Abstract
Objectives
Many patients, due to a combination of illness and sedatives, spend a considerable amount of time in a comatose state that can include time in burst suppression. We sought to determine if burst suppression measured by processed electroencephalography (pEEG) during coma in sedative-exposed patients is a predictor of post-coma delirium during critical illness.
Design
Observational convenience sample cohort
Setting
Medical and surgical ICUs in a tertiary care medical center
Patients
Cohort of 124 mechanically ventilated ICU patients
Measurements and Main Results
Depth of sedation was monitored twice daily using the Richmond Agitation-Sedation Scale and continuously monitored by pEEG. When non-comatose, patients were assessed for delirium twice daily using Confusion Assessment Method for the ICU (CAM-ICU). Multiple logistic regression and Cox proportional hazards regression were used to assess associations between time in burst suppression and both incidence and time to resolution of delirium, respectively, adjusting for time in deep sedation and a principal component score consisting of APACHE II score and cumulative doses of sedatives while comatose. Of the 124 patients enrolled and monitored, 55 patients either never had coma or never emerged from coma yielding 69 patients for whom we performed these analyses; 42 of these 69 (61%) had post-coma delirium. Most patients had burst-suppression during coma, though often short-lived [ median (intraquartile range) time in burst suppression, 6.4 (1-58) minutes]. After adjusting for covariates, even this short time in burst suppression independently predicted a higher incidence of post-coma delirium [odds ratio 4.16; 95% confidence interval (CI) 1.27-13.62; p=0.02] and a lower likelihood (delayed) resolution of delirium (hazard ratio 0.78; 95% CI 0.53-0.98; p=0.04).
Conclusions
Time in burst suppression during coma, as measured by processed EEG, was an independent predictor of incidence and time to resolution of post-coma/post-deep sedation delirium. These findings of this single center investigation support lighter sedation strategies.
Keywords: coma, sedation, delirium, electroencephalography, mechanical ventilation
“We lost her as she was gradually withdrawn from our life into the hands of nurses and delirium and morphia…” From C.S. Lewis's book Surprised by Joy, about his mother's descent into deep sedation during the dying process
Introduction
Delirium, which occurs in 60% to 80% of critically ill, mechanically ventilated patients, is independently associated with increases in hospital costs, hospital length of stay, 6- and 12-month mortality, and cognitive impairment up to a year later [1-7]. Sedative exposure represents a potentially modifiable delirium risk factor, since prior studies have shown that both deep sedation and the use of specific sedative agents, primarily benzodiazepines, are risk factors for delirium [8-10]. Other recent studies have disputed this relationship [11, 12]. Most recently it has been shown [13] that for the majority of patients, those in whom delirium persists 2 hours or longer after stopping sedation, delirium is a predictor of a poor prognosis. Expressed the other way around, there are a minority (about 1 in 10 in this study) with rapidly reversible delirium who have a prognosis similar to those never demonstrating symptoms of delirium [13]. Sedation and the methods for reducing exposure to them remain points of ongoing study and intrigue for the critical care community [14, 15]. Despite increasing knowledge of the harmful effects of deep sedation [16] and new approaches to decrease over-sedation such as the ABCDE bundle [17-20], many patients still spend considerable time in an often largely iatrogenic, unresponsive, comatose state [8, 9, 21-23].
Though bedside sedation scales, such as the Richmond Agitation-Sedation Scale (RASS) or Sedation Agitation Scale (SAS) provide intensive care unit (ICU) teams with a reliable way to assess and track level of consciousness and depth of sedation [24-26], a key limitation in current practice is that physical exam-based sedation scales are not able to gauge subtle changes in deep degrees of sedation. This limitation, at least for research purposes, can be elucidated somewhat through the use of electroencephalographic (EEG) monitoring. The EEG has been used in the ICU primarily to monitor patients with non-convulsive seizures and in several neurocritical care applications [27-30]. Newer technologies are now available that process standard EEG waveforms and provide a bispectral index (i.e., the BIS, which is a form of processed EEG or pEEG). BIS value ranges from 0 to 100, with 0 corresponding to an isoelectric EEG, values below 60 generally corresponding to an anesthetic state with amnesia, and a value of 90 to 100 expected for a fully awake and alert individual. BIS is a reliable indicator of sedation depth [31, 32] and has also been used to assess brain function [33]. Unlike clinical sedation scales, pEEG can identify the deepest levels of sedation, in the form of burst suppression, a period of minimal or isoelectric EEG activity. Previous studies have shown a high correlation between that the number of bursts/min as measured by standard EEG compared with pBIS or the BIS suppression ratio [34, 35].
