Abstract
BACKGROUND:
Successful management of chronic cough has varied in the primary research studies in the reported literature. One of the potential reasons relates to a lack of intervention fidelity to the core elements of the diagnostic and/or therapeutic interventions that were meant to be used by the investigators.
METHODS:
We conducted a systematic review to summarize the evidence supporting intervention fidelity as an important methodologic consideration in assessing the effectiveness of clinical practice guidelines used for the diagnosis and management of chronic cough. We developed and used a tool to assess for five areas of intervention fidelity. Medline (PubMed), Scopus, and the Cochrane Database of Systematic Reviews were searched from January 1998 to May 2014. Guideline recommendations and suggestions for those conducting research using guidelines or protocols to diagnose and manage chronic cough in the adult were developed and voted upon using CHEST Organization methodology.
RESULTS:
A total of 23 studies (17 uncontrolled prospective observational, two randomized controlled, and four retrospective observational) met our inclusion criteria. These articles included 3,636 patients. Data could not be pooled for meta-analysis because of heterogeneity. Findings related to the five areas of intervention fidelity included three areas primarily related to the provider and two primarily related to the patients. In the area of study design, 11 of 23 studies appeared to be underpinned by a single guideline/protocol; for training of providers, two of 23 studies reported training, and zero of 23 reported the use of an intervention manual; and for the area of delivery of treatment, when assessing the treatment of gastroesophageal reflux disease, three of 23 studies appeared consistent with the most recent guideline/protocol referenced by the authors. For receipt of treatment, zero of 23 studies mentioned measuring concordance of patient-interventionist understanding of the treatment recommended, and zero of 23 mentioned measuring enactment of treatment, with three of 23 measuring side effects and two of 23 measuring adherence. The overall average intervention fidelity score for all 23 studies was poor (20.74 out of 48).
CONCLUSIONS:
Only low-quality evidence supports that intervention fidelity strategies were used when conducting primary research in diagnosing and managing chronic cough in adults. This supports the contention that some of the variability in the reporting of patients with unexplained or unresolved chronic cough may be due to lack of intervention fidelity. By following the recommendations and suggestions in this article, researchers will likely be better able to incorporate strategies to address intervention fidelity, thereby strengthening the validity and generalizability of their results that provide the basis for the development of trustworthy guidelines.
Summary of Recommendations and Suggestions
1. In conducting studies of chronic cough in adults, we recommend that investigators, as a first step, include intervention fidelity in the design of their studies of the diagnosis and treatment of chronic cough, by addressing intervention fidelity in the following 5 areas: study design, training of providers, treatment delivery, treatment receipt, and enactment of treatment (Grade 1C).
2. In conducting studies of chronic cough in adults, we recommend, as a second step, that the training of investigators be addressed; and, all investigators should agree to employ the use of an evidence-based clinical practice guideline or an evidence-based protocol for the diagnosis and treatment of chronic cough and agree to follow an intervention manual outlining the minimum expected interventions throughout the study (Grade 1C).
3. In conducting studies of chronic cough in adults, we recommend that investigators, as a third step, establish a standardized plan for delivery and measurement of treatment through the use of an intervention manual (Grade 1C).
4. In conducting studies of chronic cough in adults, we recommend that investigators, as a fourth step, establish a standardized plan for maximizing and measuring concordance of understanding of interventions and treatment between subjects and investigators (Grade 1C).
5. In conducting studies of chronic cough in adults, as a fifth step, we recommend that investigators establish a standardized plan for evaluating and measuring the subject’s ability to enact and adhere to the treatment plan under real life circumstances (Grade 1C).
6. In conducting studies of chronic cough in adults, we recommend that investigators not make a diagnosis of idiopathic chronic cough as a distinct clinical entity unless known causes of cough have been excluded by a systematic evaluation using an evidence-based guideline and intervention fidelity has been addressed in the design and implementation of the study (Grade 1C).
7. In all patients with chronic cough, we suggest that clinicians use an evidence-based guideline that contains core elements and processes as a guide for diagnosis and treatment (Ungraded Consensus-Based Statement).
Multiple professional societies worldwide have engaged experts to develop evidence-based guidelines to assist providers in the management of chronic cough.1 Yet, according to the published literature, successful management of chronic cough has varied from 54% to 100%.2-4 Although it is not clear what accounts for this variability, one of the potential reasons5 relates to a lack of intervention fidelity to the core elements of the diagnostic and/or therapeutic interventions that were meant to be used by the investigators.
Although descriptors and definitions for intervention fidelity vary in content and detail, they are conceptually similar. Intervention fidelity has been defined as “the extent to which an intervention was delivered as conceived and planned—to arrive at valid conclusions concerning its effectiveness in achieving the target outcomes.”6 The concept of intervention fidelity is an important methodologic consideration when conducting primary research in randomized controlled clinical trials as well as nonrandomized observational studies, to ensure reliable and valid testing of an intervention.7-11 When using the randomized controlled study design, the importance of establishing a plan for standardized, consistent implementation of the intervention by both the investigator and the subject is well recognized. Randomized study designs routinely include measures for issues such as patient adherence to therapy to ensure fidelity to the intervention that is being tested. However, despite strong study designs, when interventions are flexible, dynamic, and individualized, even randomized controlled trials can be subject to problems related to intervention fidelity.12 In contrast, in the case of nonrandomized, noncontrolled, observational studies assessing the outcomes associated with the implementation of interventions in ambulatory settings, the literature addressing intervention fidelity is not as well established or, in the case of chronic cough, not addressed at all. Because observational studies may provide the best evidence for the real-world implementation of clinical practice guidelines, we decided to evaluate intervention fidelity, according to the Treatment Fidelity Workgroup of the National Institutes of Health (NIH) Behavioral Change Consortium7 recommendations, in the management of chronic cough as an important unmet need.
This systematic review addresses the use of guidelines or protocols for the diagnosis and management of chronic cough, beginning with the publication of the first formal professional society guideline published for this purpose. The first formal professional society guideline for the diagnosis and management of chronic cough was published in 1998, and this publication used a definition of ≥ 3 weeks’ duration to define chronic cough.13 Since at least the year 2000, chronic cough has been defined as being of > 8 weeks’ duration.14 The 2004 European Respiratory Society,15 the 2006 American College of Chest Physicians (CHEST),16 and most guidelines for the diagnosis and management of chronic cough that followed define chronic cough as being of > 8 weeks’ duration. For at least these reasons, the literature we reviewed varied in its definition of chronic cough.
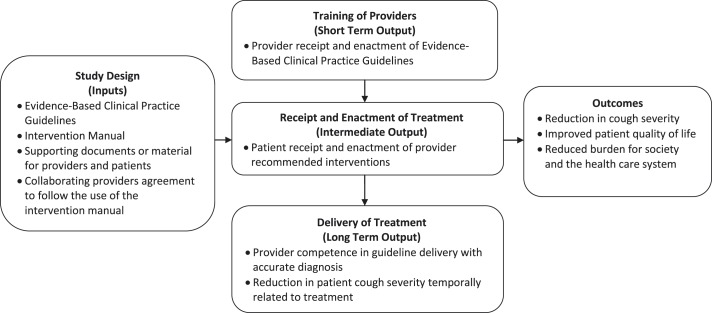
The Treatment Fidelity Workgroup of the NIH Behavioral Change Consortium7,10,11 has recommended that the following five areas be addressed to assess intervention fidelity: study design, training of providers, delivery of treatment, receipt of treatment, and enactment of treatment.7,10,11 Study design, training of providers, and delivery of treatment focus on the interventionist, whereas receipt and enactment of treatment focus on the patient receiving the intervention.7 Although these particular strategies were developed for use with behavioral interventions, we adapted them to address intervention fidelity in studies of the use of guidelines or protocols to manage chronic cough. The strategies proposed have been incorporated into a visual model (Fig 1) of the logic for the role of intervention fidelity in studies of the diagnosis and management of chronic cough in adults. The model demonstrates the importance of the five areas of intervention fidelity specific to successful cough guideline implementation. For the impact of these recommendations to be realized as intended, several outcomes need to be achieved. The short-term outcome is for the providers to receive and enact the guidelines according to the recommendations. The intermediate outcome is that patients will receive and enact the provider recommendations that are based upon the guidelines. The long-term outcome is for providers to be competent in guideline delivery by providing accurate diagnoses, with the result being that the patient achieves a reduction in cough severity so that the impact of improved quality of life and reduced burden on the system and society can be achieved. We used this logic model (Fig 1) to guide our assessment of how well authors implemented the use of guidelines or protocols in the studies in our systematic review.
Figure 1 –
Logic model for the role of intervention fidelity in the use of guidelines for the diagnosis and management of chronic cough in adults.
