Abstract
Background
Neuroendocrine tumors (NETs) are believed to be relatively rare and to follow a generally indolent course. However, liver metastases are common in NET patients and the outcome of NET liver metastasis is poor. In Western countries, streptozocin (STZ) has been established as a first-line anticancer drug for unresectable NET; however, STZ cannot be used in daily practice in Japan. The aim of the present study was to determine the status of STZ usage in Japan and to evaluate the effectiveness and safety of STZ chemotherapy in Japanese NET patients.
Methods
A retrospective multi-center survey was conducted. Five institutions with experience performing STZ chemotherapy participated in the study. The patient demographics, tumor characteristics, context of STZ chemotherapy, and patient outcome were collected and assessed.
Results
Fifty-four patients were enrolled. The main recipients of STZ chemotherapy were middle-aged patients with pancreatic NET and unresectable liver metastases. The predominant regimen was the weekly/bi-weekly intravenous administration of STZ combined with other oral anticancer agents. STZ monotherapy was used in one-fourth of the patients. The median progression-free and overall survival periods were 11.8 and 38.7 months, respectively, and sustained stable disease was obtained in some selected patients. The adverse events profile was mild and tolerable.
Conclusions
Our survey showed the clinical benefit and safety of STZ therapy for Japanese patients with unresectable NET. Therefore, we recommend that STZ, which is the only cytotoxic agent available against NET, should be used in daily practice in Japan.
Electronic supplementary material
The online version of this article (doi:10.1007/s00535-014-1006-3) contains supplementary material, which is available to authorized users.
Keywords: Neuroendocrine tumors, Streptozocin, Multi-center survey, Tumor response, Progression-free survival rate
Introduction
Neuroendocrine tumors (NETs) have been regarded as relatively rare neoplasms, but the number of patients with NET is increasing in the US [1], Europe [2], and Japan [3, 4]. The epidemiological pattern of NET is highly heterogeneous; for example, the tumor location, biological behavior (functioning NET or non-functioning NET), and percentage of distant metastases differ extensively among databases. Therefore, the clinical outcomes of the various treatment modalities also differ according to the characteristics of the study cohort.
The clinical course of well-differentiated NET (NET G1 or NET G2) is believed to be generally indolent, but some previous studies have documented that 40–95 % of NET patients are metastatic at presentation, and the 5-year survival rate is 56–83 % for metastatic intestinal NETs and 40–60 % for metastatic pancreatic NETs. Thus, optimal management of metastatic lesions, especially of liver metastases, is key to improving the outcomes of NET patients [5].
Streptozocin (STZ) was first discovered as an antibiotic derived from Streptomyces achromogenes, and was approved in the US as a cytotoxic antitumor drug for symptomatic or advanced pancreatic NET in 1982. In Western countries, STZ combined with doxorubicin (DOX) or fluorouracil (5-FU) has been established as a first-line chemotherapy for both pancreatic and gastrointestinal NETs based on several clinical trails including randomized clinical trials [6–12]. However, STZ has not been covered by the Japanese insurance system, and Japanese oncologists/gastroenterologists cannot choose this powerful option for the treatment of advanced or metastatic NETs.
Because of these specific circumstances, STZ chemotherapy has only been used in clinical trials in Japan. Therefore, the aim of the study was to investigate the actual situations in which STZ is used in Japan and to evaluate the effectiveness and safety of STZ chemotherapy among Japanese NET patients.
Methods
This study was conducted as a retrospective multi-center survey. Five institutions (The University of Tokyo Hospital, Tokyo; Osaka Saiseikai Noe Hospital, Osaka; Kyoto University Hospital, Kyoto; Japanese Red Cross Medical Center, Tokyo; and Yamagata University Hospital, Yamagata) with experience performing STZ chemotherapy participated in the present study.
Patients who were treated with STZ between September 1995 and November 2011 were included as the study subjects. The following clinicopathological factors were investigated: (1) sex, age at the start of STZ chemotherapy, date at the start of STZ chemotherapy, and performance status at the start of STZ chemotherapy; (2) clinical diagnosis, site of the primary tumor, age of tumor presentation, behavior of the tumor (functioning or non-functioning), presence or absence of metastasis, and metastatic site(s); (3) STZ treatment regimen, period of treatment, total dose of administered STZ, anti-tumor drugs used in combination with STZ; and (4) efficacy of STZ chemotherapy, adverse events, progression-free survival period, and overall survival period. The tumor response to STZ therapy was evaluated using RECIST criteria [13], and adverse events were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4.0). The survival curves were generated using Kaplan–Meier methods [14], and the differences among the curves were evaluated using a log-rank test [15]. Differences were considered significant when P < 0.05.