Whereas burst suppression is sometimes a therapeutic goal in the treatment of refractory status epilepticus, burst suppression resulting from deep sedation or metabolic derangements during critical illness is nearly always inadvertent and has been shown to be an independent predictor of death [36, 37]. Thus, avoiding burst suppression via EEG monitoring may improve outcomes for deeply sedated ICU patients. One adverse outcome that might be avoided is delirium, which is thought to be the result of diffuse cortical dysfunction characterized by a generalized slowing on EEG or pEEG, with a predominance of theta and delta waves [38, 39].
Because benzodiazepines and other potent sedatives represent a common iatrogenic cause of delirium and burst suppression [8, 10, 36, 40, 41], we hypothesized that ICU patients who spend more time in burst suppression during coma would be at higher risk for post-coma or post deep sedation delirium than patients who spend little or no time in burst suppression. Furthermore, we hypothesized that greater time in burst suppression would be associated with delayed resolution of post-coma or post deep sedation delirium.
Methods
Study Design and Patient Population
The institutional review board at Vanderbilt University Medical Center (Nashville, TN) approved this prospective observational cohort study, which was nested within a larger long-term cohort study[42]. Informed consent was obtained from each participant or an authorized surrogate decision maker prior to enrollment. From March 2007 to April 2010, all patients admitted to the medical, surgical, or cardiovascular ICU at Vanderbilt University Medical Center were screened on a daily basis for enrollment into the parent study. Patients who required at least 24 hours of mechanical ventilation or vasopressors for shock were eligible for the parent study unless they met exclusion criteria, which included preexisting severe cognitive or neurodegenerative disease, active substance abuse, or psychotic disorder; inability to see, hear, or speak and understand English; ICU admission after cardiopulmonary resuscitation or moribund status; significant time in an ICU during the 2 months prior to screening (not including the current ICU stay) or cardiac bypass surgery in the 3 months prior to screening; onset of the current episode of respiratory failure or shock more than 72 hours before enrollment or pending extubation on the day of screening; homelessness, incarceration, or residence >200 miles from Nashville, TN; earlier participation in the parent study or participation in another study that prevented co-enrollment; or lack of informed consent due to refusal or absence of an authorized surrogate.
Patients enrolled in a parent cohort study from June 2007 to February 2008 or March 2009 to February 2010, periods that corresponded with availability of study personnel and equipment required for the current study, were eligible for inclusion in the current study unless they met one of the following additional exclusion criteria: absence of respiratory failure requiring mechanical ventilation at the time of planned BIS monitoring, Child-Pugh class B or C cirrhosis, clinical indication for continuous EEG monitoring or participation in another study preventing BIS monitoring, unavailability of equipment or study personnel, or lack of informed consent due to refusal (since informed consent was obtained separately from that obtained at enrollment into the parent study).
All aspects of patient care in the ICU were managed by a team of critical care physicians per standardized protocols in place at the respective ICUs. Analgesic and sedative dosing was directed by the patient care team using a validated sedation scale, the Richmond Agitation-Sedation Score (RASS) [24, 25]. Daily awakening and breathing trials were performed at the discretion of the ICU team per the unit's sedation protocol and was not mandated by study protocol [43]. Data regarding the performance of these awakening trials was not tracked during this study.
Bispectral EEG Monitoring, Burst Suppression, and Covariates
All BIS monitoring and daily data collection were conducted by Vanderbilt University research staff. Immediately after enrollment, the skin was cleaned with isopropyl alcohol and the 4-channel BIS sensor was applied to the forehead and connected to the portable BIS Vista EEG monitor [44]. Bedside ICU nurses were trained to troubleshoot sensor positioning when the signal quality was suboptimal. Research staff checked on the BIS monitors at least twice per day to ensure data collection was continuing. Though the research personnel who conducted clinical assessments of consciousness and delirium were not blinded to the BIS numerical score at the time of each assessment, a partial cover was placed over the front of the BIS monitor such that the research personnel were blinded to the presence/absence of burst suppression, the primary predictor of interest in the current study. BIS monitoring continued until the patient was extubated, expired, or for 7 days, whichever came first. BIS scores were not used in the clinical management of patients.
The BIS software, which processes the EEG obtained by the bispectral EEG monitor, determined and recorded the bispectral index and burst suppression ratio once per minute. Time in burst suppression, our primary exposure variable, was defined as time in minutes during which the patient's pEEG waveforms were either nearly or fully isoelectric. This was determined by the BIS software, which first calculated a burst suppression ratio, which is the percent of the previous 63-second epoch of pEEG that is isoelectric (flat line). The burst suppression ratios were then averaged to give a total percent of coma time in burst suppression, and this was multiplied by total time in coma to produce time in burst suppression. The signal quality index was also recorded every minute; BIS values and suppression ratios from minutes in which the signal quality index was low (<51) were not included in the analysis; no adjustments of BIS values for EMG activity were made [44, 45].
