. 1996. Biostatistical analysis. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Zub HW, Arnoult S, Younous J, Lejeune-Henaut I, Brancourt-Hulmel M. 2012. The frost tolerance of Miscanthus at the juvenile stage: differences between clones are influenced by leaf-stage and acclimation. European Journal of Agronomy 36, 32–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.