The protocol was approved by the local ethical committee of each institution that participated in the study. The clinical data were summarized in a blinded manner.
Results
Patients
The data of 54 patients were collected. The patient cohort consisted of 24 male and 30 female patients, and the median age was 54.0 years (range 24–76 years) at the onset of the disease and 56.0 years (range 31–77 years) at the start of STZ administration (Table 1). Regarding the distribution of age at the onset of the disease, a peak occurred at between 50 and 59 years, followed by a second peak at 60–69 years (as shown in the Electronic supplementary material). The performance status of most of the patients was 0 or 1 (Table 1).
Table 1.
Patient demographics and tumor characteristics
| Parameters | No. of patients | Percent (%) |
|---|---|---|
| Sex | ||
| Male | 24 | 44.4 |
| Female | 30 | 55.6 |
| Age at onset | ||
| Mean | 52.5 | |
| Median | 54.0 | |
| Range | 24–76 | |
| Age at the beginning of STZ administration | ||
| Mean | 56.0 | |
| Median | 56.0 | |
| Range | 31–77 | |
| Performance status | ||
| 0 | 34 | 63.0 |
| 1 | 17 | 31.5 |
| 2 | 3 | 5.5 |
| 3–4 | 0 | 0.0 |
| Primary site | ||
| Pancreaticoduodenal NET | (46) | (85.2) |
| Pancreas head | 12 | 26.1 |
| Pancreas body | 10 | 21.7 |
| Pancreas tail | 19 | 41.3 |
| Head, body and tail | 1 | 2.2 |
| Duodenal | 4 | 8.7 |
| Gastrointestinal NET | (8) | (14.8) |
| Stomach | 2 | 25.0 |
| Small Intestine | 1 | 12.5 |
| Rectum | 4 | 50.0 |
| Others | 1 | 12.5 |
| Pathological diagnosis (WHO 2000) | ||
| Well-differentiated endocrine tumor | 0 | 0.0 |
| Well-differentiated endocrine carcinoma | 52 | 96.4 |
| Poorly-differentiated endocrine carcinoma/small cell carcinoma | 1 | 1.8 |
| Others | 1 | 1.8 |
| Functioning NET/non-functioning NET | ||
| Functioning | (18) | (33.3) |
| Gastrinoma | 9 | 16.7 |
| Insulinoma | 7 | 13.0 |
| Glucagonoma | 4 | 7.4 |
| Somatostatinoma | 1 | 1.9 |
| Serotonin, tachykinins producing tumor | 1 | 1.9 |
| Non-functioning | (36) | (66.7) |
| Metastatic site(s) | ||
| Liver | 53 | 98.1 |
| Lymph nodes | 26 | 48.1 |
| Peritoneum | 3 | 5.6 |
| Lung | 2 | 3.7 |
| Others | 10 | 18.5 |
Tumor characteristics
The characteristics of the tumors are summarized in Table 1. Forty-two patients had pancreatic NET (P-NET), and the duodenum and gastrointestinal tract were the original sites in 4 and 8 patients, respectively. The pathological diagnosis based on the WHO Classification 2000 was well-differentiated endocrine carcinoma in 52 patients (96.4 %). One-third of the tumors (n = 18) were functioning, with 9 gastrinomas and 7 insulinomas; the other two-thirds were non-functioning NETs. All the patients had metastatic sites: all but one patient had liver metastasis, with lymph node metastasis being the second most common site (n = 26, 48.1 %).
STZ therapy
STZ chemotherapy was used as a first-line therapy in 39 patients, as a second-line therapy in 11 patients, and as a third-line therapy in 4 patients. The treatments used prior to STZ chemotherapy included transcatheter arterial chemoembolization (TACE), octreotide, 5FU, and gemcitabine.
STZ was administered intravenously in 35 patients (64.8 %) and intra-arterially in 3 patients. Both routes were used in 15 patients. The dosing regimen was daily [350–500 mg/m2 of STZ administered for 5 consecutive days (days 1–5) every 6 weeks] in 14 patients and weekly or bi-weekly in 31 patients (350–1,000 mg/m2 of STZ administered at each treatment).