Covariates, which were determined a priori based on previous research and clinical judgment, included severity of illness and exposure to sedative medications. Severity of illness was measured at ICU admission using the Acute Physiology and Chronic Health Evaluation (APACHE) II score [46]. Sedative exposure was measured on a daily basis by recording the total 24-hour doses of lorazepam, midazolam, fentanyl, morphine, and propofol received according to the medical record.
Clinical Assessment of Consciousness and Delirium
Assessment of consciousness was performed by study staff using the Richmond Agitation-Sedation Scale (RASS) twice a day for patients in the ICU and once per day for patients who had been transferred to a general care area. The RASS is a standardized, reliable arousal scale validated for use over time in critically ill patients [24, 25]. In this study, we considered patients comatose when they were responsive to verbal stimulation with movement or eye opening but without eye contact (RASS -3), unresponsive to verbal stimuli but responsive to physical stimuli (RASS -4), or unresponsive to both verbal and physical stimuli (RASS -5). Emergence from coma was defined as the transition from coma to a RASS of -2 or higher (i.e., more awake) for 3 consecutive twice-daily assessments. The time designated as emergence from coma was the first occasion of these 3 time points in which the patient was a RASS -2 or higher. Though experts have varying opinions about what RASS level defines coma [25, 47, 48], we chose the -2 cutoff for this study to be more inclusive in order to increase power by enriching the population of patients whom we could examine for post-coma delirium.
Patients who emerged from coma were assessed for delirium by study staff using the Confusion Assessment Method for the ICU (CAM-ICU) twice a day for patients in the ICU and once per day for patients moved to a general care area. The CAM-ICU is a delirium assessment instrument that is sensitive, specific, valid and reliable in mechanically ventilated and non-ventilated ICU patients [49, 50]. The CAM-ICU was used to assess for delirium directly following RASS assessment if the patient was not comatose (i.e., not RASS -3, -4, or -5). The date and time of resolution of delirium was defined by the first CAM-ICU negative assessment that was followed by 3 or more CAM-ICU negative assessments, i.e., a 48-hour period without coma or delirium was considered to indicate resolution of brain dysfunction.
Statistical analysis
Descriptive data are presented using medians and interquartile ranges (IQRs) for continuous variables and proportions for categorical variables. Sample size was determined by availability of resources, including BIS equipment and staff familiar with lead placement. To determine whether time in burst suppression, the primary exposure variable in our models, was independently associated with post-coma delirium, we used multiple logistic regression to assess the association between time in burst suppression and post-coma delirium, as determined by the first CAM-ICU assessment done after emergence from coma (the primary outcome variable). We also used multivariable Cox proportional hazards regression to assess the association between time in burst suppression and the time to resolution of delirium (i.e., the time between emergence from coma and the onset of a 48-hour period of being continuously non-comatose and CAM-ICU negative). We adjusted in both regression models for time spent under a BIS level of 40 (a measure widely considered deep sedation) and a principal component score consisting of APACHE II at ICU admission, and cumulative doses of benzodiazepines, opiates and propofol received while comatose. Each class of sedating medications, including benzodiazepines, opiates, and propofol, was considered a separate covariate. Lorazepam and midazolam doses were summed after conversion to midazolam equivalents, and fentanyl and morphine doses were summed after conversion to fentanyl equivalents. By including both sedative doses and time spent under a BIS level of 40—with the former being an intervention determined by the ICU team and the latter representing the patient's response to this intervention—we sought to determine the specific association between burst suppression and post-coma delirium that was independent of both the amount of sedatives a patient was given and their general response as indicated by depth of sedation. The principal component score was included as a data reduction technique to allow for adjustment of key covariates while avoiding overfitting, which can occur when more covariates are included in a multivariable regression model than can be reliably fit, as determined by the number of outcome events.
Results
During enrollment, 1,626 patients were screened for the parent BRAIN-ICU study[42], of whom 252 were enrolled and thus candidates for this investigation (Figure 1); 124 of these patients received BIS monitoring, of whom 55 were excluded from the current analyses because they were either never in coma or they never emerged from coma. Thus, 69 patients were monitored with BIS while comatose and subsequently emerged from their coma into either delirium or a normal state of cognition. Patient characteristics are shown in Table 1. No patient received neuromuscular blockade during the study. Eight patients included in the analyses were defined as having coma based on the a priori definition of coma as RASS -3 or deeper; these 8 would not have met criteria for analyses if a cutoff of RASS -4 had been used. Forty-two (61%) of 69 patients had post-coma delirium; only 22 (52%) of these had resolution of delirium (i.e., were 48 hours free of delirium and coma) during the study monitoring period.
