Abstract
Polyamines (PAs), such as spermine and spermidine, modulate the activity of numerous receptors and channels in the central nervous system (CNS) and are stored in glial cells; however, little attention has been paid to their role in the regulation of connexin (Cx)-based gap junction channels. We have previously shown that PAs facilitate diffusion of Lucifer Yellow through astrocytic gap junctions in acute brain slices; therefore, we hypothesized that spermine can regulate Cx43-mediated (as the most abundant Cx in astrocytes) gap junctional communication. We used electrophysiological patch-clamp recording from paired Novikoff cells endogenously expressing Cx43 and HeLaCx43-EGFP transfectants to study pH-dependent modulation of cell–cell coupling in the presence or absence of PAs. Our results showed (i) a higher increase in gap junctional communication at higher concentrations of cytoplasmic spermine, and (ii) that spermine prevented uncoupling of gap junctions at low intracellular pH. Taken together, we conclude that spermine enhances Cx43-mediated gap junctional communication and may preserve neuronal excitability during ischemia and trauma when pH in the brain acidifies. We, therefore, suggest a new role of spermine in the regulation of a Cx43-based network under (patho)physiological conditions.
Keywords: Cx43 gap junction channels, polyamines, spermine
Introduction
Polyamines (PAs) are involved in numerous physiological processes including gene expression, protein and nucleic acid function and synthesis, protection against oxidative stress 1,2, and increasing longevity 3–5. The brain contains large amounts of PAs, mainly spermidine and spermine 6–8, and an age-dependent depletion of PAs has been reported 4,5,9. Trauma also causes release and loss of PAs in the brain 6. Intriguingly, spermidine and spermine are not synthesized in glial cells in the adult brain 10; however, they accumulate almost exclusively in glia. Astrocytes rather than neurons contain spermidine and spermine in the cortex and hippocampus 7, as well as in Müller and Bergmann glial cells in the retina 11,12 and cerebellum 7, respectively. This suggests that PAs are transported into the glia from external sources through blood and cerebrospinal fluid 1,2,8 to be further diffused through the glial network 8,13. PAs can regulate glial 8,12–16 and other cell receptors/channels 17,18 and transporters 14,15 intracellularly and extracellularly.
Intracellular PAs induce a voltage-dependent block of inwardly rectifying potassium (Kir) channels, nicotinic acetylcholine receptors, GluA-2-lacking glutamate receptor channels, GluN1/GluN2 N-methyl-d-aspartic acid receptor channels, olfactory cyclic nucleotide-gated cation channels, voltage-gated sodium channels, and transient receptor potential channels of the vanilloid subfamily, the melastatin subfamily, and canonical 8. In addition, N-methyl-d-aspartic acid and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor/Kainate channels show a double rectification and potentiation in the presence of intracellular and extracellular PAs, because PAs can weaken hydrogen block of such channels 8,18. To this list can be added PA-sensitive connexin (Cx) channels mediating cell-to-cell exchange of ions and macromolecules through gap junctions in glia 13,19,20. As most of the mentioned neuronal receptors and channels have also been found in glial cells 8, it is evident that PAs plays a similar regulatory role in astrocytes as well.
The glial network or panglial syncytium consists of Cx26, Cx30, Cx32, Cx43, Cx45, and Cx47, which have been shown to be expressed in glia and are responsible for electrical and molecular signaling. Among these Cxs, Cx43 and Cx30 play a major role because their knockout resulted in a lack of gap junctional communication in astrocytes 21. Although PAs have been shown to increase gap junctional communication between astrocytes 13, the mechanism underlying this endogenous regulation remains to be determined.
In this study, we used Novikoff cells endogenously expressing Cx43, as well as HeLaCx43 stable transfectants to investigate the interaction between PAs, acidification, and gap junctional communication. We found that spermine increased Cx43 junctional conductance (gj) in a concentration-dependent manner and reversed acidification-induced uncoupling.