Both regimens were used in 3 patients. Interestingly, the participating institutions in Eastern Japan applied a weekly or bi-weekly regimen, while the institutions in Western Japan applied a daily regimen.
Thirteen patients received STZ monotherapy, while a combination therapy was used in the other 41 patients. The combined antitumor agents included tegafur-uracil (UFT, n = 26), octreotide (n = 20), fluorouracil (5-FU, n = 15), and oral fluoropyrimidine (S-1, n = 6) (Table 2).
Table 2.
STZ therapy
| Parameters | No. of patients | Percent (%) |
|---|---|---|
| Dosing route | ||
| Intravenous (IV) | 35 | 64.8 |
| Intra-arterial (IA) | 3 | 5.6 |
| IV/IA | 15 | 27.8 |
| Unknown | 1 | 1.9 |
| Dosing regimen | ||
| Daily | 14 | 25.9 |
| Weekly/bi-weekly | 31 | 57.4 |
| Daily/weekly | 3 | 5.6 |
| Others | 6 | 11.1 |
| Antitumor agents combined with STZ | ||
| Doxorubicin | 1 | 1.9 |
| Fluorouracil (5-FU) | 15 | 27.8 |
| Oral fluoropyrimidine (S-1) | 6 | 11.1 |
| Tegafur-uracil (UFT) | 26 | 48.1 |
| Octreotide | 20 | 37.0 |
| Mitomycin C | 3 | 5.6 |
| Interferon | 1 | 1.9 |
| Sunitinib | 1 | 1.9 |
| None (STZ monotherapy) | 13 | 24.1 |
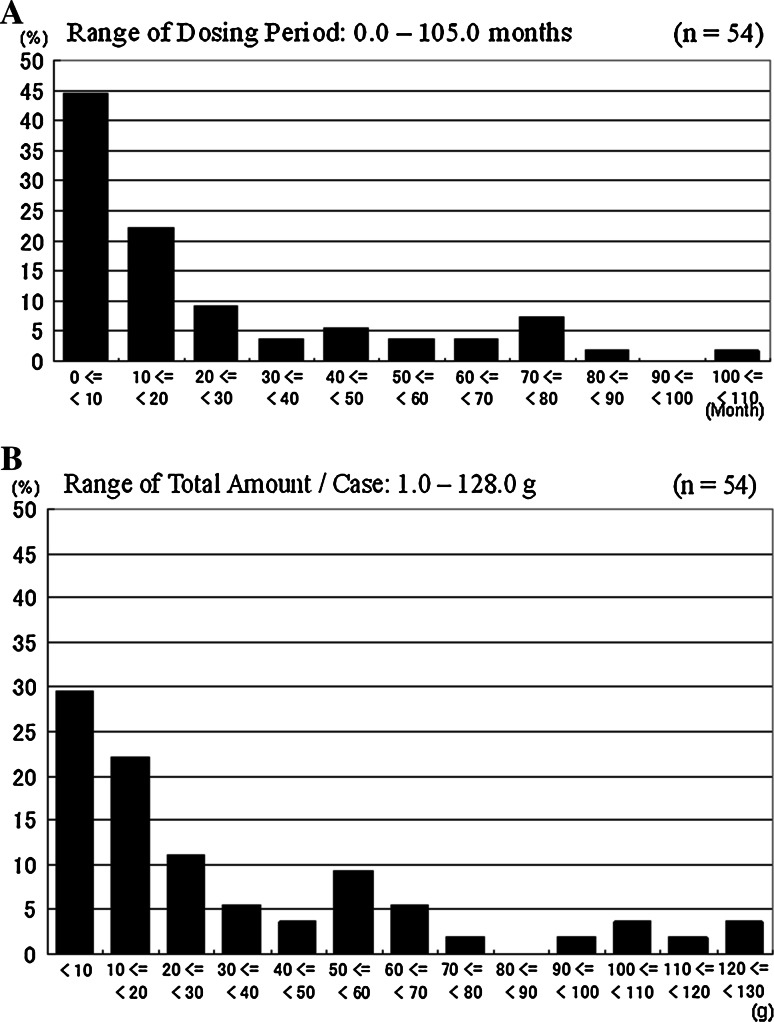
The dosing period ranged from 0 to 105 months, with a median of 12.4 months. The dosing period was within 20 months in most patients (Fig. 1a). The total amount of STZ administered ranged from 1.0 to 128.0 g (median 18.8 g) (Fig. 1b).