Fig. 1. Study flowchart.
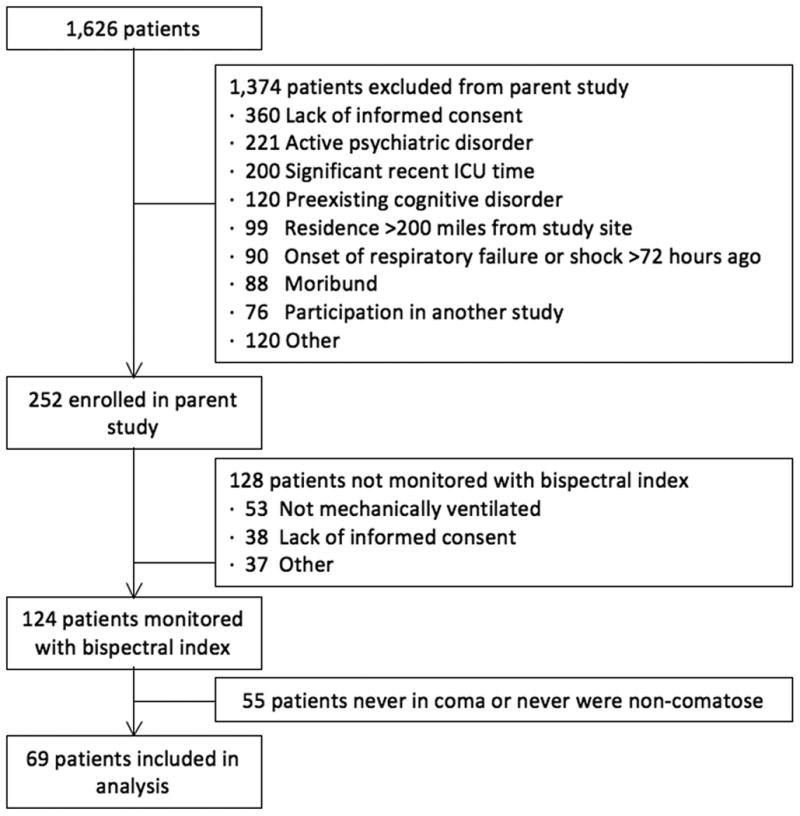
Table 1. Demographics and clinical characteristics *.
| Variable | Cohort, n=69 |
|---|---|
| Age | 57 (46-68) a |
| Female (%, n/total) | 48% (33/69) |
| Race (%, n/total) | |
| Black/African American | 14% (10/69) |
| White | 86% (59/69) |
| APACHE II | 26.0 (20.2-32.0) a |
| ICU admission diagnosis (%, n/total) | |
| Sepsis/acute respiratory distress syndrome | 35% (24/69) |
| Surgery | 29% (20/69) |
| Airway protection/upper airway obstruction | 13% (9/69) |
| Myocardial infarction/congestive heart failure | 9% (6/69) |
| GI bleed/hemorrhagic shock | 4% (3/69) |
| Other | 10% (7/69) |
| Total Lorazepam (mg) | 7 [0.8-64] |
| Total Fentanyl (mcg) | 5375 [1150-12350] |
| Total Propofol (mg) | 0 [0-3291] |
| Time in burst suppression, minutes | 6.4 (1.0-58.0) a |
| Time under BIS of 40, minutes | 67 (2-827) a |
| Post-coma delirium, (%, n/total) | 61% (42/69) |
| Time to resolution of delirium, hours | 25 (0-71) a,b |
Median (interquartile range)
Results for the 49 patients who had resolution of delirium during the study period (n=22) or were never delirious (n=27).
Abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation severity of illness score; BIS = Bispectral Index fronto-temporal EEG monitor, GI = gastrointestinal; ICU = Intensive Care Unit
After adjusting for covariates (time spent under a BIS level of 40 - as a measure widely considered “deep sedation” - and a principal component score consisting of APACHE II at ICU admission, and cumulative doses of benzodiazepines, opiates and propofol received while comatose), greater time in burst suppression during coma was a significant independent predictor of post-coma/post-deep sedation delirium [Odds ratio (95% confidence interval) 4.16 (1.27-13.62), p=0.02] and delayed resolution of delirium [Hazard Ratio (95% confidence interval) 0.78 (0.53-0.98), p=0.04], as shown in Table 2 and Figures 2 and 3. That is, multivariable analysis revealed that patients with approximately 1 hour of burst suppression (the 75th percentile for time in burst suppression in our population) had a four times higher odds of post-coma delirium than those with only 1 minute of burst suppression (the 25th percentile for time in burst suppression in our population). Similarly, patients with approximately 1 hour of burst suppression had 22% lower likelihood of resolution of delirium during the study period than those with 1 minute in burst suppression. Thus, patients were more likely to stay delirious longer if they had spent more time burst suppressed.