Materials and methods
Cell lines and cell cultures
Novikoff cells (a rat hepatoma cell line) that endogenously express Cx43 22 were seeded in uncoated 75 cm2 flasks at a density of 60 000 cells/cm2, and the culture medium was exchanged every 3 days. At confluence, the cells were dissociated by trypsinization and reseeded onto glass coverslips in 35-mm Petri dishes. The cells were maintained in Dulbecco’s modified Eagle’s medium (Sigma Chemical Co., St. Louis, Missouri, USA) supplemented with 10 mM glucose, 2 mM l-glutamine, 10% fetal calf serum, and 200 IU/ml penicillin/200 μg/ml streptomycin at 37°C (5% CO2, 95% air).
The cDNA constructs encoding Cx43-EGFP were made using HeLa cells as described by Bukauskas et al. 22. HeLa cells transfected with cDNAs encoding rat Cx43 or rat Cx43 with EGFP attached to the C terminus (Cx43-EGFP) were maintained in culture as described by Bukauskas et al. 22. Cx43-EGFP cells could be recognized by their fluorescence at 530 nm.
For electrophysiological analysis the cells were seeded onto coverslips placed in culture dishes at 1000 cells/cm2 16–24 h before the experiment.
Patch clamp from cell pairs in cell cultures
Coverslips with cultured cells were transferred to a recording chamber (RC-27L; Warner Instr. Corp., Hamden, Connecticut, USA) adapted on the stage of an Olympus upright microscope (Olympus, Shinjuku-ku, Tokyo, Japan) with infrared and fluorescence attachments. Cells were visualized using the Nomarski optical infrared attachment equipped with DIC (BX51WI; Olympus) and a DP30BW digital camera with DP Controller software (Olympus).
Two piezoelectric micromanipulators (MX7500 with MC-1000 drive; Siskiyou Inc., Grants Pass, Oregon, USA) were used for precise positioning of the micropipettes. Whole-cell recordings were performed in pairs of Novikoff and HeLaCx43-EGFP cells using a dual whole-cell voltage clamp. The extracellular perfusion solution (extracellular solution; Sigma-Aldridge, St Louis, Missouri, USA) contained (in mM): NaCl, 140; CaCl2, 2.5; MgCl2, 2; HEPES, 10; and KCl, 3 (osmolarity was kept stable at 308 mosmol/l). HEKA amplifiers (EPC-10, three channels, Germany) were used to acquire, store, and analyze the data obtained from pairs of cells.
Junctional current (Ij), measured in cell 2 was divided by voltage (Vj), applied to cell 1 to calculate junctional conductance (gj). Data records were digitized at 5 kHz and filtered at 1 kHz.
Electrodes and intracellular solutions
Patch pipettes were fabricated from Clark capillaries (outer diameter, 1.5 mm/inner diameter, 0.86 mm; #300058; Harvard Apparatus, Holliston, Massachusetts, USA) using a P-97 puller (Sutter Instr. Co., Novato, California, USA). For dual whole-cell voltage clamp recording, the patch pipettes were filled with a pipette solution containing: KCl, 140 mM; HEPES-KOH, 10 mM; EGTA, 2 mM; CaCl2, 0.2 mM; MgCl2, 1 mM; and pH 7.2 spermine, 100 μM–10 mM. The resistance of the pipette was in the range of 5–8 MΩ. Intracellular pH was adjusted by lowering the pH in the pipettes to pH 6 using MES buffer. To measure intracellular pH, cells were transfected with EGFP, and intensity of the EGFP fluorescence was used as criteria to estimated pHi.
Data analysis
Data were analyzed using Origin 9.1 software (OriginLab, Northampton, Massachusetts, USA) and are reported as mean±SEM. Significant differences between groups of data were evaluated using t-tests (P<0.05).
