Progressive disease remains the main cause of treatment failure following allogeneic stem cell transplantation (allo-SCT) for Hodgkin lymphoma (HL), and the role of donor leukocyte infusions (DLIs) in the management of disease recurrence is still being actively debated [1-3]. While published studies on this issue are sketchy, response rates (i.e. complete plus partial responses) in the 30-50% range have been reported but their durability, as well as their ultimate impact on patient survival are not always clear [4-5]. In one recent report, nineteen (79%) of 24 HL patients receiving DLIs for relapse following in vivo T-cell depletion with their alemtuzumab-based conditioning regimen experienced a response, which was durable in many cases [6]. A seemingly higher likelihood of durable responses to DLIs following alemtuzumab-based conditioning had been postulated previously [7]. The role of concomitant chemotherapy with the DLIs (if any) is unclear. We wish to contribute to this debate by providing an update on our initial reports on this topic [8, 9].
Between 1999 and 2010, a total of 27 patients with relapsed/refractory HL following unmanipulated allo-SCT received a total of 55 DLIs as immunotherapy for treatment of progressive disease (PD). We feel that the designation “donor leukocyte infusion” is preferable to “donor lymphocyte infusion”, as clearly the infused product contains additional cell types in addition to lymphocytes, such as monocytes, natural killer (NK) cells, etc. The decision between a DLI (with or without prior chemotherapy) and alternative forms of salvage therapy was made on a case-by-case basis. The DLIs did not undergo any form of manipulation or cell selection.
These patients' characteristics are outlined in Table 1. The DLI was ordinarily preceded by a taper in their immunosuppression (if still ongoing). Their median age was 30 years (19-60; M/F 19/8), and 21/27 (78%) had a history of prior autologous SCT. Seventeen patients received more than one DLI (range 2-5). Seventeen had a matched sibling/parent donor and ten a matched unrelated donor. In all but two cases the conditioning regimen included fludarabine plus cyclophosphamide or melphalan plus/minus antithymocyte globulin. The median time to PD after allo-SCT was 5 months (range 1-21). The median time from PD to the first DLI was 4 months (range 1-34). This retrospective study was approved by the University of Texas M.D. Anderson Cancer Center Institutional Review Board. All patients provided written informed consent to their treatment. In ten patients (37%) prior salvage chemotherapy was administered immediately prior to at least one of their DLIs. This was done at the discretion of the attending physician following the patient. The pre-DLI chimerism status was 2/27 mixed and 25/27 full donor. For details on response criteria definitions, please refer to our original reports [8, 9].
Table 1.
Patient characteristics.
| Patient number | 27 |
|
| |
| Age (years, range) | 30 (19-60) |
|
| |
| Men/women | 19/8 |
|
| |
| Total DLI number (range per patient) | 55 |
| One DLI vs. >1 (patients) | 10 vs. 17 |
| >1 DLI (range) | 2-5 |
|
| |
| Donor | |
| Matched related/parent | 17 (63%) |
| Matched unrelated | 10 (37%) |
|
| |
| Conditioning regimen | |
| Fludarabine with melphalan or cyclophosphamide +/- ATG* | 25 |
| Others | 2 |
|
| |
| Patients who had a previous autograft | 21 (75%) |
| No prior autograft | 6 (25%) |
|
| |
| Time to progression after autograft (months, range) | 5 (1-21) |
|
| |
| Time from disease progression after allotransplant to first DLI (months, range) | 4 (1-34) |
|
| |
| Concomitant chemotherapy** | |
| Yes | 10 (37%) |
| No | 17 (63%) |
DLI: Donor leukocyte infusion. ATG: Antithymocyte globulin.
For patients receiving a matched unrelated donor transplant.
Chemotherapy administered prior to at least one of the DLIs.