Table 2. Burst suppression time and its relationship to brain dysfunction.
| N | 25th percentile | 75th percentile | Ratio (CI) | p value | |
|---|---|---|---|---|---|
| Odds of Emergence into delirium* | |||||
| - Time in burst suppression during coma, minutes | 69 | 1.2 | 58.2 | OR 4.16 (1.27-13.62) | 0.02 |
| Time to resolution of delirium** | |||||
| - Time in burst suppression during coma, minutes | 69 | 1.2 | 58.2 | HR 0.78 (0.53-0.98) | 0.04 |
Logistic regression tested the relationship between time in burst suppression during coma and the odds of emergence from coma into delirium. To illustrate this relationship in a meaningful manner, we have used the 25th and 75th IQRs noted above: patients with approximately 1 hour of burst suppression during coma (75th percentile) had odds of emerging into delirium that were four times as high as those with only 1 minute of burst suppression during coma (25th percentile).
Cox proportional hazard model tested the relationship between time in burst suppression during coma and length of time until resolution of brain dysfunction, defined as 48 hours free of delirium and coma and expressed as a Hazard Ratio. Patients with approximately 1 hour time in burst suppression during coma (75th percentile) had 20% lower likelihood of resolution of delirium compared to those with 1 minute in burst suppression during coma (25th percentile). Said another way, they were more likely to stay delirious longer if they had spent more time burst suppressed.
Fig. 2. Odds of Post-Coma Delirium by Time Burst Suppressed.
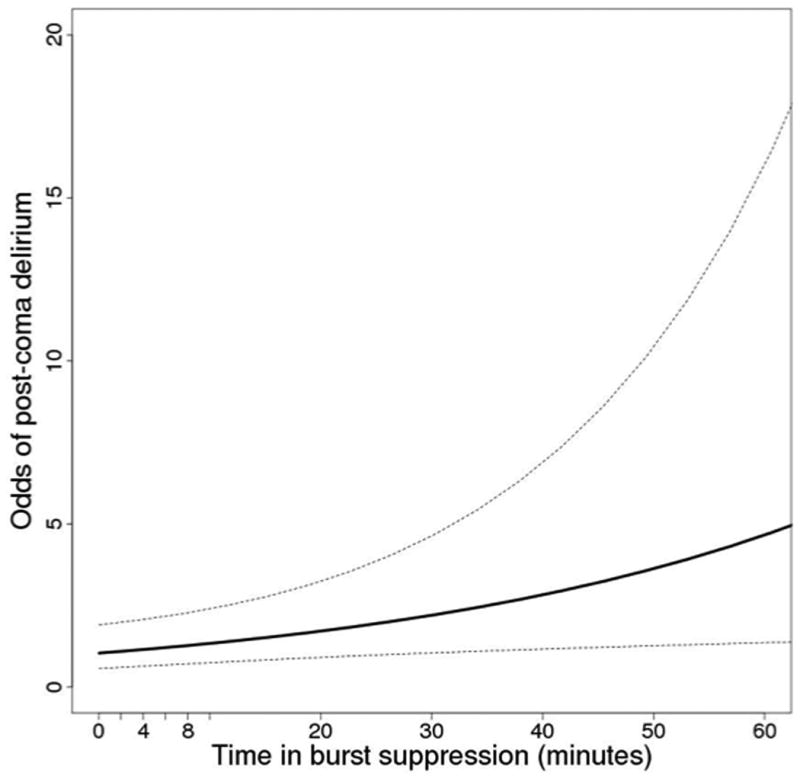
Association between time in burst suppression and the probability of post-coma delirium after adjustment for time at deep (BIS < 40) sedation, severity of illness, and total exposure to benzodiazepines, propofol, and narcotics. A patient with 60 minutes of burst suppression will have 4 times greater odds of emerging into delirium than a patient with 1 minute of burst suppression. *Odds ratios are based on results from a logistic regression adjusting for a fixed time at BIS less than 40 and the principal component comprised of the covariates listed above as described in methods.
Fig. 3. