Abstract
BACKGROUND:
Low levels of physical activity (PA) are associated with poor outcomes in people with COPD. Interventions to increase PA could improve outcomes.
METHODS:
We tested the efficacy of a novel Internet-mediated, pedometer-based exercise intervention. Veterans with COPD (N = 239) were randomized in a 2:1 ratio to the (1) intervention group (Omron HJ-720 ITC pedometer and Internet-mediated program) or (2) wait-list control group (pedometer). The primary outcome was health-related quality of life (HRQL), assessed by the St. George’s Respiratory Questionnaire (SGRQ), at 4 months. We examined the SGRQ total score (SGRQ-TS) and three domain scores: Symptoms, Activities, and Impact. The secondary outcome was daily step counts. Linear regression models assessed the effect of intervention on outcomes.
RESULTS:
Participants had a mean age of 67 ± 9 years, and 94% were men. There was no significant between-group difference in mean 4-month SGRQ-TS (2.3 units, P = .14). Nevertheless, a significantly greater proportion of intervention participants than control subjects had at least a 4-unit improvement in SGRQ-TS, the minimum clinically important difference (53% vs 39%, respectively, P = .05). For domain scores, the intervention group had a lower (reflecting better HRQL) mean than the control group by 4.6 units for Symptoms (P = .046) and by 3.3 units for Impact (P = .049). There was no significant difference in Activities score between the two groups. Compared with the control subjects, intervention participants walked 779 more steps per day at 4 months (P = .005).
CONCLUSIONS:
An Internet-mediated, pedometer-based walking program can improve domains of HRQL and daily step counts at 4 months in people with COPD.
TRIAL REGISTRY:
Clinical Trials.gov; No.: NCT01102777; URL: www.clinicaltrials.gov
COPD is the third most common cause of death in the United States; it is the only disease among the top 10 that continues to increase in prevalence.1‐3 The GOLD (Global Initiative for Chronic Obstructive Lung Disease) guidelines state, “all COPD patients benefit from regular physical activity.”3 However, people with COPD are significantly less physically active than healthy people.4,5 Decreased physical activity (PA) is associated with higher levels of systemic inflammation and increased risk of acute exacerbations, hospital admissions, and death, independent of lung function in COPD.6‐11 Physical inactivity is a major contributor to skeletal muscle dysfunction in COPD.12
Interventions that increase PA could favorably impact outcomes in people with COPD. Supervised pulmonary rehabilitation programs improve exercise capacity and health-related quality of life (HRQL) in people with COPD.13 However, low rates of referral, geographic distance from a medical center, and need for travel limit enrollment in and adherence to pulmonary rehabilitation programs.14‐16 Benefits of pulmonary rehabilitation decline to baseline at 6 to 12 months after program completion.17 It is also unclear whether the benefits translate directly into increases in daily physical activities.18 Novel exercise programs are needed to promote PA in people with COPD.
Exercise programs implemented via the Internet have the potential to be widely accessible and promote sustained behavior change.19,20 We developed an Internet-mediated, pedometer-based walking program called Taking Healthy Steps (THS) to promote PA in people with COPD.21‐25 THS provides iterative step-count feedback, individualized step-count goals, education on disease self-management, motivational support, and an online community of social support. A 3-month single-arm study showed this intervention to be safe, engaging, and able to increase daily step counts.22
In the current study, we extend our work by assessing the efficacy of THS to improve HRQL and daily step counts in a randomized controlled trial. The conceptual framework and design of this study has previously been described.25 The 12-month intervention is composed distinctly of an initial intensive 4-month phase followed by an 8-month maintenance phase. In this article, we present the results at 4 months.
Materials and Methods
Participants and Study Design
Potential participants were identified from a national database of veterans who had received medical services in the previous year and had a COPD diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code of 491.x, chronic bronchitis; 492.x, emphysema; or 496.x, chronic airway obstruction NEC). Veterans from the United States and Puerto Rico were enrolled between December 2011 and January 2013.25 We excluded veterans from one of the 21 Veterans Integrated Service Networks, where another study using the THS platform was recruiting participants. This study was conducted in accordance with the amended Declaration of Helsinki. The coordinating center was at the Ann Arbor VA Healthcare System, Ann Arbor, Michigan. Ethical approval was granted by the VA Ann Arbor Human Studies Subcommittee (VA# 0008).