For further clarification of the five areas of intervention fidelity,7,10 the area of study design focuses on establishing clarity of the theoretical underpinnings of the study. Using the management of cough as an example, for this review, the theoretical underpinnings referred to the published evidence-based guidelines or protocols referenced by the author as providing the theoretical rationale for the interventions used in the study. Identification of the supporting published evidence-based guideline or protocol allows one to assess the extent of its relationship to the interventions and therefore the degree to which the guideline has been implemented. The area of training of providers refers to educating providers to help them in the maintenance of standardized delivery of the intervention by the interventionist throughout the study. This could be accomplished through the use of an intervention manual. Delivery of treatment would relate to the treatment interventions outlined by the identified guideline being used by the provider. Receipt of treatment would refer to verifying that the patient and the interventionist have concordance of understanding of the treatment recommended, whereas enactment of treatment would be exemplified by whether the patient has been able to engage in the use of the recommended treatment in daily life. Enactment of treatment in daily life is a concept that is more broad than that of adherence.7 For example, a patient may try out new recipes to develop the ability to adhere to an antireflux diet; although this may be important to an individual’s adherence to the diet recommendation, it is not an equivalent.7 Receipt and enactment of treatment pertain to the patient rather than the interventionist.7,10
The purpose of this study was to systematically evaluate the literature for the use of previously published clinical practice guidelines or protocols for the diagnosis and management of chronic cough in adults. This systematic review included: assessing these studies for elements of intervention fidelity, summarizing findings, and establishing recommendations and/or suggestions for future investigators performing clinical research on chronic cough in adults. We hypothesized that routinely incorporating intervention fidelity as a methodologic strategy should improve the reliability and validity of the outcomes of studies using guidelines or protocols in the treatment of chronic cough.
To fulfill our purposes and to test this hypothesis, we set out to accomplish four specific aims:
Develop and pilot a tool that assesses five areas of intervention fidelity in the identified studies.
Systematically review the literature on studies that diagnosed and treated an initially unexplained chronic cough in adults using a guideline or protocol and determine whether the five areas of intervention fidelity were addressed in the identified studies and to what degree.
Assess whether intervention fidelity was used to the extent that readers can be confident that the diagnoses made were valid.
Use these findings to provide recommendations and/or suggestions for those conducting research of any design in the area of chronic cough.
Materials and Methods
Systematic Review
The Executive Committee of the CHEST Expert Cough Panel convened a writing committee to develop recommendations or suggestions that pertain to the assessment of intervention fidelity in studies of the use of guidelines or protocols to diagnose and manage chronic cough in adults. This writing committee based its recommendations or suggestions on a systematic review contained within this article. This systematic review follows the “Methodologies for the Development of the Management of Cough: CHEST Guideline and Expert Panel Report.”17,18
Eligibility Criteria:
The key clinical question, associated PICOTS elements (ie, population, intervention, comparator, outcome, timing, setting), and study selection criteria were developed (Table 1), and a systematic review of the literature was performed with the intent of identifying studies that met the following criteria: (1) addressed chronic cough in adults; (2) used evidence-based clinical practice guidelines or protocols to diagnose and manage chronic cough; (3) diagnosed explained and unexplained chronic cough; (4) included any study design with the exception of case reports and letters to the editor because of lack of necessary methodologic details for assessing intervention fidelity; and (5) articles published in English and during or after 1998, the year of the publication of the first cough clinical practice guideline.13
TABLE 1 ] .
Key Clinical Question and PICOTS/Study Selection Criteria
| Key Question: In Studies of Subjects With Chronic Cough, Did the Authors State That Evidence-Based Clinical Practice Guidelines or an Evidence-Based Protocol Were Followed for Diagnosis and Management, and Did Fidelity to the Guidelines/Study Improve Outcomes? |
| PICOTS/study selection criteria |
| Population |
| Adult patients receiving clinical evaluation and management for chronic cough |
| Includes both subjects with chronic cough that is ultimately resolved (explained) or that remains unresolved, unresponsive, intractable, refractory, idiopathic, unexplained |
| Intervention |
| Application of evidence-based guidelines or protocols for the diagnosis and management of chronic cough (includes subjects fulfilling the definition of chronic cough, fidelity to the recommended diagnostic evaluations performed and management strategies used, intervention fidelity to the study) |
| Use of validated or standardized outcome measures |
| Comparators |
| Diagnosis and management of chronic cough that is not faithful to evidence-based guidelines or protocols |
| Use of nonvalidated or nonstandardized measures to establish outcomes |
| Outcomes |
| Diagnosis of explained and unexplained (idiopathic) chronic cough |
| Subjective or objective improvement in cough severity |
| Subjective or objective improvement in cough-specific quality of life |
| Timing |
| Chronic cough, with cough of ≥ 3 wk duration |
| Setting |
| Outpatient |
| Specialty or primary care |
| Study design |
| Any clinical trial or comparative study, randomized or not |
| English language only |
| Date of the first published guideline for the management of cough (1998) forward |
| Human |
| All sample sizes |
| Studies with enough detail related to full cough guideline or protocol use to assess for intervention fidelity; exclude case series submitted as letters to the editor |
PICOTS = population, intervention, comparator, outcome, timing, setting.
Study Identification:
We conducted a systematic review of the literature using PubMed and Scopus on May 27, 2014, searching the literature from January 1, 1998, to May 27, 2014. A total of 4,022 studies were initially identified from the combined search (see Fig 219 for diagram of study selection). The Cochrane Database of Systematic Reviews was hand searched for the same time period to reassure that all relevant reviews were included in the PubMed search. The search strategy was designed by experienced academic librarians (Nancy Harger, MLS, and Judy Nordberg, MLIS) working in collaboration with clinical experts (C. T. F. and R. S. I.). The search strategy for each database is described in e-Table 1 (1.7MB, pdf) . The reference lists of narrative and systematic reviews were searched for relevant citations.
Figure 2 –
Flow diagram of study selection. PICOTS = population, intervention, comparator, outcome, timing, setting. (From Moher et al.19)
All titles and abstracts returned by the initial search were reviewed independently and in duplicate by two reviewers (C. T. F. and R. S. I.). A mediator was available to settle any potential disagreements. Potentially eligible studies underwent full text review following the same process.
Quality Assessment:
Final full text articles meeting the inclusion criteria were subjected to independent and duplicate quality assessment, based upon potential methodologic biases. The quality of studies was assessed with an adapted tool routinely used by CHEST to assess randomized controlled clinical trials in the development of their clinical practice guidelines. This tool, created by R. L. D. and associates, was developed for quality assessment of intervention studies, including randomized controlled trials and observational studies.18
Data Extraction:
Two reviewers worked independently and in duplicate to extract and enter data into a predesigned evidence table to ensure consistent and complete data extraction. The following data items were extracted: study design, primary aim, setting, population (eg, number of participants, age, sex, cough duration), number lost to follow-up or excluded, time to follow-up, number diagnosed with unexplained or unresolved cough, and description of patient outcome assessments. Additional data specific to the five areas of intervention fidelity were collected in a separate data extraction table. A separate tool, developed as part of this work (e-Appendix 1 (1.7MB, pdf) ), was used to rate intervention fidelity for each study. All studies were assessed for intervention fidelity using the tool, which included eight elements that addressed the five areas of intervention fidelity. The following eight elements were sought in each study:
• Three elements for intervention fidelity strategies in study design: (1) Was the guideline or protocol used to guide the study published, and was it clearly identified? (2) Did the authors identify the diagnostic methods for screening for causes of chronic cough according to the guideline or protocol cited or referenced (eg, cough duration and diagnostic methods used)? and (3) Were standardized or validated tools used to measure patient-reported outcomes?
• One element for training of providers: Was there formal training of providers related to the guideline or protocol used, and was an intervention manual used to guide providers?
• Two elements for delivery of treatment: (1) Were the core treatment interventions consistent with the guideline or protocol used to develop the intervention manual and/or to guide the study? and (2) Was there assessment of response to treatment at specified timeframes?
• One element for receipt of treatment: Was there any mention and/or measurement of concordance of patient and provider understanding of the problem and/or the treatment recommendations?
• One element for enactment of treatment: Was there any mention and/or measurement of patients’ ability to engage in the treatment recommendations in daily life?
Each of the eight elements was rated for presence (yes or no) and for degree of presence using a rating scale ranging from 0, strongly disagree, to 6, strongly agree. Total intervention fidelity scores were computed as the sum of the eight item ratings that could range from 0 to 48, with ≤ 23 = poor, 24 to 35 = fair, and 36 to 48 = good.
Data Analysis:
The final studies that met the PICOTS criteria were carefully reviewed for homogeneity of study characteristics and key clinical information. They were analyzed in detail for the use of guidelines or protocols to manage chronic cough and the presence of our previously identified intervention fidelity elements. Findings are described for the five areas of interest according to our intervention fidelity tool. Because of the heterogeneity of study designs and quality, a meta-analysis could not be performed. Using the results of this systematic review as their basis, recommendations and suggestions were developed and submitted to the full panel for voting according to the CHEST Organization’s methods previously published.17,18
Clinical Practice Guideline Recommendations and Suggestions
The methodology used by the CHEST Guideline Oversight Committee to select the Expert Cough Panel Chair and the international panel of experts to perform the systematic review, synthesize the evidence, and develop the recommendations and suggestions has been published.17,18
Grading:
In addition to the quality of the evidence, the recommendation grading also includes strength of recommendation dimension. In the context of practice recommendations, a strong recommendation applies to almost all patients, whereas a weak recommendation is conditional and only applies to some patients. In the context of research recommendations, such as the ones in this guideline, we intended a strong recommendation (Grade 1) to imply that we recommend using intervention fidelity strategies in all studies when patients with chronic cough are diagnosed and managed. The strength of a recommendation in this paper is based on consideration of three factors: balance of benefits to harms, patient values and preferences, and resource considerations. Harms incorporate risks and burdens to the patients, which can include convenience or lack of convenience, difficulty of administration, and invasiveness. These, in turn, impact patient preferences. The resource considerations go beyond economics and should also factor in time and other indirect costs. The authors of these recommendations have considered these parameters in determining the strength of the recommendations and associated grades.