Results
Spermine enhances Cx43-mediated gap junctional communication
Recently, in acute brain slices it was shown that spermine added intracellularly facilitated the spread of Lucifer Yellow in the astrocytic syncytium 13. The number of fluorescent cells increased about 10-fold 13 when spermine (1 mM) was added in the astrocyte. The most abundant Cx in astrocytes is Cx43 21; therefore, we focused on Cx43-expressing cells and examined the effect of spermine on junctional current (Ij) using whole-cell patch-clamp recordings from Novikoff and HeLaCx43-EGFP cell pairs.
Typically it takes about 2 min to dialyze and fully replenish glial cells with PAs through a patch pipette 16. We found enhanced gap junctional communication in Novikoff (Fig. 1d and e) and HeLaCx43-EGFP cells (Fig. 1a–c; by 214±25%, P<0.05, n=9) with the presence of spermine in both pipettes. In contrast, if patch pipettes did not contain spermine, we observed a run-down of Ij (Fig. 1a and d). The latter suggests that washout of endogenous PAs from cells (any proliferating cells contain endogenous PAs) triggers moderate uncoupling of the cells. While keeping the internal pHi stable at 7.2, we observed that the gap junctional communication increased with a rise in spermine concentration in the pipette solution from 1 to 5 and 10 mM by 164±20, 229±34, and 384±55%, respectively (P<0.05, n=5 in each group; Fig. 1d and e).
Fig. 1.
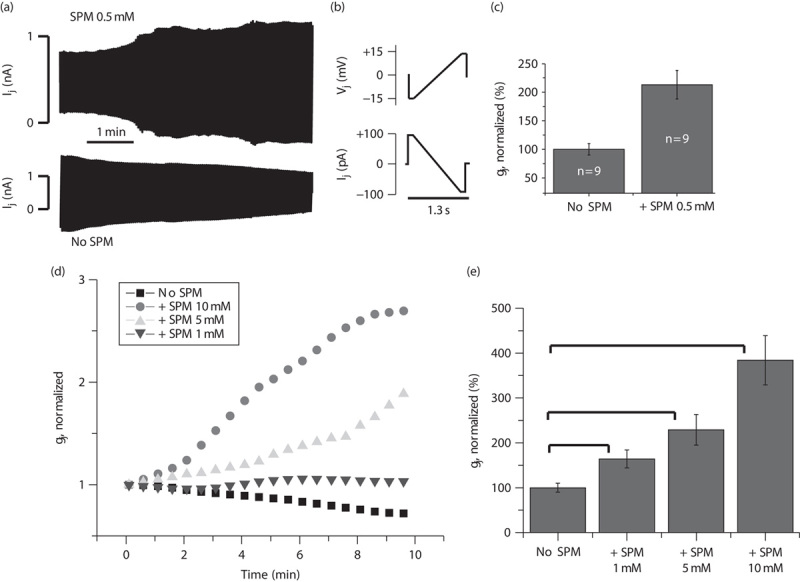
Effect of spermine (SPM) on Cx43 gap junction channels. (a) Examples of junctional current (Ij) records from HeLaCx43-EGFP cell pairs in response to a repeated step-ramp-step protocol shown in (b). The presence of SPM in the pipette solution caused an increase, whereas the absence of SPM produced a decrease in Ij over time. (b) Voltage (Vj) protocol used to study Ij changes over time. (c) Summarized data after 10 min of recording in the presence or absence of 0.5 mM SPM in the pipette solution. Gap junctional conductance was calculated and normalized. Error bars represent SEM. *Significant difference (P<0.05, n=9 in each group). (d) Averaged junctional conductance (gj) measurements in Novikoff cell pairs using different intracellular concentrations of SPM: no SPM, 1 mM SPM, 5 mM SPM, and 10 mM SPM. (e) Summarized data of experiments shown in (d) at 10 min of the recordings. Error bars represent SEM. *Significant difference between groups (P<0.05, n=5 in each group).