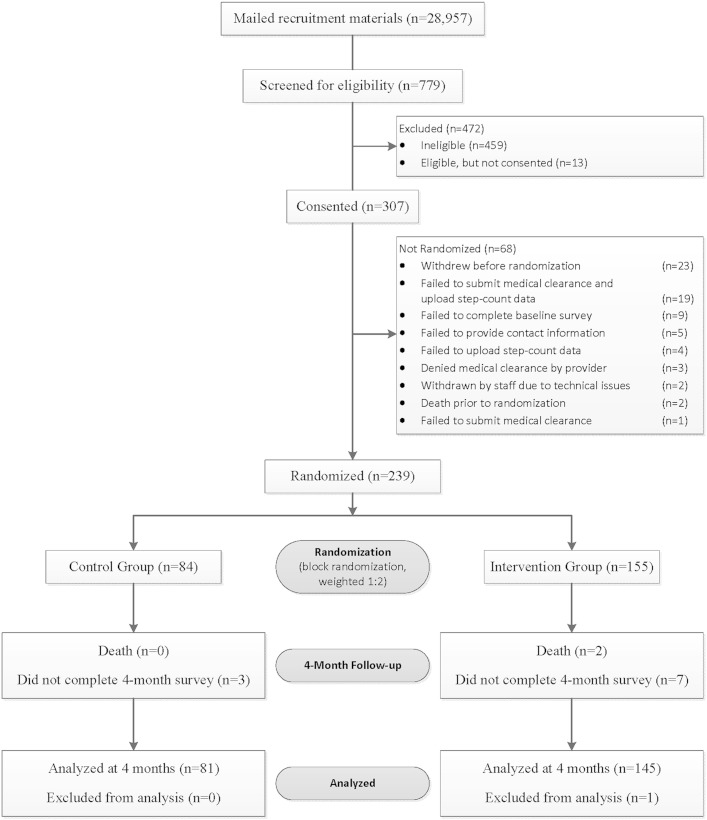
A random sample of 28,957 veterans (one-half urban, one-half rural) were sent a recruitment letter.25 Zip codes were matched with the rural-urban commuting area codes to determine urban or rural residence.26 Interested participants completed an online questionnaire that assessed inclusion criteria, including age ≥ 40 years old; diagnosis of COPD, emphysema, or chronic bronchitis based on ICD-9-CM codes; able to walk a minimum of one block; sedentary (< 150 min of self-reported PA per week); has a health-care provider who can give medical clearance; competent to give informed consent; checks e-mail weekly; has access to a computer with an Internet connection, a USB port, and Windows XP, Vista, 7, or 8; and not involved in another pedometer-based walking program.25 After participants submitted their responses, a computer algorithm determined eligibility. Eligible participants provided informed consent online; 239 participants were randomized in a 2:1 ratio to intervention or control groups (Fig 1).25 Group assignment was computer generated, and randomization was stratified by dyspnea and urban vs rural residence. Participants were enrolled in the study for 12 months. Dyspnea was assessed using the modified Medical Research Council (mMRC) scale (range 0-4, with 4 indicating the most severe level of dyspnea).27 Outcomes were assessed online. Online questions assessed demographics, comorbidities, oxygen use, and smoking status. Participants in both groups could report adverse events at any time and were prompted each month to answer an online question querying the occurrence of new or worsening symptoms or medical problems.
Figure 1 –
Consolidated Standards of Reporting Trials diagram.
Outcomes
HRQL was assessed with the St. George’s Respiratory Questionnaire (SGRQ), composed of a summary total score (SGRQ-TS) and three domain scores: Symptoms (frequency and severity), Activities (that cause or are limited by breathlessness), and Impact (social functioning and psychologic disturbances resulting from airways disease).28 Scores range from 0 to 100, with lower scores indicating better HRQL. A change of 4 units is the minimum clinically important difference for the SGRQ-TS.29 The SGRQ has been well validated in people with COPD.30
Daily step count was assessed with the Omron HJ-720 ITC pedometer (Omron Healthcare, Inc). Our previous work has shown the Omron pedometer to be highly accurate in the clinic and feasible for use in a PA intervention in people with COPD.21,22 At study entry, participants wore the pedometer for 1 week, with a sticker covering the digital display to prevent feedback, and uploaded baseline step-count data. Days were considered valid wear days if at least 100 steps and 8 h of step counts were recorded.22,31 Daily step count at 4 months was calculated using at least 5 days of valid data within a period of 7 consecutive days from a window of ± 14 days around day 121. Participants in both arms had the step-count assessment at 4 months conducted in the same way. They received feedback from the face of the pedometer during the assessment period while they continued in the study.