Ten of 27 (37%) patients had a complete/partial response (CR/PR) following at least one of their DLIs. Six of 27 patients (22%) achieved CR/CRU (complete response, unknown), and four of 27(15%) achieved a PR. The median response duration was 7.5 months (range 0.5-20). Of these ten responders, ten (100%) developed graft-vs.-host disease (GVHD) and half of them (50%) had received concomitant chemotherapy. Of the ten patients who received only one DLI, two (20%) had chemotherapy prior to their DLIs. One (10%) had a CRU and the other had stable disease (SD) (10%). Of the remaining eight that did not have chemo prior to DLI, 2 (20%) had CRU, 3 (30%) had PR, 2 (20%) had SD and 1 (10%) had PD. The median CD3+ cell dose administered was 49.8 ×106/ kg (range 0.05-285). GVHD developed (or flared) following the DLI in 45/55 cases (82%). After the DLI mixed chimerism (n=2) was converted to complete (or near-complete) donor chimerism.
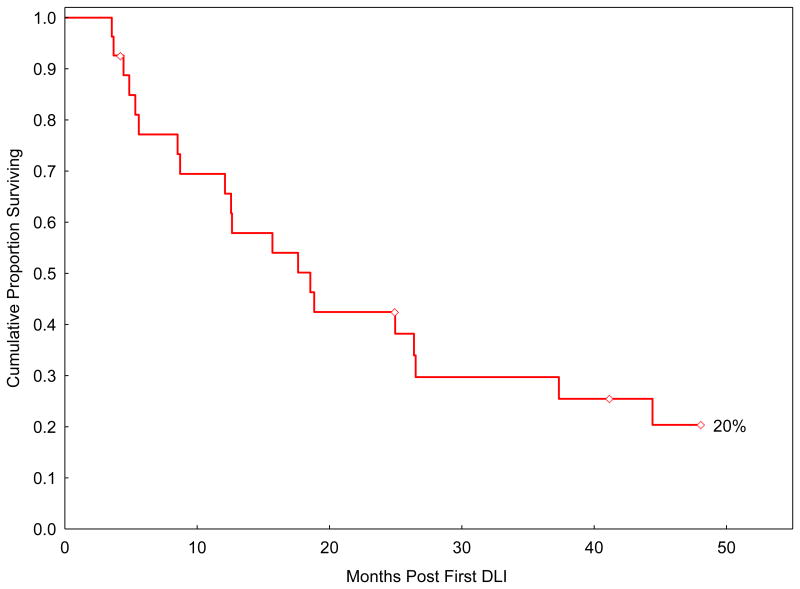
At the latest follow-up (March 2011), five patients (18%) are alive (two in CR). For these five survivors, the follow-up after the first DLI is 41 months (range 4-62). Four of them (80%) did not receive chemotherapy with their DLI(s), while one did. Twenty-two patients expired. Their median survival after the first DLI is 14 months (range 4-64). Causes of death included PD (n=15; 68%) and non-relapse mortality (n=7; 32%). In the latter group, causes of death included intracranial hemorrhage (n=1), pneumonia (n=4), fungal sepsis (n=1), and chronic GVHD (n=1). The actuarial estimate for overall survival at 4 years from the first DLI for the whole group (n=27) is 20% (95% CI 7-38) (Figure 1).
Figure 1.
Overall 4-year survival from the first donor leukocyte infusion (DLI) for the whole group (n=27).
We readily acknowledge the many limitations of this study. They include the retrospective nature of the analysis itself, a small sample size, the long duration of the study period, patient selection and heterogeneity, as well as concomitant chemotherapy administration in some of the cases. Nevertheless, these data suggest that DLIs for immunotherapy of recurrent HL following unmanipulated allo-SCT have significant (albeit not always durable) activity, and a subset of patients become long-term survivors. They also indicate that administration of multiple DLIs is feasible in selected patients. Responses were always associated with the development of GVHD, a finding which would support the presence of a graft-vs.-HL effect. The role (if any) of concomitant chemotherapy cannot be clarified from these limited data. PD (and not GVHD) was the main cause of mortality following DLIs.