Spermine prevents acidification-induced uncoupling of Cx43 gap junctions
Hydrogen cations (H+) are known blockers of Cxs, including Cx43 23,24. We found that PAs not only increase Cx43-mediated gap junctional communication (Fig. 1), but also protect Cx43 gap junctions from acidification-induced uncoupling (Fig. 2). For this, we recorded Ij from Novikoff cells, in which internal acidification was induced by reducing the pHi of the pipette solution to 6 (Fig. 2). Within a few minutes after patch opening, Ij declined on average to 35±10% (P<0.05, n=4; Fig. 2a and b). This gj reduction was rescued by PAs added to the pipette (Fig. 2a and b). At a concentration of spermine greater than 1 mM, the blocking effect at pHi=6 was fully removed (Fig. 2a and b).
Fig. 2.
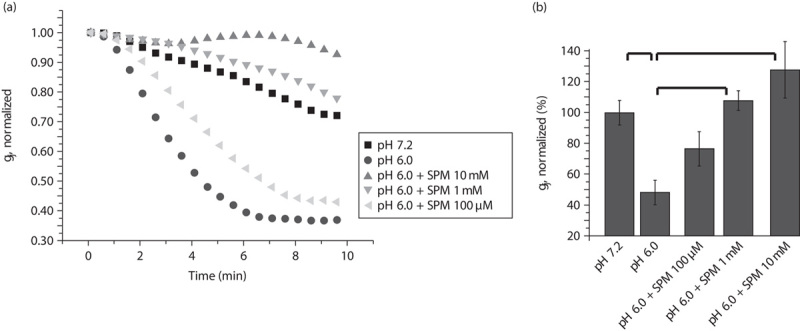
Spermine (SPM) rescues uncoupling of Cx43 gap junctions induced by intracellular acidification (pHi6) of Novikoff cells. (a) Averaged and normalized junctional conductance (gj) measured in Novikoff cells. Intracellular acidification (pHi=6) significantly enhanced gj decay compared with gj changes at pHi=7.2. SPM added in the patch pipette solution transformed gj decay into gj increase even at pHi=6. (b) Summarized data from (a) at 10 min of the recordings. Error bars represent SEM. *Significant difference (P<0.05, n=4 in each group).
Discussion
Our data (Figs 1 and 2) show that spermine (i) produces robust enhancement of communication through Cx43 gap junctions under normal conditions and (ii) can unblock closure of these gap junctions during cytoplasmic acidification of Cx43-expressing cells. A similar enhancement of astrocyte-to-astrocyte communication under normal conditions was observed in brain slices 13. Astrocytes also predominantly express Cx43 in addition to Cx30 21. Taken together, these data show the unique capability of spermine to enhance Cx43-mediated gap junctions; the specific channels expressed in brain astrocytes, not in neurons.
The large amount of PAs in the brain 7 and retina 12 is accumulated almost exclusively in the glia, not in neurons. Spermine accumulating in the glia can regulate neurons in two ways: (i) opening glial cell Cx43 gap junctions will contribute to activation of the glial syncytium and to better potassium, water, and glutamate buffering 21 during neuronal activity, and (ii) when spermine opens large pores made from Cx43 hemichannels in astrocytes, it will allow permeation of different neuroactive substances such as ATP, glutamate, and others through these pores. In addition, we propose that, spermine may exit the glial cytoplasm through the Cx43 hemichannels and modulate neuronal channels and receptors, because many neuronal channels are very sensitive to spermine 18. The conditions under which spermine is liberated from cytoplasmic buffers, as well as the functional role of spermine in regulation of glial Cx43 hemichannels, require additional study.