Intervention Group
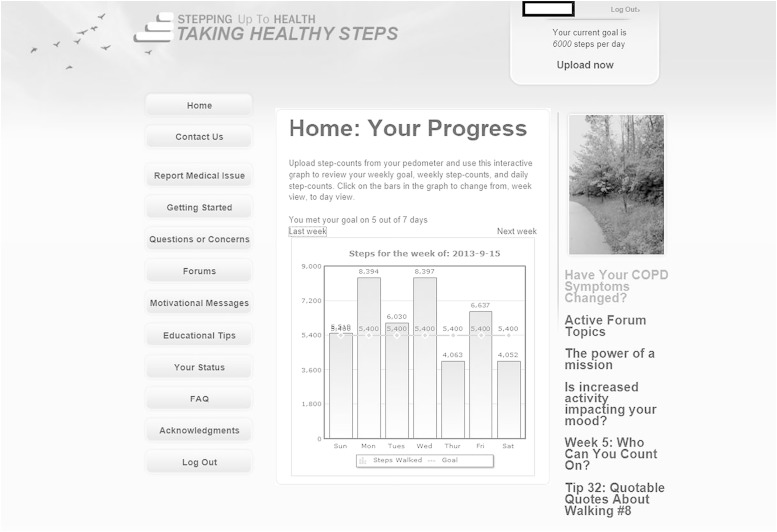
The objective of the THS intervention was to encourage participants to increase their walking.21,22,25 Participants were instructed to wear the pedometer every day and upload step-count data at least once a week. Each week’s goal was the lowest of three numbers: (1) the average of the most recent 7 days of step counts + 600 steps, (2) the previous goal + 600 steps, or (3) 10,000 steps per day. They received access to the website with four key components (Fig 2). Iterative step-count feedback allowed self-monitoring; weekly goal-setting was individualized, dynamic, and concrete; education and motivational content enhanced disease self-management and self-efficacy; and an online community forum provided social support.21,22,25,32,33 The 12-month intervention was composed distinctly of an initial intensive 4-month phase followed by a maintenance 8-month phase.
Figure 2 –
Example of home page on study website.
Wait-list Control Group
Control subjects were instructed to wear the pedometer every day, upload step-count data at least monthly, and report adverse events. They received no instructions about exercise, were not assigned step-count goals, and had access to a webpage that only showed a count of what week they were in the study. At the end of the 12-month study, they were given the option to participate in the Internet-mediated intervention.
Statistical Analysis
To detect a between-group difference in mean change of 4.3 units (SD = 10) in SGRQ-TS with 80% power using a two-tailed 0.05-level test and accounting for a 2:1 ratio of enrollment, at least 192 evaluable participants were needed.25 The final analysis used the intent-to-treat approach. The analysis excluded one outlier in the THS group whose change in SGRQ-TS was 4.0 SDs greater than the mean for change in SGRQ-TS. Two-sample t tests or χ2 tests compared baseline subject characteristics between groups. Between-group differences in SGRQ scores and daily step counts at 4 months were assessed using linear regression models with an indicator for group assignment as the primary predictor, adjusting for baseline values of the outcome, mMRC dyspnea score (dichotomized 0-1 and 2-4), and urban vs rural residence. The proportion of participants who achieved at least a 4-unit improvement in SGRQ-TS was compared between groups using a χ2 test. Paired t tests determined unadjusted within-group changes in SGRQ scores and daily step counts at 4 months compared with baseline.
Secondary analyses were performed to identify baseline predictors of change in SGRQ-TS and change in daily step counts at 4 months and to assess if the intervention effects on the outcomes depended on any of the predictors. We considered age, baseline daily step count, mMRC dyspnea score, current smoking status, current oxygen use, and number of comorbidities as potential predictors. We identified the predictors of change in 4-month outcomes by fitting separate models for each potential predictor with the indicator for intervention group, and the predictor and baseline values of the outcome variable as independent variables. We then assessed if each of the potential predictors modified the intervention effect by further including an interaction of the predictor by intervention group indicator, using separate regression models for each potential predictor.
Results
Participant Characteristics
Participant characteristics include mean age 67 ± 9 years, male sex (94%), rural residence (45%), mMRC dyspnea score ≥ 2 (31%), current smokers (25%), and supplemental oxygen use (24%) (Table 1). There were no significant differences in baseline characteristics between study groups.
TABLE 1 ] .
Baseline Participant Characteristics
| Characteristic | Intervention (n = 154) | Control Group (n = 84) | Total (N = 238) |
| Age, mean (SD), y | 67.0 (8.6) | 66.4 (9.2) | 66.8 (8.8) |
| Male sex | 146 (94.8) | 77 (91.7) | 223 (93.7) |
| Residence | |||
| Urban | 83 (53.9) | 47 (56.0) | 130 (54.6) |
| Rural | 71 (46.1) | 37 (44.1) | 108 (45.4) |
| Hispanic (n = 235) | 5 (3.3) | 1 (1.2) | 6 (2.6) |
| Race | |||
| Black | 7 (4.6) | 3 (3.6) | 10 (4.2) |
| White | 142 (92.2) | 79 (94.0) | 221 (92.9) |
| Other/combined | 5 (3.3) | 2 (2.4) | 7 (2.9) |
| Current smoker | 41 (26.6) | 18 (21.4) | 59 (24.8) |
| Oxygen use | 35 (22.7) | 21 (25.0) | 56 (23.5) |
| SGRQ | |||
| Symptoms (n = 236) | 57.2 (19.1) | 56.0 (19.9) | 56.8 (19.3) |
| Activities (n = 236) | 62.3 (20.2) | 64.2 (18.0) | 62.9 (19.5) |
| Impact (n = 236) | 32.2 (16.5) | 34.1 (17.9) | 32.9 (17.0) |
| Total (n = 233) | 45.6 (15.4) | 46.8 (15.6) | 46.0 (15.4) |
| Baseline daily step counts, mean (SD) | 3,488 (2,316) | 3,521 (2,058) | 3,499 (2,224) |
| mMRC dyspnea score | |||
| 0-1 | 108 (70.1) | 57 (67.9) | 165 (69.3) |
| 2-4 | 46 (29.9) | 27 (32.1) | 73 (30.7) |
Data presented as No. (%) unless otherwise specified. N = 238 unless otherwise noted. There was no significant between-group difference for all characteristics. mMRC = modified Medical Research Council; SGRQ = St. George’s Respiratory Questionnaire.