The findings of the systematic review were used to support the evidence-graded recommendations or suggestions. A highly structured, consensus-based Delphi approach was used to provide expert advice on all guidance statements. The total number of eligible voters for each guidance statement varied based on the number of individuals recused from voting because of their potential conflicts of interest. Transparency of process was documented. Further details of the methods related to conflicts of interest and transparency have been published elsewhere.17,18
Results
Systematic review results are addressed first and categorized according to the study aims. This is followed by the results of the process for establishing guideline recommendations or consensus-based suggestions.
Systematic Review
Specific Aim 1:
Develop and pilot a tool that assesses five areas of intervention fidelity in the identified studies and the degree to which they were used.
As the tool (e-Appendix 1 (1.7MB, pdf) ) was trialed in the review, an iterative process was used and three adjustments to the tool were made. Adjustments included the following: (1) For individual elements, additional detail was included for a better understanding, and wording was clarified. It was also noted that although an item was often present, it may or may not have been well described or clearly implemented. (2) To address the variability in the degree that an item was described or implemented, the dichotomous rating scale (present or not) was supplemented with the 0- to 6-point rating scale (ie, 0 = strongly disagree, to 6 = strongly agree) described in detail under data extraction. Last, this aim was additionally modified by using italic lettering as shown previously.
Specific Aim 2:
Systematically review the literature on studies that diagnosed and treated an initially unexplained chronic cough in adults, using a guideline or protocol, and determine whether the five areas of intervention fidelity were addressed in the identified studies and to what degree.
Characteristics of Included Studies:
The diagram20 outlining the flow of study selection is shown in Figure 2.19 From our systematic review, 23 studies met our focused criteria.4,21-42 Table 2 contains the characteristics of the individual studies. The methodologic quality indicators assessing study quality resulted in 47.1% (eight of 17)21,28,30-32,34,38,39 of the prospective observational studies being rated as fair or as having moderate risk of bias, whereas 52.9% (nine of 17)22-26,35-37,40 were rated as poor or as having high risk of bias. Studies not using validated or standardized outcome measures to assess cough were rated as poor because of potential for bias. Because of lack of blinding, 100% (two of two) of the randomized controlled trials29,41 were rated as fair and as having at least a moderate risk of bias. Because of the retrospective nature, lack of use of standardized or previously validated outcome measures being used to assess for change in cough,4,33,42 or unclear subject selection methods,27 100% (four of four) of the retrospective observational studies were rated as poor or as having a high risk for bias. The combined dropout rate for the 23 studies was 10.2% (421 of 4,110).
TABLE 2 ] .
Characteristics of Individual Studies by Design
| Study/Year | Setting | Study Population N = Population (% Male) | No. Analyzed N = Population n = Sample (% Male) | N = Population Analyzed or n = Subset Analyzed; Age, Mean ± SD or Median (Range), y | N = Population n = Subset Analyzed; Cough Duration Mean ± SD or Median (Range) | Follow-up Mean or Median (Range) | Lost to Follow-up or Excluded/Enrolled % (n/N) | Unresolved Cougha Number (%) Based on No. Analyzed |
| Prospective observational studies | ||||||||
| Al-Mobeireek et al21/2002 | Chest Clinic of Dallah Hospital, Riyadh, Saudi Arabia | N = 136 (?) consecutive new patients with cough > 3 wk assessed by one chest specialist | n = 100 (55) | n = 100; 39.75 ± 15.13 (?) | n = 100; 7.5 wk median (3-1,300 wk) | 34 (median) wk (1-219 wk) | 26.5 (36 of 136) | 0 (0) (noted 4 resolved on their own and 1 patient with bronchiectasis did not have substantial improvement) |
| Ayik et al22/2003 | Outpatient clinic, Ege University School of Medicine, Barnova, Turkey | N = 43 (?) consecutive new nonsmoking patients with cough > 4 wk meeting 7 criteria | n = 36 (11.1) | n = 36; 45.4 ± 14.3 (16-69) | n = 36; 31.3 ± 52.3 mo (1-240 mo) | ? (?) | 16.3 (7 of 43) | 8 (22.2) |
| Brightling et al23/1999 | Referrals to a single respiratory physician, Leicester, England | N = 91 (?) patients with cough > 3 wk and no clinical or radiologic evidence of significant lung disease; not clear if consecutive | N = 91 (?) | N = 91; ? (28-76) | ? | ? (?) | 0 (0 of 91) | 6 (6.6) |
| Fujimura et al24/2005 | Respiratory medical clinics in the research group at Kanazawa University Hospital and at 11 related hospitals in the Hokuriku area of Japan. | N = 248 (44.3) consecutive immunocompetent patients complaining of cough of ≥ 8 wk visiting a respiratory clinic for diagnosis and treatment of chronic cough | n = 176 (?) | N = 248; 50.4 ± 17.8 (15-97) | ? (?) | ? (?) | 29.0 (72 of 248) | 11 (6.25) |
| Kastelik et al25/2005 | Hull Cough Clinic, Castle Hill Hospital, University of Hull, Cottingham, England | 148 (?) Consecutive patients referred to a cough clinic presenting with cough > 8 wk | n = 131 (34) | 60 (median) (16-88) | n = 131; 5.9 y median (0.2-65) | ? (?) | 11.5 (17 of 148) | 9 (7) |
| Lai et al26/2013 | 9 General hospitals respiratory medical clinics located in 8 cities from 5 geographic areas in China. | N = 826 (?) consecutive immunocompetent current nonsmokers with cough ≥ 8 wk, with no radiologic evidence of lung disease. | n = 704 (44.7) | n = 704; 40.4 ± 12.8 (?) | n = 704; 12 mo median (2-696) | ? (?) | 14.8 (122 of 826) | 64 (8.4) |
| Lee et al28/2007 | Internal medicine clinic at Cheju National University Hospital, Korea. | N = 378 (49.2) patients complaining of cough > 4 wk meeting 4 criteria | N = 378 (49.2) | N = 378; 51.2 ± 16.12 (?) | N = 378; 2 mo median (1-36) | ? (?) | 0 (0 of 378) | 12 (3.17) noted these were “idiopathic or psychogenic” |
| Marchesani et al30/1998 | Outpatient Department of Respiratory Diseases, City Hospital, Osimo, Italy | N = 92 (21.7) consecutive patients with cough ≥ 4 wk, resistant to conventional therapy and lack of obvious cause. | n = 87 (?) | N = 92; 51 ± 1.9 (mean ± SEM) | N = 92; 32.7 ± 4.5 mo (?) | ? (?) | 5.4 (5 of 92) | 8 (9.2) |
| McGarvey et al32/1998 | Chest clinic at Belfast City Hospital, Ireland | N = 43 (32.6) lifetime nonsmoking patients with nonproductive cough of > 3 wk meeting 7 criteria | N = 43 (32.6) | N = 43; 47.5 (18-77) | N = 43; 67 mo mean (2-240) | ? (?) | 0 (0 of 43) | 8 (18.6) |
| Ojoo et al34/2013 | Hull Cough Clinic, Castle Hill Hospital, University of Hull, Cottingham, England | N = 112 (34.8) sequential consenting adult patients not taking ACE inhibitors with a history of chronic cough ≥ 8 wk | n = 92 (?) | N = 112; 56.2 mean (?) | N = 112; 3 y median (0.25-64) | 16.9 ± 10.8 (?) weeks (mean) clinical protocol | 10.7 (12 of 112) | 7 (7.6) |
| Palombini et al35/1999 | University outpatient clinic, Universidade Federal do Rio Grande do Sul Porto Alegre, Brazil | N = 78 (34.6) consecutive nonsmoking immunocompetent patients referred for evaluation of chronic cough of ≥ 3 weeks with normal or near-normal chest radiograph | N = 78 (34.6) | N = 78; 57 ± 16.7 (15-81) | N = 78; 72 ± 96.4 (1-480) mo | 2.7 ± 2.3 visits (mean) (2-480 d) | 0 (0 of 78) | 5 (6.4) |
| Plaza et al36/2006 | Patients were recruited in a clinic facility within one of the primary public health-care delivery centers in Barcelona, Spain | N = 57 (35) consecutive nonsmoking immunocompetent patients seeking medical attention for chronic cough of > 3 wk | N = 57 (45) | N = 57; 62 (16-88) | N = 57; ? (1-620 mo) | ? (?) | 0 (0 of 57) | 1 (2) |
| Ribeiro et al31/2006 | General chest clinic at Hospital in Sao Paulo, Brazil, Federal University of Sao Paulo. | N = 147 (30.6) consecutive immunocompetent patients with cough > 8 wk and no clinical or radiographic evidence of significant lung disease meeting additional 3 criteria | n = 137 (?) | N = 147; 48 ± 18 (18-79) | N = 147; 24 wk median (8-540) | ? (?) | 6.8 (10 of 147) | 1 (0.72) |
| Smyrnios et al37/1998 | Pulmonary Clinic at the University of Massachusetts Medical School, Worcester, MA | N = 30 (60) patients ≥ 64 y of age with a cough of ≥ 3 wk evaluated by one provider (subgroup from a larger study) | N = 30 (60) | N = 30; 70.4 ± 5 (64-83) | N = 30; 77 ± 140 mo (1-600) | 3.3 (mean) (?) visits over 12 wk | 0 (0 of 30) | 0 (0) |