Indeed, multiple biological effects of PAs have been reported, including increasing longevity 3, memory 4,5, cell proliferation, and differentiation; regulation of receptors and channels 16,18; modulation of transporters 14,15, behavior, and learning; and antinociceptive, neuroprotective, antidepressant, and antioxidant effects. Glial cells and their PA-dependent proteins such as PA transporters, Kir4.1 channels, and Cx43 are involved in these processes 8. As highlighted in numerous studies, the mechanistic aspects of PA functions in the brain remain poorly identified; however, there are many links and correlations between diseases, syndromes, and disorders of the central nervous system and PAs: glioblastoma multiforme, neuropathic pain, migraines, global amnesia, autism, depression, suicidal tendencies, stress, anxiety, sleeplessness, memory loss, and drug addiction are among a host of devastating neurological diseases and disorders linked to the glia and PAs for which a prevention or cure must be found 8. Epidemiological studies have demonstrated a correlation between development of Alzheimer’s, Parkinson’s, and Huntington’s diseases, along with amyotrophic lateral sclerosis and aging, and depletion of PAs in the brain, and these data suggest the involvement of the glia and PAs in brain (dys)function 2,8,13.
Since their original discovery by Leeuwenhoek, the PAs spermine and spermidine have attracted the attention of scientists and clinicians 1,2. Partly because of neurological complications in anticancer treatment using a block of polyamine biochemistry, this approach failed, and later studies have shown that this could have been predicted because of the existence of multiple effects of PAs on receptors and channels in the brain 8,13,17,18.
However, as free endogenous PAs in millimolar concentrations were found almost exclusively in the glia, not in neurons 7,11,12,15,16, and as PAs are colocalized with Cx43, the following questions arise: What are the mechanisms of regulating Cx43 by Pas? and What is the role of PA content (loss/restoration) in CNS function? Interestingly, whereas Cx40 is blocked by PAs 25, the Cx43 gap junctions are opened by PAs (Figs 1 and 2). Glutamate residues at positions 9 and 13 of Cx40 are responsible for spermine block. If these glutamate residues are replaced with lysine, the block by spermine is eliminated 25.
Our data spotlight wider areas in which PAs and Cx43 are both involved. Outside the central nervous system, Cx43 is most abundantly expressed in an assortment of cell types, including cardiomyocytes, epithelial cells, and hepatocytes, and it plays an important role in many (patho)physiological processes. This makes PAs attractive for study of Cx43 regulation in relevance to diseases.
Conclusion
In this study, we focused specifically on Cx43 and the polyamine spermine, and their interaction. We found that spermine augments the communication through Cx43 gap junctions under normal conditions and rescues Cx43 gap junctions from acidification-induced uncoupling. Thus, spermine not only regulates Cx43 gap junction permeability, but may prevent the Cx43-based network from uncoupling during ischemia, neuronal hyperexcitability, or other pathological conditions that lead to acidification.
Acknowledgements
This work was supported by NIH grants: SC2 GM095410 from NIGMS (to Y.V.K.); R01 NS065201 from NINDS (to S.N.S.); R01 NS072238 (to F.F.B.); V PPOHA P031S130068, G12 RR03035-Project-A from NIMHD (to J.B. and M.I.) and G12 MD007583 from NIMHD (for core facilities at UCC).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pegg AE, Casero RA., Jr Current status of the polyamine research field. Methods Mol Biol 2011; 720:3–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pegg AE. The function of spermine. IUBMB Life 2014; 66:8–18. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 2009; 11:1305–1314. [DOI] [PubMed] [Google Scholar]
- 4.Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci 2013; 16:1453–1460. [DOI] [PubMed] [Google Scholar]