Of the 238 participants, 221 had complete SGRQ data at baseline and 4 months. At 4 months, SGRQ-TS could not be calculated for 13 participants (6%): nine THS participants and four control subjects. There was no significant difference in baseline SGRQ-TS (46 ± 15 vs 46 ± 15, P = .89) or baseline daily step counts (3,475 ± 2,202 vs 3,546 ± 2,006, P = .83) between those for whom SGRQ-TS could not be calculated (n = 13) vs those for whom an SGRQ-TS was calculated at 4 months (n = 225).
Health-Related Quality of Life
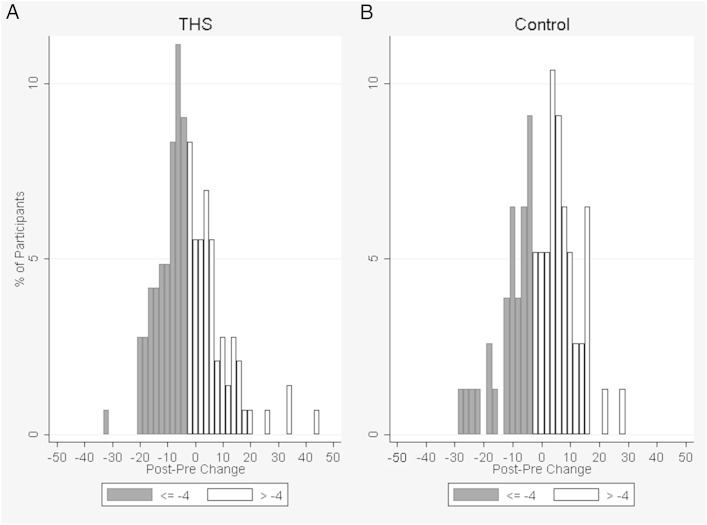
There was no significant between-group difference in SGRQ-TS (2.3 units, P = .14) at 4 months (Table 2). Nevertheless, a significantly greater proportion of intervention participants had a ≥ 4-unit improvement in SGRQ-TS than the control group participants (53% vs 39%, respectively; P = .050) (Fig 3). For domain scores, intervention participants had a significantly lower (reflecting better HRQL) mean than the control subjects by 4.6 units (P = .046) for Symptoms and by 3.3 units (P = .049) for Impact, adjusting for the baseline domain score, mMRC dyspnea score, and urban vs rural residence (Table 2). There was no significant difference in Activities score between the two groups.
TABLE 2 ] .
Within-Group Changes and Between-Group Differences in SGRQ Scores and Daily Step Counts at 4 Months
| Outcome Variable | Group | Na | Baseline Mean (SD) | 4-mo Mean (SD) | Within-Group | Between-Group (THS − Control Group) | ||
| Post-Pre Change Mean (SD) | P Value | Adjusted Differenceb (95% CI) | P Value | |||||
| SGRQ | ||||||||
| Total score | THS | 144 | 45.6 (15.4) | 42.4 (17.6) | −3.2 (11.1) | < .001 | −2.3 (−5.3, 0.8) | .142 |
| Control | 77 | 45.9 (15.0) | 45.0 (16.0) | −0.8 (10.9) | .50 | … | … | |
| Symptoms | THS | 145 | 57.4 (18.8) | 50.2 (22.9) | −7.2 (17.3) | < .001 | −4.6 (−9.0, −0.1) | .046 |
| Control | 79 | 55.0 (19.6) | 53.0 (20.6) | −2.0 (15.6) | .27 | … | … | |
| Activities | THS | 145 | 62.2 (20.5) | 60.4 (22.7) | −1.9 (14.7) | .13 | −0.6 (−4.5, 3.3) | .779 |
| Control | 79 | 63.2 (17.6) | 61.9 (21.4) | −1.3 (13.7) | .40 | … | … | |
| Impact | THS | 144 | 32.0 (16.5) | 29.3 (18.0) | −2.8 (12.8) | .01 | −3.3 (−6.7, −0.2) | .049 |
| Control | 80 | 33.2 (17.4) | 33.6 (16.6) | 0.4 (13.3) | .78 | … | … | |
| Daily step counts | ||||||||
| THS | 133 | 3,475 (2,202) | 3,922 (2,491) | 447 (1,817) | .005 | 779 (241, 1,317) | .005 | |
| Control | 68 | 3,546 (2,006) | 3,200 (2,489) | −346 (1,949) | .15 | … | … | |
THS = Taking Healthy Steps. See Table 1 legend for expansion of other abbreviations.