| Wei et al38/2009 | Department of Respiratory Medicine, Tongji Hospital, Shanghai, China | N = 287; n = 104 (32.7) elderly (> 60 y) and n = 183 (42.1) nonelderly consecutive nonsmoking consenting patients referred for isolated persistent cough > 8 wk meeting 4 criteria | N = 281 (?) n = 102 (?) elderly (> 60 y) and n = 179 (?) nonelderly | Elderly n = 104; 66 ± 60 vs nonelderly 39 ± 11 | Elderly n = 102; 5 mo median (2-252) vs nonelderly n = 183; 4 (2-240) | ? (?) | 2.1 (6 of 287) 2 of 104 elderly and 4 of 183 nonelderly |
Elderly 9 (8.8) vs nonelderly 13 (7.3); n = 281; total unexplained 22 (7.8) |
| Yu et al39/2008 | Respiratory clinic, Tongji Hospital, Shanghai, China | N = 102 (40.2) consecutive nonsmoking patients with cough of > 8 wk meeting > 10 criteria | n = 97 (?) | n = 97; 49 ± 16 y (18-82) | 4 mo median (2-120) in the 90 patients with controlled cough and 3.5 mo median (3-36) in the unimproved 12 patients | ? (?) | 4.9 (5 of 102) | 7 (7.2) |
| Yu et al40/2011 | Respiratory clinic, Tongji Hospital, Shanghai, China | N = 125 (?) consecutive consenting nonsmoking patients with cough of > 8 wk meeting > 10 criteria | n = 109 (34) | n = 109; 50 ± 14 y (?) | n = 109; 6 mo median (2-480) | ? (?) | 12.8 (16 of 125) | 3 (2.75) |
| Randomized clinical trials | ||||||||
| Field et al41/2009 | Ambulatory university-affiliated tertiary care center clinic, Calgary, AB, Canada | N = 198 (?); MD arm 103, CRE arm 95 consenting immunocompetent patients with cough ≥ 4 wk (only 1 was < 8 wk) and no radiographic abnormality of clinical evidence meeting 8 criteria | n = 151; 78 (30.8); MD arm vs CRE arm 73 (28.8) | n = 151; MD arm 46.1 ± 12.7 vs CRE arm 53.6 ± 13.1 P = .0005 | n = 151; 16 mo median (1-840) vs CRE arm 15 mo (2-360) | ? (?) CREs arm average of 4.9 contacts vs 2.7 for MD arm P < .0001). |
23.7 (47 of 198) 25 of 103 MD arm 22 of 95 CRE arm |
56 (37.1) |
| Wei et al29/2010 | Respiratory Clinic, Tongji Hospital, Tongji Shanghai, China | N = 240 (?); 120 (36) modified empirical therapy 120 (42) primary empirical 3-step therapy for chronic cough, consecutive nonsmoking consenting patients with cough ≥ 8 wk meeting > 10 criteria | n = 106 (?) modified and n = 108 (?) primary | n = 120; 45 ± 15 modified n = 120; 45 ± 15 primary | n = 120; 3 mo median (2-120) modified; n = 120; 3 mo median (2-360) primary | ? (?) | 10.8 (26 of 240) 14 of 120 modified 12 of 120 primary |
n = 214; 19 (8.9) n = 106; 11 (10.4); n = 108; 8 (7.4) |
| Retrospective observational studies | ||||||||
| Haque et al4/2005 | Royal Brompton Cough Clinic, London, England | N = 100 (29) Chart review of consecutive patients referred for chronic cough ≥ 8 wk | N = 100 (29) | N = 100; 57 median (19-81) | N = 100; 48 mo median (2-384) | ? (?) | 0 (0 of 100) | 42 (42) |
| Levine27/2008 | Independent community-based cough center in Southern California | N = 390 (33.6) patients with chronic cough > 8 wk | n = 350 (?) | N = 390 women, median 62.1 (7-85); men median 58.9 (8-82) | N = 390; 78 mo median (2 to 720) | ? (?) | 10.3 (40 of 390) | 64 (18.6) |
| McGarvey et al33/1998 | Chest clinic at Belfast City Hospital, Ireland | N = 124 (44.4) review all of the medical records of patients with chronic cough for > 3 wk with no history of chronic respiratory disease to account for the cough for one year | N = 124 (44.4) | N = 124; 54 y median (13-86) | N = 124; 14.1 mo mean (range, 3 wk -240 mo) | ? (?) | 0 (0 of 124) | 10 (8) |
| Ogawa et al42/2009 | Saiseikai Kanazawa Hospital, Kanazawa, Japan | N = 70 (47.1) retrospective chart review of consecutive new patients with cough ≥ 8 wk | N = 70 (47.1) | N = 70; 51 median (22-80) | N = 70; 4.5 mo median (range, 2-181) | ? (?) | 0 (0 of 70) | 19 (27.1) |
? = not reported. ACE = angiotensin-converting enzyme; CRE = certified respiratory educator; MD = medical doctor.
The term unresolved cough encompasses a variety of diagnoses: idiopathic, not improved, unresponsive, chronic idiopathic cough, unresolved, “idiopathic or psychogenic”, nonresponders, uncontrolled, unexplained, cause not determined.
The 23 studies (Table 2) that composed this systematic review included 3,636 patients in the analysis of studies published between 1998 and 2013. There were 2,627 subjects in the 17 prospective uncontrolled observational studies,21-26,28,30-32,34-40 644 in the four retrospective studies,4,27,33,42 and 365 in the two randomized and controlled studies.29,41 There was little homogeneity in all key study characteristics extracted. Only 52.2% (12 of 23)4,24-27,29,31,34,38-40,42 of the 23 studies defined chronic cough as ≥ 8 weeks’ duration. The ages of patients in the 23 studies were reported with variable statistics, such as mean or median and SD (± SD) or SEM, and with variability related to reporting on those in a subgroup analysis vs those initially enrolled. Of the 23 studies, only two, both retrospective, included patients under the age of 15 years.27-33 When sex was reported, the percent of male subjects across the 23 studies ranged from 11.1%22 to 60%.37 No study reported race, and only one study reported ethnicity.21 Cough duration was variably reported as mean or median with a wide range. Data related to time to patient follow-up were rarely reported. Unexplained cough, idiopathic cough, or cough unable to be resolved ranged from 021,37 to 42%.4
Areas of Intervention Fidelity:
The term intervention fidelity or a conceptually similar term was not identified in any of the studies assessed. No study identified a plan specifically addressing intervention fidelity to the study plan using the strategies as outlined by the Treatment Fidelity Workgroup of the NIH Behavioral Change Consortium as part of the study methods. Despite this finding, study design elements that were conceptually similar to those outlined by the Treatment Fidelity Workgroup were identified, and, using our five-area (eight-element) intervention fidelity tool, the studies were rated for the presence and degree to which they were used. A description of the elements of intervention fidelity present in the individual studies is provided in e-Table 2 (1.7MB, pdf) . Table 3 provides a summary of the findings from the 23 studies described in e-Table 2 (1.7MB, pdf) . Table 4 provides average scores for overall and individual element degree of intervention fidelity identified in the studies.
TABLE 3 ] .
Summary of Findings Related to Intervention Fidelity by Elements, Unresolved Chronic Cough, and Degree of IF
| Study Design IF | Delivery of Treatment | Enactment of Treatment | Outcome | Degree of IF | ||||||||
| Clinical Study Design (n) | Cough ≥ 8 wk, No. of Total | Predominate Guideline/Protocol Intervention, No. of Total | Screening Based on GERD | Validated Outcome Measure or Standardized Rating Scale, No. of Total | Training of Providers | GERD Management Based on Guideline/Protocol | Time to Initial Follow-up Clearly Defined | Receipt of Treatment | Side Effects, No. of Total | Adherence, No. of Total | Unresolved Cougha of Total No. Patients (%) | Overall IF Score |
| Prospective observational studies (17)21-26,28,30-32,34-40 | 8 of 17 | 8 of 17 single | 5 of 17 inconsistent | 8 of 17 cough rating scale | 1 of 17 | 10 of 17 inconsistent | 9 of 17 | 0 of 17 | 2 of 17 | 2 of 17 | 172 of 2,627 (6.55) | 7 fair |
| 7 of 17 multiple | 8 of 17 consistent | 0 of 17 validated QoL questionnaire | 3 of 17 consistent | 10 poor | ||||||||
| 2 of 17 unclear | 4 of 17 unclear | 4 of 17 unclear | ||||||||||
| Randomized clinical trials (2)29,41 | 1 of 2 | 1 of 2 single (1 arm unclear) | 1 of 2 unclear | 1 of 2 cough rating scale | 1 of 2 | 2 of 2 inconsistent | 1 of 2 | 0 of 2 | 1 of 2 | 0 of 2 | 75 of 365 (20.55) | 1 fair |
| 1 of 2 multiple | 1 of 2 inconsistent | 2 of 2 validated QoL questionnaire | 1 poor | |||||||||
| Retrospective studies (4)4,27,33,42 | 3 of 4 | 2 of 4 single | 1 of 4 inconsistent | 1 of 4 cough rating scale | 0 of 4 | 3 of 4 inconsistent | 1 of 4 | 0 of 4 | 0 of 4 | 0 of 4 | 135 of 644 (20.96) | 1 fair |
| 1 of 4 no guideline or unclear | 3 of 4 unclear | 1 of 4 validated QoL questionnaire | 1 of 4 unclear | 3 poor | ||||||||
| 1 of 4 unclear | ||||||||||||
| Overall summary (23) | 12 of 23 | Heterogeneous | Inconsistent | Primarily nonvalidated | Rare | Inconsistent/unclear | Inconsistent | Nonexistent | Rare | Rare | 382 of 3,636 (10.51) | poor |
GERD = gastroesophageal reflux disease (see e-Table 3 (1.7MB, pdf) , criteria were based on the published guideline or protocol used and protocols varied by study); IF = intervention fidelity; QoL = quality of life.