- 5.Sigrist SJ, Carmona-Gutierrez D, Gupta VK, Bhukel A, Mertel S, Eisenberg T, Madeo F. Spermidine-triggered autophagy ameliorates memory during aging. Autophagy 2014; 10:178–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilad GM, Gilad VH. Polyamine uptake, binding and release in rat brain. Eur J Pharmacol 1991; 193:41–46. [DOI] [PubMed] [Google Scholar]
- 7.Laube G, Veh RW. Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia 1997; 19:171–179. [DOI] [PubMed] [Google Scholar]
- 8.Skatchkov SN, Woodbury-Fariña MA, Eaton M. The role of glia in stress: polyamines and brain disorders. Psychiatr Clin North Am 2014; 37:653–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura K, Shiina R, Kashiwagi K, Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem 2006; 139:81–90. [DOI] [PubMed] [Google Scholar]
- 10.Krauss M, Langnaese K, Richter K, Brunk I, Wieske M, Ahnert-Hilger G, et al. Spermidine synthase is prominently expressed in the striatal patch compartment and in putative interneurones of the matrix compartment. J Neurochem 2006; 97:174–189. [DOI] [PubMed] [Google Scholar]
- 11.Biedermann B, Skatchkov SN, Brunk I, Bringmann A, Pannicke T, Bernstein HG, et al. Spermine/spermidine is expressed by retinal glial (Müller) cells and controls distinct K+ channels of their membrane. Glia 1998; 23:209–220. [DOI] [PubMed] [Google Scholar]
- 12.Skatchkov SN, Eaton MJ, Krusek J, Veh RW, Biedermann B, Bringmann A, et al. Spatial distribution of spermine/spermidine content and K(+)-current rectification in frog retinal glial (Müller) cells. Glia 2000; 31:84–90. [DOI] [PubMed] [Google Scholar]
- 13.Benedikt J, Inyushin M, Kucheryavykh YV, Rivera Y, Kucheryavykh LY, Nichols CG, et al. Intracellular polyamines enhance astrocytic coupling. Neuroreport 2012; 23:1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sala-Rabanal M, Li DC, Dake GR, Kurata HT, Inyushin M, Skatchkov SN, Nichols CG. Polyamine transport by the polyspecific organic cation transporters OCT1, OCT2, and OCT3. Mol Pharm 2013; 10:1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiasa M, Miyaji T, Haruna Y, Takeuchi T, Harada Y, Moriyama S, et al. Identification of a mammalian vesicular polyamine transporter. Sci Rep 2014; 4:6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kucheryavykh YV, Shuba YM, Antonov SM, Inyushin MY, Cubano L, Pearson WL, et al. Complex rectification of Müller cell Kir currents. Glia 2008; 56:775–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe S, Kusama-Eguchi K, Kobayashi H, Igarashi K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J Biol Chem 1991; 266:20803–20809. [PubMed] [Google Scholar]
- 18.Williams K. Interactions of polyamines with ion channels. Biochem J 1997; 325 (Pt 2):289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res 2006; 25:397–424. [DOI] [PubMed] [Google Scholar]
- 20.Zayas-Santiago A, Agte S, Rivera Y, Benedikt J, Ulbricht E, Karl A, et al. Unidirectional photoreceptor-to-Müller glia coupling and unique K+ channel expression in Caiman retina. PLoS One 2014; 9:e97155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gosejacob D, Dublin P, Bedner P, Hüttmann K, Zhang J, Tress O, et al. Role of astroglial connexin30 in hippocampal gap junction coupling. Glia 2011; 59:511–519. [DOI] [PubMed] [Google Scholar]
- 22.Bukauskas FF, Bukauskiene A, Bennett MV, Verselis VK. Gating properties of gap junction channels assembled from connexin43 and connexin43 fused with green fluorescent protein. Biophys J 2001; 81:137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirst-Jensen BJ, Sahoo P, Kieken F, Delmar M, Sorgen PL. Characterization of the pH-dependent interaction between the gap junction protein connexin43 carboxyl terminus and cytoplasmic loop domains. J Biol Chem 2007; 282:5801–5813. [DOI] [PubMed] [Google Scholar]
- 24.Palacios-Prado N, Briggs SW, Skeberdis VA, Pranevicius M, Bennett MV, Bukauskas FF. pH-dependent modulation of voltage gating in connexin45 homotypic and connexin45/connexin43 heterotypic gap junctions. Proc Natl Acad Sci USA 2010; 107:9897–9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musa H, Fenn E, Crye M, Gemel J, Beyer EC, Veenstra RD. Amino terminal glutamate residues confer spermine sensitivity and affect voltage gating and channel conductance of rat connexin40 gap junctions. J Physiol 2004; 557 (Pt 3):863–878. [DOI] [PMC free article] [PubMed] [Google Scholar]