Two hundred twenty-one participants had complete SGRQ data at baseline and 4 mo; five additional patients had responses at baseline and 4 mo that allowed calculation of at least one domain change score.
Adjusted for baseline value of outcome, mMRC dyspnea score, and urban vs rural residence.
Figure 3 –
A, B, Change in SGRQ-Total Score by group. X-axis represents 4-mo minus baseline values. Decrease in score represents improvement. A, THS group. B, Control group. SGRQ = St. George’s Respiratory Questionnaire; THS = Taking Healthy Steps.
Intervention participants showed an improvement in SGRQ-TS of 3.2 units at 4 months, which was a statistically significant change compared with baseline (P < .001) (Table 2). For domain scores in the intervention group, Symptoms improved by 7.2 units (P < .001) and Impact by 2.8 units (P = .01) at 4 months, whereas there was no significant change in Activities score. The control group showed no significant changes in the SGRQ-TS and domain scores at 4 months compared with baseline (Table 2).
Oxygen use, high mMRC dyspnea score, and low baseline step counts were each associated with smaller improvements in SGRQ-TS at 4 months. Sicker participants, specifically those who used oxygen, had mMRC dyspnea score ≥ 2, and had low baseline step counts, had smaller improvements in the SGRQ-TS compared with healthier people who did not use oxygen, had mMRC dyspnea score of 0 or 1, and had high baseline step counts. We did not find any baseline predictors to modify our findings for the intervention effect on SGRQ-TS at 4 months.
Daily Step Counts
Compared with the control subjects, intervention participants walked 779 more steps per day at 4 months (P = .005), adjusting for baseline daily step counts, mMRC dyspnea score, and urban vs rural residence. Intervention group participants significantly increased their mean daily step counts by 447 steps (P = .005) at 4 months, an increase of 13% from baseline. In contrast, control group participants had a decrease of 346 daily step counts at 4 months (P = .15) (Table 2). We found none of the potential baseline predictors to modify the intervention effect on daily step counts and none of the predictors to be associated with change in daily step counts except age. Each 1-year increase in age was associated with a 33-point decrease in change in daily step counts (P = .03).
Safety
All serious adverse events, including two deaths in the intervention group, were unrelated to the research study. The number of adverse events must be interpreted in the context of (1) the intervention group having twice as many participants as the control group, and (2) the intervention group being prompted four times as often to report medical events as the control group. Even taking these points into consideration, the number of musculoskeletal adverse events differed significantly between the two groups: 41 events in the intervention group and four events in the control group (P = .003). Musculoskeletal events, which were all mild and resolved without treatment, were expected risks that were disclosed in the informed consent. In addition, in the intervention group, six adverse events were pulmonary, three cardiac, and five other. In the control group, one adverse event was pulmonary, one cardiac, and three other. The “other” category included falls, hypoglycemia, foot blisters, nerve pain, neuropathy, and feet numbness. There were nine reports of COPD-related adverse events that did not require hospitalization, including eight in the intervention group and one in the control group.
Study Adherence
In the intervention group, 94% of participants completed the survey and 86% uploaded valid step-count data at 4 months, compared with 95% and 81%, respectively, in the control group. Intervention participants logged into the website an average of 5.7 days per month, a frequency higher than the expected 4 days per month, as participants were instructed to log in to the website and upload their daily step counts at a minimum of once per week.
Discussion
An Internet-mediated, pedometer-based walking program can improve domains of HRQL and daily step counts at 4 months in people with COPD. Although there was no statistically significant improvement in SGRQ-TS in the intervention group compared with the control group, we found that a greater proportion of intervention participants than control subjects had a clinically significant improvement in SGRQ-TS. Furthermore, we showed that our intervention can significantly improve the SGRQ Symptoms and Impact domains. Finally, intervention participants walked an average of 779 more steps per day, compared with control subjects. Oxygen use, dyspnea, and baseline step counts predicted change in SGRQ-TS, whereas age predicted change in daily step counts at 4 months.
The magnitude of change in SGRQ scores in our intervention group was similar to that reported in large COPD clinical trials that examined the efficacy of pharmacological therapies. Our observed improvement of 3.2 units in SGRQ-TS at 4 months was similar to the mean reduction of 3.0 units reported in the Toward a Revolution in COPD Health (TORCH) study averaged over 3 years among those receiving salmeterol and fluticasone.34,35 Similarly, the Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) study examined the use of tiotropium and demonstrated mean absolute changes in SGRQ-TS ranging from 2.3 to 3.3 units in the treatment arm.36,37 Although not statistically significant, our between-group difference in SGRQ-TS at 4 months was 2.3 units, similar to the between-group difference of 2.7 units in the UPLIFT study.36,37 Our results suggest that a behavioral lifestyle modification to increase PA is associated with improvements in SGRQ-TS of a magnitude similar to those described in drug therapy studies.