The term unresolved cough encompasses a variety of diagnoses, including idiopathic, not improved, unresponsive, chronic idiopathic cough, unresolved, “idiopathic or psychogenic,” nonresponders, uncontrolled, unexplained, cause not determined.
TABLE 4 ] .
Summary of Findings: Degree of Intervention Fidelity by Design
| Individual Element Score Range 0-6a | Range 0-8 | Range 0-48b | ||||||||
| Clinical Study Design (n) | Study Design | Training of Providers | Delivery of Treatment | Receipt of Treatment | Enactment of Treatment | |||||
| Element | 1a | 1b | 1c | 2 | 3a | 3b | 4 | 5 | No. Elements Present | Overall IF Score |
| Prospective observational studies (17) | 5.06 | 4.71 | 2.00 | 3.06 | 3.00 | 4.00 | 0.06 | 0.53 | 5.29 | 22.41 |
| Randomized clinical trials (2) | 3.50 | 3.00 | 5.00 | 3.50 | 2.50 | 6.00 | 0.00 | 1.00 | 6.00 | 24.50 |
| Retrospective observational studies (4) | 3.75 | 1.75 | 1.00 | 1.50 | 1.75 | 2.00 | 0.00 | 0.00 | 3.50 | 11.75 |
| Overall summary (23) | 4.70 | 4.04 | 2.09 | 2.83 | 2.74 | 3.83 | 0.04 | 0.48 | 5.04 | 20.74 |
1a = guideline/protocol; 1b = screening appropriate to 1a; 1c = validated/standardized cough outcome measure used; 2 = providers with expanded knowledge of guidelines/protocols and education provided and manual used; 3a = core management interventions appropriate to 1a; 3b = response assessed; 4 = reference to patient understanding as part of methods; 5 = reference to patient’s ability to use interventions in daily life. See Table 3 legend for expansion of abbreviations.
For the individual IF element scores, 0 = no fidelity to 6 = highest degree of fidelity possible; all scores are displayed as averages.
For the overall IF score ratings: good (36-48), fair (24-35), poor (≤ 23); all scores are displayed as averages.
As shown in Table 4, the overall degree of presence of the intervention fidelity elements was poor. The mean total score was poor for the prospective observational studies, fair for the randomized clinical trials, and poor for the retrospective study designs.
Intervention fidelity average summary ratings pertaining to elements of the three areas relating to the interventionists (items 1a, 1b, 1c, 2, 3a, 3b) ranged from 2.09 to 4.70 on a scale of 0 to 6 (Table 4). These summary scores were higher than the average scores for the two areas relating to patients (items 4, 5) that included receipt of treatment (0.04) and enactment of treatment (0.48).
Study Design:
This area consisted of three elements. First, “was the guideline or protocol used to guide the study published and was it clearly identified?” Forty-seven percent (eight of 17)22-24,26,30,34,36,37 of the prospective observational studies, 50% (one of two)41 of the randomized controlled studies, and 50% (two of four)4,42 of the retrospective studies were primarily based upon a single guideline or protocol (Table 3). Forty-one percent (seven of 17)21,28,31,35,38-40 of the prospective observational studies, 50% (one of two)29 of the randomized clinical trials, and 25% (one of four)27 of the retrospective studies were underpinned by multiple guidelines or protocols. The guideline or protocol underpinnings were not clear for two25,32 of the prospective observational studies and one of the retrospective observational studies.33 Table 4 displays the degree of fidelity for this study design item for the described use of a published guideline or protocol. Because 52.2% (12 of 23) of all studies were primarily underpinned by the merging of more than one guideline or protocol or having it unclear as to what the basis for diagnosis and management was, it is not possible to determine if they were uniformly true to a published guideline or protocol. Average fidelity scores for this item by study design ranged from 3.75 to 5.06 (Table 4).
Of the prospective observational studies,22,28,30-32,38-40 47.1% (eight of 17) had extensive exclusion criteria, as did 100% of the randomized controlled trials.29,41 These exclusionary criteria were not consistent from study to study. For example, some studies excluded smokers22,29,32,36,38-40 or even former smokers of many years,22 or were unclear on smoking as an exclusion,23,24 whereas others did not exclude smokers.4,21,25,27,28,30,31,33-35,37,41
Second, “did the authors identify the diagnostic methods for screening for causes of chronic cough according to the guideline or protocol cited or referenced?” The average score for this item by study design ranged from 1.75 to 4.71 (Table 4). Basing diagnostic testing on screening for gastroesophageal reflux disease (GERD), only 47.0% (eight of 17)24,26,30-32,34,35,37 of the prospective observational studies, 0% (zero of two)29,41 of the randomized controlled studies, and 0% (zero of four)4,27,33,42 of the retrospective studies appeared to be consistent with the most recent guidelines cited in the article (Table 3, e-Table 3 (1.7MB, pdf) ). Table 3 contains overall summary data, and e-Table 3 (1.7MB, pdf) contains individual study data specific to the diagnosis and management of GERD. Diagnostic methods were evaluated based upon the most recent published guideline or protocol cited by the authors, and these varied by study. In summary, only 34.7% (eight of 23) appeared to use diagnostic criteria for GERD that were consistent with the most recent protocol or guideline referenced by the authors.
Third, “were standardized or validated tools used to measure patient reported outcomes?” The average score for this element by study design ranged from 1.00 to 5.00 (Table 4). Forty-seven percent (eight of 17)21,28,30-32,34,38,39 of the prospective observational studies, 100% (two of two)29,41 of the randomized controlled studies, and 25% (one of four)27 of the retrospective studies used a subjective previously validated or standardized subjective cough severity rating scale (Table 3). Although validated or standardized scales were used in these studies, in two of the prospective observational studies it was not clear how they were used in determining the diagnosis of cough.32,34 A minority of studies, 34.8% (eight of 23), specified, with variable clarity, what degree of change constituted acceptable improvement.21,27-30,38,39,41
Although multiple studies based response to treatment of cough as being no longer troublesome, none of the prospective observational studies and only 25% (one of four)27 of the retrospective studies mentioned any type of cough-specific quality-of-life scale as an outcome variable (Table 3). On the other hand, 100% (two of two)29,41 of the randomized controlled studies measured cough-specific quality of life using a validated questionnaire as an outcome measure.
Training of Providers:
This area consisted of one element with three parts: “was there formal training of providers related to the guideline or protocol used, and was an intervention manual used to guide providers?” Average scores for this item by study design ranged from 1.50 to 3.50 (Table 4). Only one prospective observational study26 mentioned that multiple providers in all sites had received education and that quality control was used; yet, this study did not mention the use of a manual to guide intervention delivery (Table 3). One randomized controlled trial reported, in the methods section, that one arm of the trial was guided by an algorithm and with prescribed follow-up.41 No other study mentioned any provider education or the use of an intervention manual to direct the use of the guideline or protocol. There was no mention of whether there was deviation from the protocol or guideline on the part of the interventionist in any of the studies reviewed.
As summarized from Table 2, 56.5% (13 of 23)21,23,24,26,29-33,37-40 of the studies were conducted in general respiratory clinics, 17.4% (four of 23)4,25,27,34 in cough specialty clinics, and 26.1% (six of 23) in a variety of other types of primary care, hospital, or general medicine clinics.22,28,35,36,41,42 All but three of the studies21,23,37 appeared to have multiple physicians participating in patient management.
Delivery of Treatment:
This area included two elements. First, “were the core treatment interventions consistent with the guideline or protocol used to develop the intervention manual and/or to guide the study?” The average scores for this item by study design ranged from 1.75 to 3.00 (Table 4). As previously noted, a single multicenter study with multiple providers reported training and quality control but not the use of an intervention manual to guide the providers, and it was not clear if the training pertained to both diagnostic and management interventions.26 No other study noted any education of providers or the use of a manual to direct care. Treatment descriptions varied in detail, content, and consistency with the primary guideline cited, with one study23 providing a table that very clearly associated diagnoses with the history, examination, investigations, and treatment.
Looking specifically for treatment of GERD, at least one component of the most current recommendations referenced by the authors was not noted as being used for 58.8% (10 of 17)21,23,28,30-32,34,35,38,40 of the prospective observational studies (Table 3, e-Table 3 (1.7MB, pdf) ). Management recommendations were evaluated based upon the most recent published guideline or protocol cited by the authors, and these varied by study. An additional 23.5% (four of 17)22,25,36,39 of the prospective observational studies did not include enough information to assess for this item. Only 17.6% (three of 17)24,26,37 of studies of this design appeared to consistently apply all treatments for GERD as recommended by the most recent guideline or protocols cited. Additionally, in 100% (two of two)29,41 of the randomized controlled studies and 75% (three of four)4,27,42 of retrospective studies, at least one component of GERD treatment specified by the most current recommendations referenced by the authors was not noted. One retrospective study did not provide enough information to make a determination regarding GERD treatment.33 These findings resulted in only 13.0% (three of 23) of the studies clearly being consistent to the most recent guideline or protocol regarding GERD treatment.