Fig. 1.
Distribution of the dosing period (a) and the total amount of STZ administered (b) (n = 54)
The tumor response as evaluated according to the RECIST criteria is shown in Table 3. The tumor response was CR in 2 patients, PR in 11 patients, SD in 9 patients, PD in 25 patients, and unknown in 7 patients, with a response rate of 27.7 %. The response to STZ monotherapy was CR in 1 patient, PR in 4 patients, SD in 1 patient, PD in 8 patients, and unknown in 4 patients, with a response rate of 35.7 %.
Table 3.
Tumor response, evaluated according to the RECIST criteria
| Tumor response according to RECIST criteria |
All cases | Pancreaticoduodenal NET | Gastrointestinal NET | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subtotal | STZ monotherapy | Combination therapy |
Subtotal | STZ monotherapy | Combination therapy | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| 54 | 46 | 14 | 32 | 8 | 4 | 4 | ||||||||
| CR | 2 | 4.3 | 2 | 5.3 | 1 | 11.1 | 1 | 3.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PR | 11 | 23.9 | 9 | 23.7 | 3 | 33.3 | 6 | 20.7 | 2 | 25.0 | 1 | 25.0 | 1 | 25.0 |
| SD | 9 | 19.6 | 8 | 21.1 | 1 | 11.1 | 7 | 24.1 | 1 | 12.5 | 0 | 0.0 | 1 | 25.0 |
| PD | 25 | 54.3 | 20 | 52.6 | 5 | 55.6 | 15 | 51.7 | 5 | 62.5 | 3 | 75.0 | 2 | 50.0 |
| UK | 7 | 7 | 4 | 3 | 0 | 0 | 0 | |||||||
UK unknown
Documented adverse events included nausea (n = 12, 22.2 %), vomiting (n = 7, 13.0 %), and lethargy (n = 4, 7.4 %). Other adverse hematological, hepatobiliary, or nervous system events were observed in a few patients. Grade 3 adverse events were observed in 6 patients (3 nausea and 3 vomiting), but no grade 4 adverse events were documented (Table 4). New-onset diabetes mellitus was not documented, but the control of the disease was impaired during STZ therapy in one patient who had been treated for diabetes mellitus.
Table 4.
Adverse events
| Adverse events | n | % | CTCAE grade | ||||
|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | Unknown | |||
| Gastointestinal disorder | |||||||
| Abdominal pain | 1 | 1.9 | – | 1 | – | – | – |
| Diarrhea | 2 | 3.7 | 1 | 1 | – | – | – |
| Epigastric pain | 1 | 1.9 | 1 | – | – | – | – |
| Nausea | 12 | 22.2 | 5 | 4 | 3 | – | – |
| Acute pancreatitis | 1 | 2.9 | – | 1 | – | – | – |
| Vomiting | 7 | 13.0 | 1 | 3 | 3 | – | – |
| Hematolymphoid system disorder | |||||||
| Leukopenia | 1 | 1.9 | 1 | – | – | – | – |
| Neutropenia | 2 | 3.7 | 1 | 1 | – | – | – |
| Thrombocytopenia | 1 | 1.9 | 1 | – | – | – | – |
| Ocular lesion | |||||||
| Abnormal ocular sensation | 1 | 1.9 | – | – | – | – | 1 |
| Hepatobiliary system disorder | |||||||
| Liver function abnormality | 1 | 1.9 | 1 | – | – | – | – |
| Nerve system disorder | |||||||
| Syncope | 1 | 1.9 | – | – | 1 | – | – |
| Headache | 1 | 1.9 | – | – | – | – | 1 |
| Others | |||||||
| Lethargy | 4 | 7.4 | 3 | 1 | – | – | – |
| Back pain | 1 | 1.9 | – | 1 | – | – | – |
STZ therapy was discontinued in 46 patients. The reasons for the discontinuation were tumor progression in 43 patients, conversion to other treatments in 2 patients, and a severe adverse event in 1 patient.