There was no significant change in the Activities domain, although there were improvements in the Symptoms and Impact domains. It is possible that 4 months was too short a time period for the improvements in pulmonary symptoms to be translated into increases in PA, as assessed by the Activities domain. This finding is consistent with our previous work showing that psychologic factors such as fear, motivation, and confidence, and the presence of other symptoms such as back and leg pain can contribute to why people with COPD do not walk more.32 In the current study, we also showed that dyspnea, number of comorbidities, and current oxygen use did not predict change in daily step counts at 4 months. Taken together, our results suggest that improving pulmonary symptoms alone does not necessarily lead to increased PA. Additional studies that comprehensively address cognitive and behavioral factors using our intervention or other novel approaches are needed.
We acknowledge that conventional pulmonary rehabilitation programs provide exercise regimens that are more rigorous than our intervention and that result in greater improvements in HRQL and exercise capacity.13,38,39 Nevertheless, there have been mixed results as to whether these improvements are associated with increased PA in the home environment after the supervised program ends.13,40‐42 We propose that our intervention can complement conventional pulmonary rehabilitation programs and facilitate the PA essential to COPD self-management programs.43 In addition, our study significantly extends the modalities potentially used to deliver telehealthcare in COPD to include the Internet.44 A combination of telephone calls and face-to-face contact and use of cell phones and personal digital assistants have previously been used to promote PA in people with COPD.44‐47 A study of a PA intervention that combined a pedometer with in-person counseling showed an increase in daily step counts of 11% at 3 months.48 Our intervention using the Internet and a pedometer poses minimal burden on the user and health-care resources.
We intentionally and a priori chose a 2:1 ratio for randomization for two reasons: (1) to maximize the critical mass of participants enrolled at any particular time point who would use the online community forum; and (2) to have fewer subjects in the control group to minimize any potential ethical issue, since PA is clearly beneficial for general health. Our power analysis accounted for the 2:1 randomization ratio. The intervention is composed distinctly of an initial 4-month intensive phase followed by an 8-month maintenance phase. The 4-month results reflect the efficacy of the full intervention, and the 12-month results reflect long-term maintenance by the intervention. Given the distinct aims of the 4-month intensive phase and the 8-month maintenance phase, we believe that presenting the results at 4 months is well justified.
We demonstrated excellent adherence and retention in the study, with participants logging into the website more frequently than expected from our instructions. It was feasible for veterans with COPD to use a website and a pedometer. Participants remained engaged with the THS program, and all but seven participants completed primary outcome assessments at 4 months. Overall, the THS intervention was safe, but minor musculoskeletal adverse events occurred more frequently, which is to be expected when individuals with COPD start an exercise program. In our study, 459 subjects were ineligible, with the top three reasons, which were not mutually exclusive, being: not sedentary (n = 202), could not walk a block (n = 120), or no compatible computer access (n = 161). The most recent GOLD guidelines recommend regular PA for all patients with stable COPD; thus, our intervention has the potential to benefit all patients with COPD. Once implemented, we envision that every patient with COPD could be offered the opportunity to use our intervention. The point of contact may be in the clinic, at the time of hospital discharge, or during a pulmonary rehabilitation program. Face-to-face contact with a health-care provider recommending the intervention would increase frequency of uptake compared with our research study, which was completely automated.
Our study has several limitations. We studied mainly white men who had little to no dyspnea, limiting the generalizability of our results. Spirometric confirmation of the COPD diagnosis was not made at study entry. A major strength of our study is the randomized controlled trial design with balanced groups at baseline. Any potential misclassification of asthma as COPD was most likely balanced between groups and would not bias the primary results. Our method of using the ICD-9-CM code to identify COPD has been previously shown to have diagnostic accuracy.49 Furthermore, our baseline mean SGRQ-TS of 46 ± 15 is strikingly similar to the baseline mean SGRQ-TS of 46 ± 17 in UPLIFT, 49 ± 17 in TORCH, and 50 ± 20 in the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study, arguing against any significant error in the COPD diagnosis.34,36,50 We did not ascertain information about acute exacerbations prior to study entry that may impact HRQL and daily step counts. Again, since the randomization process balanced baseline characteristics between the two groups, any effects of acute exacerbations were most likely the same in both groups. We acknowledge that response behavior to questions administered by a computer may differ from administration by paper and pencil.
We did not account for season of enrollment, which may have influenced the secondary outcome of daily step counts. Season is a complex variable to quantify, since we enrolled patients from across the United States. There are small seasonal changes in Florida or Arizona but large seasonal changes in North Dakota and Montana, for example. We believe that the balanced randomization would minimize any confounding introduced by season. Finally, the control group could view their daily step counts on the pedometer. The effect of the intervention on daily step counts could potentially have been greater if the control group had not received feedback and possibly motivation from the pedometer.
In summary, an Internet-mediated, pedometer-based walking program improves HRQL domains and increases daily step counts in people with COPD. Our results show that this intervention is safe and feasible and has the potential to provide a widely accessible and sustainable exercise program.