The second element of this area included: “was there assessment of response to treatment at specified timeframes?” Average scores for this item by study design ranged from 2.00 to 6.00 (Table 4). Although most guidelines and protocols noted the need for reassessment and revision of the intervention plan, patient follow-up for reassessment posttreatment, when reported, varied greatly, with initial follow-up for those studies reporting data ranging from 5 days27 to 3 months.24,30 With respect to follow-up, although multiple studies included time for response to treatment as part of diagnostic criteria, many were not clear regarding time to initial follow-up and reassessment of response to treatment. Of the observational studies, 52.9% (nine of 17)22,23,26,28,30,31,34,38,39 clearly included a time for initial follow-up as part of their methods, as did 50% (one of two)29 of the randomized controlled trials and 25% (one of four)27 of the retrospective observational studies (Table 3). As noted under characteristics of studies, few provided data related to this element.
Receipt of Treatment:
This area included one element: “Was there any mention and/or measurement of concordance of patient and provider understanding of the problem and/or treatment recommendations?” The average scores for this item by study design ranged from 0.00 to 0.06 (Table 4). No study of any design specifically reported systematically assessing for or measuring patient understanding (Table 3). Although one study21 mentioned the need for patient education, noting that > 30% of patients lacked an awareness of previous diagnoses, there was no mention of measuring patient understanding of the interventions used in any of the studies reviewed. One study noted that patients were instructed as to how to follow the treatment algorithm through to the next phone call but made no mention of addressing understanding.28 An additional study promoted the need for protocol simplicity and sequential therapy to enhance patient adherence but did not address this issue in the methods.39 One randomized controlled study mentioned that certified respiratory educators followed an algorithm that included biweekly patient contact with explanation of differential diagnoses and the rationale for each intervention but made no mention of assessment of patient understanding.41
Enactment of Treatment:
This area included one element: “Was there any mention and/or measurement of patient’s ability to engage in the treatment recommendations in daily life?” The average scores for this item by study design ranged from 0.00 to 1.00 (Table 4). No study of any design specifically reported systematically assessing for or measuring patient ability to engage in interventions in their daily life (Table 3).
In evaluating the 23 studies for this area, reasons for not enacting treatment were classified into those related to nonadherence and those related to side effects (Table 3). Although adherence and side effects are not synonymous with enactment, they give us insight into this area, as enactment was not measured in any study. Of the prospective observational studies, two provided data related to patient nonadherence but did not report whether adherence was systematically evaluated for within their methods.21,32 Of these, one noted a 23% relapse rate due to nonadherence that was addressed during the study; however, reasons for nonadherence were not described.21 The other study reported relapse of symptoms in six patients with postnasal drip syndrome and relapse in one patient secondary to stopping treatment of GERD.32 The latter study also reported a patient who could not tolerate a proton pump inhibitor secondary to side effects and whose cough resolved with a change in therapy; although this study reported side effects, it was not clear if this was systematically addressed.32
Additionally, two studies reported systematically assessing for side effects (Table 3). Although no association was made with adherence, one prospective observational study reported 10% of patients having side effects from treatment that included drowsiness and abdominal discomfort, with no patients dropping out secondary to this issue.39 Of the randomized controlled trials, one reported assessing for and measuring side effects, noting the occurrence of 57 adverse events (eg, drowsiness, abdominal discomfort, dry mouth, dysuria, palpitations, or fatigue) with the use of their modified protocol and 74 similar adverse events with their cited standard protocol.29 Two patients withdrew from this study because of side effects.29
The four retrospective studies did not report data related specifically to patient adherence or side effects or generally related to the patient’s ability to use recommended interventions in daily life. One retrospective study noted that at 1 year, 44% of those contacted by phone had a cough that persisted.33
One study noted that smoking cessation was not addressed because patients were unlikely to quit because of cough.28 Although no related data were supplied, two studies reported the potential impact of cost and access to care, respectively,26,40 and one mentioned the impact of culture adversely affecting the application of care.26 Only one study, a randomized controlled trial, mentioned teaching any physical skills (eg, inhaler use) in the deployment of recommended interventions.41
Unresolved Cough as an Outcome:
Unresolved cough encompasses terms such as idiopathic, not improved, unresponsive, chronic idiopathic cough, unresolved, “idiopathic or psychogenic,” nonresponders, uncontrolled, unexplained, and cause not determined, and averaged 10.5% with a range of 6.6% to 21.0% by study design (see Table 3). A final diagnosis of unresolved cough ranged from 0% to 6.6% in the three single-provider studies.21,23,37 For all studies, response to specific therapy was a criterion for establishing a diagnosis. It was not possible to determine whether unresolved cough in these studies referred to patients who were managed by guidelines or protocols and had no diagnosis or whether it included subjects who may have had an established diagnosis but did not respond to appropriate therapy.
Specific Aim 3:
Assess whether intervention fidelity was used to the extent that one can be confident that the diagnoses made were valid.
As revealed in Table 4, intervention fidelity in the 23 studies selected for review was overall poor. The highest degree of intervention fidelity was in the two randomized controlled trials, yet they could only be rated as fair. Of the five areas assessed, those related to receipt and enactment of treatment by the patients were barely addressed in the studies. Although the areas of study design, training of providers, and delivery of treatment were present to a modest degree, they were still inadequately addressed. Had we measured the methodologic intervention fidelity strategies, as specifically described by Treatment Fidelity Workgroup of the NIH Behavioral Change Consortium, rather than using conceptually similar elements, the findings of this study related to intervention fidelity would have been worse.
These findings suggest that in studies of the diagnosis and treatment of patients with chronic cough that is initially of unknown cause before being worked up, intervention fidelity strategies were not systematically used as part of the methods. Addressing the five areas of intervention fidelity has been proposed as being important to verifying treatment integrity, and treatment integrity is important to the validity of outcomes in intervention studies. In the area of study design, although most studies clearly cited one or more published guidelines or protocols as theoretically underpinning the study, they were not always clearly tied to the diagnostic or management interventions used. When assessing the methods used to determine whether chronic cough may be due to GERD, one or more of the criteria for establishing the diagnosis based upon the most recent guideline cited by the authors was often not present. Additionally, response to specific treatment was cited as at least part of the criteria for establishing a diagnosis, and, most often, this was not established using standardized or previously validated tools to ensure valid measurement of outcomes. Training of providers was rarely mentioned in the studies assessed, and the use of an intervention manual was never mentioned. In the area of delivery of treatment, when assessing the treatments delivered for GERD, they were most often missing at least one element of that proposed by what appeared to be the most recent of the guidelines or protocols cited by the authors, and they were therefore not true to the proposed theoretical underpinnings of the study. Receipt and enactment of treatment by the patient were also rarely addressed. Because treatment integrity was not verified, we cannot be confident that the diagnoses established, based upon improvement in cough with specific treatment, were reliable and valid.
Summary of Evidence and Interpretation From the Systematic Review:
This review suggested that in studies of the diagnosis and treatment of patients with chronic cough that is initially of unknown cause before being evaluated, intervention fidelity strategies were not systematically used. Therefore, one cannot be sure of the reliability and validity of study results. Our results lend credence to our hypothesis that routinely incorporating intervention fidelity as a methodologic strategy should improve the reliability and validity of the outcomes of studies using guidelines or protocols in the treatment of chronic cough in adults. The diagnostic and therapeutic interventions were used in different ways, and it was not possible to be confident that core elements of guidelines were actually delivered and received as intended. Our results also support the supposition that the variability in success in treating chronic cough, as reported in the literature, may be due in part to guidelines or protocols not being implemented as planned by interventionists and patients.
Strengths and Limitations
The strengths of this systematic review include the novelty of addressing intervention fidelity in studies of the management of chronic cough and doing so using the most up to date and rigorous systematic review methodology. Strengths also include the development of a new tool to systematically assess for the presence of elements of and the degree of intervention fidelity in studies using guidelines for the diagnosis and management of chronic cough.
The limitations relate to the fact that the use of intervention fidelity strategies in studies using guidelines to diagnose and manage cough is an emerging area of study; therefore, tools and methods for extracting data are in their infancy. The data extracted are also limited based upon their subjective nature and what was documented. Other limitations relate to the lack of direct mention of intervention fidelity strategies in the methods of the studies reviewed. Therefore, we had to assess for elements that were conceptually similar. In addition, there was a lack of comparative studies, very few randomized controlled clinical trials, the likelihood of publication bias, absence of validated tools to assess cough outcomes, heterogeneity regarding the populations studied based upon variable definitions of chronic cough, and the small number of patients enrolled in the studies. Although the locations where the studies were carried out were culturally diverse, we took this into account by only holding the authors accountable for what they said they did.
Our inability to pool data due to the heterogeneity of the studies for meta-analysis was also a limitation. Because of these limitations, it was not possible to correlate the degree of intervention fidelity with the number of patients with unresolved chronic cough. For example, although Table 3 reveals that there were fewer patients diagnosed with unresolved chronic cough in the prospective observational studies (6.55%) compared with those in the randomized controlled clinical trials (20.55%), this does not seem intuitively plausible and may be an artifact due to the bias associated with the less frequent use of reliable and validated patient outcome measures in the observational studies. It is also possible that randomized controlled trials do not allow for adequate flexibility and individualization associated with guideline implementation and, therefore, do not provide the best assessment of real-life settings, further supporting the need for the use of intervention fidelity strategies.
Recommendations and Suggestions
Based upon the systematic review, the Expert Cough Panel was able to make a series of recommendations and/or suggestions for the use of intervention fidelity, by those conducting research, in studies of adults with chronic cough who are being diagnosed and managed using an evidence-based clinical practice guideline or protocol. The recommendations or suggestions are presented in stepwise fashion to provide a systematic plan in logical sequential order so that all five areas of intervention fidelity are addressed from creation of the study design through activation of the intervention fidelity strategies.