Patient outcome
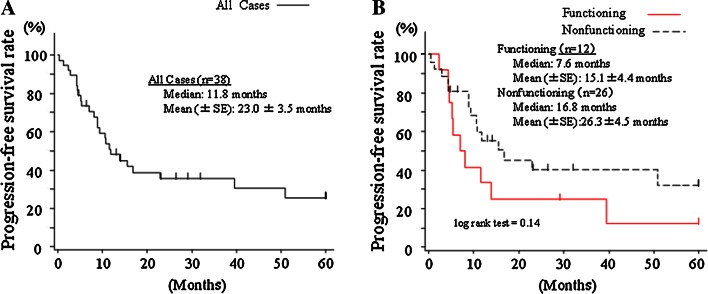
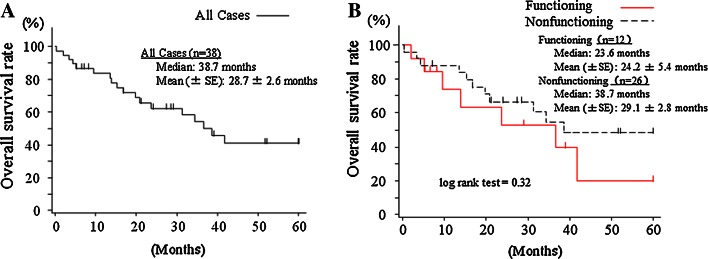
Data regarding patient outcome were available for 38 patients. The progression-free and overall survival curves are shown in Figs. 2 and 3. The median progression-free period was 11.8 months in all of the patients (mean 23.0 ± 3.5 months), 7.6 months in the functioning NET patients, and 16.8 months in the non-functioning NET patients (P = 0.14). Meanwhile, the median survival period was 38.7 months in all of the patients (mean 28.7 ± 2.6 months), 23.6 months in the functioning NET patients, and 38.7 months in the non-functioning NET patients (P = 0.32).
Fig. 2.
Progression-free survival curves for all of the patients (n = 38) (a) and stratified according to functioning (n = 12) and non-functioning (n = 26) tumors (b)
Fig. 3.
Overall survival curves for all the patients (n = 38) (a) and curves stratified according to functioning (n = 12) and non-functioning (n = 26) tumors (b)
The median amount of STZ administered was 18.8 g. When the patients were stratified according to the amount of STZ (≥18.8 or <18.8 g), both the overall survival rate and the progression-free survival rate were better in the patients who received ≥18.8 g STZ (see the Electronic supplementary material 2).
The overall and progression-free survival outcomes were similar among the patients who received a daily regimen and those receiving a weekly/bi-weekly regimen (data not shown). In addition, the outcomes did not differ between patients with pancreaticoduodenal NET (n = 46) and those with gastrointestinal NET (n = 8) (see the Electronic supplementary material 3).
Discussion
The present study was a retrospective multi-center cohort study in patients with unresectable NET receiving STZ chemotherapy. This is the first attempt to determine the circumstances surrounding chemotherapy for NET patients in Japan. The five participating centers were high-volume centers treating NET patients in Japan, and most of the patients who received STZ therapy before 2011 were thought to have been included in the study.
During the study period (from 1995 to 2011), octreotide was the only antitumor agent against NET available in Japan until everolimus and sunitinib began to be covered by the Japanese insurance system. STZ is not yet covered by the Japanese insurance system:, so STZ therapy had only been conducted on a clinical trial basis using imported STZ at all of the institutions that participated in the present study. One of the aims of our study was to encourage the approval of STZ use in a daily clinical setting in Japan.
The results of the present study revealed that the main recipients of STZ chemotherapy were patients with P-NET (well-differentiated endocrine carcinoma based on the WHO Classification 2000) with liver metastases. The dosing routes and dosing regimens varied among the regions and institutions, but an intravenous weekly/bi-weekly regimen was popularly applied. The original regimen proposed by Moertel et al. [7] was a combination therapy of STZ with doxorubicin or STZ with fluorouracil (5-FU); however, in the present study, various antitumor agents were combined with STZ, and STZ monotherapy was applied in one-fourth of the patients. The reasons for this were likely twofold: first, the use of oral anticancer drugs, such as S-1 and UFT, is popular in Japan; second, the use of other cytotoxic anticancer drugs has not been approved.