Acknowledgments
Author contributions: C. R. R. was the primary investigator and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. M. L. M., R. K., H. Q. N., M. D. C., D. E. G., N. D. G., and C. R. R. were involved in the conception and design of all stages of the study; M. L. M., C. H. M., R. K., P. R., H. Q. N., M. D. C., and C. R. R. were involved in study data collection; R. J. C., C. H. M., R. K., P. R., R. G. H., H. M. K., and N. D. G. conducted study analyses; and all authors contributed to the writing and revising of the manuscript and read and approved the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design, collection, analysis or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication.
Other contributions: We thank the veterans for their participation in this research study.
ABBREVIATIONS
- HRQL
health-related quality of life
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- mMRC
modified Medical Research Council
- PA
physical activity
- SGRQ
St. George’s Respiratory Questionnaire
- SGRQ-TS
St. George’s Respiratory Questionnaire total score
- THS
Taking Healthy Steps
- UPLIFT
Understanding Potential Long-term Impacts on Function with Tiotropium
Footnotes
FUNDING/SUPPORT: This study was funded by the Department of Veterans Affairs, Health Services Research and Development Service [Grant IIR 09-366 to Dr Richardson]; the Department of Veterans Affairs, Rehabilitation Research and Development Service [Career Development Award F6847W to Dr Moy]; and the National Institutes of Health Heart, Lung, and Blood Institute [Grant T32 HL007749-20 to Dr Martinez].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765-773. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Deaths from chronic obstructive pulmonary disease—United States, 2000-2005. MMWR Morb Mortal Wkly Rep. 2008;57(45):1229-1232. [PubMed] [Google Scholar]
- 3.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-365. [DOI] [PubMed] [Google Scholar]
- 4.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972-977. [DOI] [PubMed] [Google Scholar]
- 5.Moy ML, Danilack VA, Weston NA, Garshick E. Daily step counts in a US cohort with COPD. Respir Med. 2012;106(7):962-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen HQ, Chu L, Amy Liu IL, et al. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(5):695-705. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61(9):772-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moy ML, Teylan M, Danilack VA, Gagnon DR, Garshick E. An index of daily step count and systemic inflammation predicts clinical outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(2):149-157. [DOI] [PubMed] [Google Scholar]
- 9.Moy ML, Teylan M, Weston NA, Gagnon DR, Danilack VA, Garshick E. Daily step count is associated with plasma C-reactive protein and IL-6 in a US cohort with COPD. Chest. 2014;145(3):542-550. [DOI] [PubMed] [Google Scholar]
- 10.Moy ML, Teylan M, Weston NA, Gagnon DR, Garshick E. Daily step count predicts acute exacerbations in a US cohort with COPD. PLoS One. 2013;8(4):e60400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331-342. [DOI] [PubMed] [Google Scholar]
- 12.Maltais F, Decramer M, Casaburi R, et al. ; ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15-e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spruit MA, Singh SJ, Garvey C, et al. ; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64. [DOI] [PubMed] [Google Scholar]
- 14.Fischer MJ, Scharloo M, Abbink JJ, et al. Drop-out and attendance in pulmonary rehabilitation: the role of clinical and psychosocial variables. Respir Med. 2009;103(10):1564-1571. [DOI] [PubMed] [Google Scholar]
- 15.Garrod R, Marshall J, Barley E, Jones PW. Predictors of success and failure in pulmonary rehabilitation. Eur Respir J. 2006;27(4):788-794. [DOI] [PubMed] [Google Scholar]
- 16.Martinez CH, Raparla S, Plauschinat CA, et al. Gender differences in symptoms and care delivery for chronic obstructive pulmonary disease. J Womens Health (Larchmt). 2012;21(12):1267-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. Am J Respir Crit Care Med. 2003;167(6):880-888. [DOI] [PubMed] [Google Scholar]
- 18.Cindy Ng LW, Mackney J, Jenkins S, Hill K. Does exercise training change physical activity in people with COPD? A systematic review and meta-analysis. Chron Respir Dis. 2012;9(1):17-26. [DOI] [PubMed] [Google Scholar]
- 19.Kuijpers W, Groen WG, Aaronson NK, van Harten WH. A systematic review of web-based interventions for patient empowerment and physical activity in chronic diseases: relevance for cancer survivors. J Med Internet Res. 2013;15(2):e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moy ML, Janney AW, Nguyen HQ, et al. Use of pedometer and Internet-mediated walking program in patients with chronic obstructive pulmonary disease. J Rehabil Res Dev. 2010;47(5):485-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moy ML, Weston NA, Wilson EJ, Hess ML, Richardson CR. A pilot study of an Internet walking program and pedometer in COPD. Respir Med. 2012;106(9):1342-1350. [DOI] [PubMed] [Google Scholar]