1. In conducting studies of chronic cough in adults, we recommend that investigators, as a first step, include intervention fidelity in the design of their studies of the diagnosis and treatment of chronic cough, by addressing intervention fidelity in the following 5 areas: study design, training of providers, treatment delivery, treatment receipt, and enactment of treatment (Grade 1C).
2. In conducting studies of chronic cough in adults, we recommend, as a second step, that the training of investigators be addressed; and, all investigators should agree to employ the use of an evidence-based clinical practice guideline or an evidence-based protocol for the diagnosis and treatment of chronic cough and agree to follow an intervention manual outlining the minimum expected interventions throughout the study (Grade 1C).
3. In conducting studies of chronic cough in adults, we recommend that investigators, as a third step, establish a standardized plan for delivery and measurement of treatment through the use of an intervention manual (Grade 1C).
4. In conducting studies of chronic cough in adults, we recommend that investigators, as a fourth step, establish a standardized plan for maximizing and measuring concordance of understanding of interventions and treatment between subjects and investigators (Grade 1C).
5. In conducting studies of chronic cough in adults, as a fifth step, we recommend that investigators establish a standardized plan for evaluating and measuring the subject’s ability to enact and adhere to the treatment plan under real life circumstances (Grade 1C).
6. In conducting studies of chronic cough in adults, we recommend that investigators not make a diagnosis of idiopathic chronic cough as a distinct clinical entity unless known causes of cough have been excluded by a systematic evaluation using an evidence-based guideline and intervention fidelity has been addressed in the design and implementation of the study (Grade 1C).
7. In all patients with chronic cough, we suggest that clinicians use an evidence-based guideline that contains core elements and processes as a guide for diagnosis and treatment (Ungraded Consensus-Based Statement).
Areas for Future Research and Clinical Practice
To advance the field and provide trustworthy guidelines to guide clinical practice, there are a number of potential future research issues that should be addressed. They are enumerated below:
• To improve the internal and external validity of future studies seeking to diagnose and manage chronic cough in adults, researchers should use the recommendations and suggestions related to intervention fidelity made in this document. If researchers are not able to use these recommendations, they should document why there were not able to do so.
• To carry out the future studies, tools need to be developed to guide and monitor the intervention fidelity strategies provided in our recommendations and suggestions such as an intervention manual (see example in e-Appendix 2 (1.7MB, pdf) ) and a tool to measure interventionist-patient concordance of understanding of management (see example in e-Appendix 2 (1.7MB, pdf) ). The feasibility of using the tools in e-Appendix 2 (1.7MB, pdf) has previously been reported.43 In addition to suggesting appropriate tools, the intervention manual in e-Appendix 2 (1.7MB, pdf) also provides suggested steps for researchers to follow to carry out clinical studies that satisfy the five areas of intervention fidelity.
• Benefits and harms associated with patient care should be considered in future studies using intervention fidelity strategies. At this time, benefits are believed to greatly outweigh harms, because not following current guidelines may result in patients not getting maximal benefit out of being evaluated for chronic cough. Additionally, there may be a potential for diagnostic mislabeling, and patients may be exposed to unnecessary harm associated with interventions that may not have been indicated. Guidelines are meant to guide patient care, and as such, they do not preclude the need to adjust care to the individual patient situation. By measuring receipt and enactment of treatment, in particular, we are likely to develop greater insight into the benefits and harms associated with the use of these guidelines.
Conclusions
Since publication of the 2006 Chest Cough Guidelines, and based upon this systematic review, it is clear that some of the variability in the reporting of successful management patients with chronic cough may be due to lack of intervention fidelity. Using these results, the Expert Cough Panel has been able to make a series of recommendations and suggestions directed at researchers for carrying out future studies of chronic cough in adults. By following the recommendations and suggestions in this article, patients will likely benefit, as their providers will be managing them according to more reliable and valid studies. Improved research will strengthen the evidence used in clinical practice guidelines that clinicians use when counseling patients regarding benefits and harms associated in their management.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: C. T. F. and R. S. I. contributed as topic editors for this article; R. L. D. contributed as the appointed methodologist and guided the systematic review that formed the basis for the recommendations; C. T. F., R. L. D., and R. S. I. contributed to the development of the key questions using the PICOTS format, review of the data, and elaboration of recommendations, including their grading; C. T. F. and R. S. I. drafted, wrote, reviewed, and approved the manuscript; and R. L. D. reviewed and approved the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Drs French and Irwin are codevelopers of the Cough Quality-of-Life Questionnaire and have received no funding related to this in the last 4 years. Dr Irwin was also a member of the Advisory Panel for the AHRQ systematic review on assessing cough severity and a reviewer of the final AHRQ document. Although Dr Irwin is the Editor in Chief of CHEST, he reports that the entire review process and acceptance decision of this manuscript was carried out by others, independent of him. Although Ms Diekemper is a staff methodologist working for ACCP, she has no conflicts of interest related to the content of this manuscript.
Endorsements: This guideline has been endorsed by the American Association for Respiratory Care, the American Thoracic Society, the Canadian Thoracic Society, and the Irish Thoracic Society.
Collaborators: Todd M. Adams, MD; Kenneth W. Altman, MD, PhD; Alan F. Barker, MD; Surinder S. Birring, MBChB, MD; Fiona Blackhall, MD, PhD; Donald C. Bolser, PhD; Louis-Philippe Boulet, MD, FCCP; Sidney S. Braman, MD, FCCP; Christopher Brightling, MBBS, PhD, FCCP; Priscilla Callahan-Lyon, MD; Brendan J. Canning, PhD; Anne B. Chang, MBBS, PhD, MPH; Remy Coeytaux, MD, PhD; Terrie Cowley; Paul Davenport, PhD; Rebecca L. Diekemper, MPH; Satoru Ebihara, MD, PhD; Ali A. El Solh, MD, MPH; Patricio Escalante, MD, FCCP; Anthony Feinstein, MPhil, PhD; Stephen K. Field, MD; Dina Fisher, MD; Cynthia T. French, PhD, FCCP; Peter Gibson, MBBS; Philip Gold, MD, MACP, FCCP; Michael K. Gould, MD, FCCP; Cameron Grant, MBChB, PhD; Susan M. Harding, MD, FCCP; Anthony Harnden, MBChB; Adam T. Hill, MBChB, MD; Richard S. Irwin, MD, Master FCCP; Peter J. Kahrilas, MD; Karina A. Keogh, MD; Andrew P. Lane, MD; Kaiser Lim, MD; Mark A. Malesker, PharmD, FCCP; Peter Mazzone, MD, MPH, FCCP; Stuart Mazzone, PhD, FCCP; Douglas C. McCrory, MD, MHS; Lorcan McGarvey, MD; Alex Molasiotis, PhD, RN; M. Hassan Murad, MD, MPH; Peter Newcombe, PhD; Huong Q. Nguyen, PhD, RN; John Oppenheimer, MD; David Prezant, MD; Tamara Pringsheim, MD; Marcos I. Restrepo, MD, FCCP; Mark Rosen, MD, Master FCCP; Bruce Rubin, MEngr, MD, MBA; Jay H. Ryu, MD, FCCP; Jaclyn Smith, MBChB, PhD; Susan M. Tarlo, MBBS, FCCP; Anne E. Vertigan, PhD, MBA; Gang Wang, MD, PhD; Miles Weinberger, MD, FCCP; Kelly Weir, MsPath.
Role of sponsors: CHEST was the sole supporter of these guidelines, this article, and the innovations addressed within.
Other contributions: We thank the CHEST Organization for providing resources in the support of this work. We also thank Nancy Harger, MLS, and Judy Nordberg, MLIS, Education and Clinical Services Librarians working in the University of Massachusetts Medical School Library in Worcester, MA, for their assistance and guidance in conducting the literature searches.
Additional information: The e-Appendixes and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- CHEST
American College of Chest Physicians
- GERD
gastroesophageal reflux disease
- NIH
National Institutes of Health
- PICOTS
population, intervention, comparator, outcome, timing, setting
Footnotes
DISCLAIMER: American College of Chest Physician guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at http://www.chestnet.org/Guidelines-and-Resources/Guidelines-and-Consensus-Statements/CHEST-Guidelines.