That being said, the long-term prognosis for DLI-treated HL patients after an allo-SCT is clearly unsatisfactory. The therapeutic landscape in this area is changing rapidly. The role of DLIs should be reassessed in the contest of new and effective agents currently increasingly available in the salvage setting for HL, such as brentuximab (SGN-35) and panobinostat [10, 11]. While their track record in treating recurrences following allo-SCT is still limited, the preliminary data look quite encouraging [12]. These agents would avoid the significant morbidity and mortality associated with the development of GVHD, which seems required in many (and possibly most) cases to achieve a response.
References
- 1.Greaves PJ, Gribben JG. Demonstration of durable graft versus lymphoma effects in Hodgkin's lymphoma. J Clin Oncol. 2011;29:952–953. doi: 10.1200/JCO.2010.33.2437. [DOI] [PubMed] [Google Scholar]
- 2.Peggs KS, Hunter A, Chopra R, et al. Clinical evidence of a graft-versus-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet. 2005;365:1906–1908. doi: 10.1016/S0140-6736(05)66659-7. [DOI] [PubMed] [Google Scholar]
- 3.Porter DL, Stadtmauer EA, Lazarus HM. ‘GVHD’: graft-versus-host disease or graft-versus-Hodgkin's disease? An old acronym with new meaning. Bone Marrow Transplant. 2003;31:739–746. doi: 10.1038/sj.bmt.1703895. [DOI] [PubMed] [Google Scholar]
- 4.Peggs KS, Anderlini P, Sureda A. Allogeneic transplantation for Hodgkin lymphoma. Br J Haematol. 2008;143(4):468–80. doi: 10.1111/j.1365-2141.2008.07349.x. [DOI] [PubMed] [Google Scholar]
- 5.Robinson SP, Sureda A, Canals C, et al. Reduced-intensity conditioning allogeneic stem cell transplantation for Hodgkin's lymphoma: identification of prognostic factors predicting outcome. Haematologica. 2009;94:230–238. doi: 10.3324/haematol.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peggs KS, Kayani I, Edwards N, et al. Donor lymphocyte infusions modulate relapse risk in mixed chimeras and induce durable salvage in relapsed patients after T-cell-depleted allogeneic transplantation for Hodgkin's lymphoma. J Clin Oncol. 2011;29:971–978. doi: 10.1200/JCO.2010.32.1711. [DOI] [PubMed] [Google Scholar]
- 7.Peggs KS, Sureda A, Qian W, et al. Reduced-intensity conditioning for allogeneic haematopoietic stem cell transplantation in relapsed and refractory Hodgkin lymphoma: impact of alemtuzumab and donor lymphocyte infusions on long-term outcome. Br J Haematol. 2007;139:70–80. doi: 10.1111/j.1365-2141.2007.06759.x. [DOI] [PubMed] [Google Scholar]
- 8.Anderlini P, Acholonu SA, Okoroji GJ, et al. Donor leukocyte infusions in relapsed Hodgkin's lymphoma following allogeneic stem cell transplantation: CD3+ cell dose, GVHD and disease response. Bone Marrow Transplant. 2004;34:511–514. doi: 10.1038/sj.bmt.1704621. [DOI] [PubMed] [Google Scholar]
- 9.Anderlini P, Saliba R, Acholonu S, et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin's lymphoma: the updated M.D. Anderson Cancer Center experience. Haematologica. 2008;93(2):257–64. doi: 10.3324/haematol.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 11.Sureda A, Engert A, Browett PJ, et al. Interim results for the phase II study of panobinostat (LBH589) in patients with relapsed/refractory Hodgkin's lymphoma (HL) after autologous hematopoietic stem cell transplant (AHSCT) J Clin Oncol. 2010;28(Suppl. 15) Abstract 8007. [Google Scholar]
- 12.Gopal AK, Ramchandren R, Berryman RB, et al. Brentuximab vedotin (SGN-35) treatment in relapsed CD30-positive Hodgkin lymphoma patients following allogeneic stem cell transplant: a multi-centre case series. Bone Marrow Transpl. 2011;46(Suppl. 1) Abstract O267. [Google Scholar]