- 23.Richardson CR, Buis LR, Janney AW, et al. An online community improves adherence in an internet-mediated walking program. Part 1: results of a randomized controlled trial. J Med Internet Res. 2010;12(4):e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson CR, Mehari KS, McIntyre LG, et al. A randomized trial comparing structured and lifestyle goals in an internet-mediated walking program for people with type 2 diabetes. Int J Behav Nutr Phys Act. 2007;4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez CH, Moy ML, Nguyen HQ, et al. Taking Healthy Steps: rationale, design and baseline characteristics of a randomized trial of a pedometer-based Internet-mediated walking program in veterans with chronic obstructive pulmonary disease. BMC Pulm Med. 2014;14(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weeks WB, Kazis LE, Shen Y, et al. Differences in health-related quality of life in rural and urban veterans. Am J Public Health. 2004;94(10):1762-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580-586. [DOI] [PubMed] [Google Scholar]
- 28.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321-1327. [DOI] [PubMed] [Google Scholar]
- 29.Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75-79. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan RM, Ries AL, Reilly J, Mohsenifar Z; National Emphysema Treatment Trial Research Group. Measurement of health-related quality of life in the national emphysema treatment trial. Chest. 2004;126(3):781-789. [DOI] [PubMed] [Google Scholar]
- 31.Demeyer H, Burtin C, Van Remoortel H, et al. Standardizing the analysis of physical activity in patients with COPD following a pulmonary rehabilitation program. Chest. 2014;146(2):318-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danilack VA, Weston NA, Richardson CR, Mori DL, Moy ML. Reasons people with COPD do not walk and relationship with daily step count. COPD. 2014;11(3):290-299. [DOI] [PubMed] [Google Scholar]
- 33.Resnick PJ, Janney AW, Buis LR, Richardson CR. Adding an online community to an internet-mediated walking program. Part 2: strategies for encouraging community participation. J Med Internet Res. 2010;12(4):e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calverley PM, Anderson JA, Celli B, et al. ; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775-789. [DOI] [PubMed] [Google Scholar]
- 35.Jones PW, Anderson JA, Calverley PM, et al. ; TORCH investigators. Health status in the TORCH study of COPD: treatment efficacy and other determinants of change. Respir Res. 2011;12:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tashkin DP, Celli B, Senn S, et al. ; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543-1554. [DOI] [PubMed] [Google Scholar]
- 37.Troosters T, Celli B, Lystig T, et al. ; UPLIFT Investigators. Tiotropium as a first maintenance drug in COPD: secondary analysis of the UPLIFT trial. Eur Respir J. 2010;36(1):65-73. [DOI] [PubMed] [Google Scholar]
- 38.Finnerty JP, Keeping I, Bullough I, Jones J. The effectiveness of outpatient pulmonary rehabilitation in chronic lung disease: a randomized controlled trial. Chest. 2001;119(6):1705-1710. [DOI] [PubMed] [Google Scholar]
- 39.Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000;355(9201):362-368. [DOI] [PubMed] [Google Scholar]
- 40.Egan C, Deering BM, Blake C, et al. Short term and long term effects of pulmonary rehabilitation on physical activity in COPD. Respir Med. 2012;106(12):1671-1679. [DOI] [PubMed] [Google Scholar]
- 41.Pitta F, Troosters T, Probst VS, Langer D, Decramer M, Gosselink R. Are patients with COPD more active after pulmonary rehabilitation? Chest. 2008;134(2):273-280. [DOI] [PubMed] [Google Scholar]
- 42.Steele BG, Belza B, Cain KC, et al. A randomized clinical trial of an activity and exercise adherence intervention in chronic pulmonary disease. Arch Phys Med Rehabil. 2008;89(3):404-412. [DOI] [PubMed] [Google Scholar]
- 43.Rice KL, Dewan N, Bloomfield HE, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2010;182(7):890-896. [DOI] [PubMed] [Google Scholar]
- 44.McLean S, Nurmatov U, Liu JL, Pagliari C, Car J, Sheikh A. Telehealthcare for chronic obstructive pulmonary disease: Cochrane Review and meta-analysis. Br J Gen Pract. 2012;62(604):e739-e749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borycki E. M-health: can chronic obstructive pulmonary disease patients use mobile phones and associated software to self-manage their disease? Stud Health Technol Inform. 2012;172:79-84. [PubMed] [Google Scholar]
- 46.Liu WT, Wang CH, Lin HC, et al. Efficacy of a cell phone-based exercise programme for COPD. Eur Respir J. 2008;32(3):651-659. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen HQ, Gill DP, Wolpin S, Steele BG, Benditt JO. Pilot study of a cell phone-based exercise persistence intervention post-rehabilitation for COPD. Int J Chron Obstruct Pulmon Dis. 2009;4:301-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hospes G, Bossenbroek L, Ten Hacken NH, van Hengel P, de Greef MH. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling program. Patient Educ Couns. 2009;75(2):274-278. [DOI] [PubMed] [Google Scholar]
- 49.Cooke CR, Joo MJ, Anderson SM, et al. The validity of using ICD-9 codes and pharmacy records to identify patients with chronic obstructive pulmonary disease. BMC Health Serv Res. 2011;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurst JR, Vestbo J, Anzueto A, et al. ; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128-1138. [DOI] [PubMed] [Google Scholar]