Our results showed that the response rate was 27.7 % for all of the enrolled patients, and a subgroup analysis showed that the response rate was 28.2 % for pancreaticoduodenal NET patients and 25.0 % for gastrointestinal NET patients, respectively. In addition, STZ monotherapy was associated with a response rate of 35.7 % (40.0 % for pancreaticoduodenal NET and 25.0 % for gastrointestinal NET). These figures were comparable with those obtained in Western series in which radiological measurements were used to evaluate tumor response [10–12].
The dosing period was less than 10 months in 45 % of the patients, and 10–20 months in 22 % of the patients. As a result, the total amount of STZ adminstered was less than 20 g in over 50 % of the patients (Fig. 1b). These results corresponded to a median progression-free period of 11.8 months (Fig. 2). The figure of 11.8 months was similar to that obtained in studies examining everolimus and sunitinib [16, 17]. However, the progression-free survival curve in STZ therapy patients reached a plateau about 2 years after the start of the therapy (Fig. 2), showing a difference from the everolimus and sunitinib studies. This finding suggested that sustained stable disease can be expected in some selected patients receiving STZ, and that these patients can undergo STZ chemotherapy for a long period because of the mild adverse event profile. Actually, some patients in our study received STZ therapy for over 5 years. As expected, the outcomes were better among the patients who received a larger dose of STZ (see the Electronic supplementary material 2). These results also support the idea that long-term STZ chemotherapy is associated with long-term SD maintenance. In our analyses, the progressions and overall survivals were comparable between the patients with functioning NET and those with non-functioning NET, suggesting that STZ is applicable to all NET patients with the same dosing regimen.
Our survey showed that the adverse events associated with STZ chemotherapy were acceptable. Studies using animal models showed that high-dose STZ administration induced impaired glucose tolerance, leading to diabetes mellitus. In the present survey, new-onset diabetes mellitus induced by STZ was not documented. In addition, STZ therapy was discontinued because of a severe adverse event in only one patient. This mild adverse event profile can likely be attributed to the relatively low-dose regimens performed in our series (350–500 mg/m2 in the daily regimen, and 350–1,000 mg/m2 regimen in the weekly/bi-weekly regimen).
In conclusion, our survey showed the clinical benefit and safety of STZ therapy for pancreaticoduodenal and gastrointestinal NET. Therefore, we recommend that STZ, the only cytotoxic agent available for NET, should be used in daily practice in Japan.
Electronic supplementary material
Below are the links to the electronic supplementary material.
Acknowledgments
This study was conducted by the NET Chemotherapy Investigation and Research Group in cooperation with NobelPharma Co. Ltd.
The context of the study was presented at the 49th Annual Meeting of the Japan Society of Clinical Oncology, November 2011, Nagoya.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the US. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Hauso O, Gustafsson BI, Kidd M, et al. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008;113:2655–2664. doi: 10.1002/cncr.23883. [DOI] [PubMed] [Google Scholar]
- 3.Ito T, Sasano H, Tanaka M, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234–243. doi: 10.1007/s00535-009-0194-8. [DOI] [PubMed] [Google Scholar]
- 4.Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2014;6. [DOI] [PubMed]
- 5.Frilling A, Modlin IM, Kidd M, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15:e8–e21. doi: 10.1016/S1470-2045(13)70362-0. [DOI] [PubMed] [Google Scholar]
- 6.Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1980;303:1189–1194. doi: 10.1056/NEJM198011203032101. [DOI] [PubMed] [Google Scholar]
- 7.Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 8.Rivera E, Ajani JA. Doxorubicin, streptozocin, and 5-fluorouracil chemotherapy for patients with metastatic islet-cell carcinoma. Am J Clin Oncol. 1998;21:36–38. doi: 10.1097/00000421-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 9.von Schrenck T, Howard JM, Doppman JL, et al. Prospective study of chemotherapy in patients with metstatic gastrinoma. Gastroenterology. 1988;94:1326–1334. doi: 10.1016/0016-5085(88)90670-1. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson B, Skogseid B, Lundqvist G, et al. Medical treatment and long-term survival in a prospective study of 84 patients with endocrine pancreatic tumors. Cancer. 1990;65:1883–1890. doi: 10.1002/1097-0142(19900501)65:9<1883::AID-CNCR2820650902>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Cheng PN, Saitz LB. Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer. 1999;86:944–948. doi: 10.1002/(SICI)1097-0142(19990915)86:6<944::AID-CNCR8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762–4771. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Boqaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan E, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:257–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 15.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 16.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of oancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.