FUNDING/SUPPORT: CHEST was the sole supporter of these guidelines, this article, and the innovations addressed within.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Contributor Information
on behalf of the CHEST Expert Cough Panel:
Todd M. Adams, Kenneth W. Altman, Alan F. Barker, Surinder S. Birring, Fiona Blackhall, Donald C. Bolser, Louis-Philippe Boulet, Sidney S. Braman, Christopher Brightling, Priscilla Callahan-Lyon, Brendan J. Canning, Anne B. Chang, Remy Coeytaux, Terrie Cowley, Paul Davenport, Rebecca L. Diekemper, Satoru Ebihara, Ali A. El Solh, Patricio Escalante, Anthony Feinstein, Stephen K. Field, Dina Fisher, Cynthia T. French, Peter Gibson, Philip Gold, Michael K. Gould, Cameron Grant, Susan M. Harding, Anthony Harnden, Adam T. Hill, Richard S. Irwin, Peter J. Kahrilas, Karina A. Keogh, Andrew P. Lane, Kaiser Lim, Mark A. Malesker, Peter Mazzone, Stuart Mazzone, Douglas C. McCrory, Lorcan McGarvey, Alex Molasiotis, M. Hassan Murad, Peter Newcombe, Huong Q. Nguyen, John Oppenheimer, David Prezant, Tamara Pringsheim, Marcos I. Restrepo, Mark Rosen, Bruce Rubin, Jay H. Ryu, Jaclyn Smith, Susan M. Tarlo, Anne E. Vertigan, Gang Wang, Miles Weinberger, and Kelly Weir
References
- 1.Irwin RS, French CT, Lewis SZ, Diekemper RL, Gold PM; on behalf of the CHEST Expert Cough Panel. Overview to the management of cough: CHEST guideline and expert panel report. Chest. 2014;146(4):885-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis. 1981;123(4 pt 1):413-417. [DOI] [PubMed] [Google Scholar]
- 3.Irwin RS, Ownbey R, Cagle PT, Baker S, Fraire AE. Interpreting the histopathology of chronic cough: a prospective, controlled, comparative study. Chest. 2006;130(2):362-370. [DOI] [PubMed] [Google Scholar]
- 4.Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough: a discrete clinical entity? Chest. 2005;127(5):1710-1713. [DOI] [PubMed] [Google Scholar]
- 5.Irwin RS. Unexplained cough in the adult. Otolaryngol Clin North Am. 2010;43(1):167. [DOI] [PubMed] [Google Scholar]
- 6.Song MK, Happ MB, Sandelowski M. Development of a tool to assess fidelity to a psycho-educational intervention. J Adv Nurs. 2010;66(3):673-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellg AJ, Borrelli B, Resnick B, et al. ; Treatment Fidelity Workgroup of the NIH Behavior Change Consortium. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23(5):443-451. [DOI] [PubMed] [Google Scholar]
- 8.Moncher FJ, Prinz RJ. Treatment fidelity in outcome studies. Clin Psychol Rev. 1991;11(3):247-266. [Google Scholar]
- 9.Santacroce SJ, Maccarelli LM, Grey M. Intervention fidelity. Nurs Res. 2004;53(1):63-66. [DOI] [PubMed] [Google Scholar]
- 10.Resnick B, Bellg AJ, Borrelli B, et al. Examples of implementation and evaluation of treatment fidelity in the BCC studies: where we are and where we need to go. Ann Behav Med. 2005;29(suppl):46-54. [DOI] [PubMed] [Google Scholar]
- 11.Resnick B, Inguito P, Orwig D, et al. Treatment fidelity in behavior change research: a case example. Nurs Res. 2005;54(2):139-143. [DOI] [PubMed] [Google Scholar]
- 12.Stein KF, Sargent JT, Rafaels N. Intervention research: establishing fidelity of the independent variable in nursing clinical trials. Nurs Res. 2007;56(1):54-62. [DOI] [PubMed] [Google Scholar]
- 13.Irwin RS, Boulet L-P, Cloutier MM, et al. Managing cough as a defense mechanism and as a symptom: a consensus panel report of the American College of Chest Physicians. Chest. 1998;114(2_suppl managing):133S-181S. [DOI] [PubMed] [Google Scholar]
- 14.Irwin RS, Madison JM. The diagnosis and treatment of cough. N Engl J Med. 2000;343(23):1715-1721. [DOI] [PubMed] [Google Scholar]
- 15.Morice AH, Fontana GA, Sovijarvi AR, et al. ; ERS Task Force. The diagnosis and management of chronic cough. Eur Respir J. 2004;24(3):481-492. [DOI] [PubMed] [Google Scholar]
- 16.Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1_suppl):1S-23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis SZ, Diekemper R, Ornelas J, Casey KR. Methodologies for the development of CHEST guidelines and expert panel reports. Chest. 2014;146(1):182-192. [DOI] [PubMed] [Google Scholar]
- 18.Lewis SZ, Diekemper RL, French CT, Gold PM, Irwin RS; on behalf of the CHEST Expert Cough Panel. Methodologies for the development of the management of cough: CHEST guideline and expert panel report. Chest. 2014;146(5):1395-1402. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-W94. [DOI] [PubMed] [Google Scholar]
- 21.Al-Mobeireek AF, Al-Sarhani A, Al-Amri S, Bamgboye E, Ahmed SS. Chronic cough at a non-teaching hospital: are extrapulmonary causes overlooked? Respirology. 2002;7(2):141-146. [DOI] [PubMed] [Google Scholar]
- 22.Ayik SO, Başoğlu OK, Erdínç M, Bor S, Veral A, Bílgen C. Eosinophilic bronchitis as a cause of chronic cough. Respir Med. 2003;97(6):695-701. [DOI] [PubMed] [Google Scholar]
- 23.Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999;160(2):406-410. [DOI] [PubMed] [Google Scholar]
- 24.Fujimura M, Abo M, Ogawa H, et al. Importance of atopic cough, cough variant asthma and sinobronchial syndrome as causes of chronic cough in the Hokuriku area of Japan. Respirology. 2005;10(2):201-207. [DOI] [PubMed] [Google Scholar]
- 25.Kastelik JA, Aziz I, Ojoo JC, Thompson RH, Redington AE, Morice AH. Investigation and management of chronic cough using a probability-based algorithm. Eur Respir J. 2005;25(2):235-243. [DOI] [PubMed] [Google Scholar]
- 26.Lai K, Chen R, Lin J, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest. 2013;143(3):613-620. [DOI] [PubMed] [Google Scholar]
- 27.Levine BM. Systematic evaluation and treatment of chronic cough in a community setting. Allergy Asthma Proc. 2008;29(3):336-342. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Kim M, Kim JH, Lee YR, Kim S, Kim Y. A cheaper, faster way to resolve chronic cough. J Fam Pract. 2007;56(8):641-646. [PubMed] [Google Scholar]
- 29.Wei W, Yu L, Wang Y, et al. Efficacy and safety of modified sequential three-step empirical therapy for chronic cough. Respirology. 2010;15(5):830-836. [DOI] [PubMed] [Google Scholar]
- 30.Marchesani F, Cecarini L, Pela R, Sanguinetti CM. Causes of chronic persistent cough in adult patients: the results of a systematic management protocol. Monaldi Arch Chest Dis. 1998;53(5):510-514. [PubMed] [Google Scholar]
- 31.Ribeiro M, De Castro Pereira CA, Nery LE, Beppu OS, Silva CO. A prospective longitudinal study of clinical characteristics, laboratory findings, diagnostic spectrum and outcomes of specific therapy in adult patients with chronic cough in a general respiratory clinic. Int J Clin Pract. 2006;60(7):799-805. [DOI] [PubMed] [Google Scholar]
- 32.McGarvey LP, Heaney LG, Lawson JT, et al. Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic protocol. Thorax. 1998;53(9):738-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGarvey LP, Heaney LG, MacMahon J. A retrospective survey of diagnosis and management of patients presenting with chronic cough to a general chest clinic. Int J Clin Pract. 1998;52(3):158-161. [PubMed] [Google Scholar]
- 34.Ojoo JC, Everett CF, Mulrennan SA, Faruqi S, Kastelik JA, Morice AH. Management of patients with chronic cough using a clinical protocol: a prospective observational study. Cough. 2013;9(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palombini BC, Villanova CA, Araújo E, et al. A pathogenic triad in chronic cough: asthma, postnasal drip syndrome, and gastroesophageal reflux disease. Chest. 1999;116(2):279-284. [DOI] [PubMed] [Google Scholar]
- 36.Plaza V, Miguel E, Bellido-Casado J, Lozano MP, Ríos L, Bolíbar I. Usefulness of the guidelines of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) in identifying the causes of chronic cough[in Spanish]. Arch Bronconeumol. 2006;42(2):68-73. [DOI] [PubMed] [Google Scholar]
- 37.Smyrnios NA, Irwin RS, Curley FJ, French CL. From a prospective study of chronic cough: diagnostic and therapeutic aspects in older adults. Arch Intern Med. 1998;158(11):1222-1228. [DOI] [PubMed] [Google Scholar]
- 38.Wei W, Yu L, Lu H, et al. Comparison of cause distribution between elderly and non-elderly patients with chronic cough. Respiration. 2009;77(3):259-264. [DOI] [PubMed] [Google Scholar]
- 39.Yu L, Qiu Z, Lü H, Wei W, Shi C. Clinical benefit of sequential three-step empirical therapy in the management of chronic cough. Respirology. 2008;13(3):353-358. [DOI] [PubMed] [Google Scholar]
- 40.Yu L, Qiu ZH, Wei WL, et al. Discrepancy between presumptive and definite causes of chronic cough. Chin Med J (Engl). 2011;124(24):4138-4143. [PubMed] [Google Scholar]
- 41.Field SK, Conley DP, Thawer AM, Leigh R, Cowie RL. Effect of the management of patients with chronic cough by pulmonologists and certified respiratory educators on quality of life: a randomized trial. Chest. 2009;136(4):1021-1028. [DOI] [PubMed] [Google Scholar]
- 42.Ogawa H, Fujimura M, Takeuchi Y, Makimura K. The importance of basidiomycetous fungi cultured from the sputum of chronic idiopathic cough: a study to determine the existence of recognizable clinical patterns to distinguish CIC from non-CIC. Respir Med. 2009;103(10):1492-1497. [DOI] [PubMed] [Google Scholar]
- 43.French CL. Examining Change in Symptoms of Depression, Anxiety, and Stress in Adults After Treatment of Chronic Cough: A Dissertation [dissertation]. University of Massachusetts Medical School; Graduate School of Nursing Dissertations 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement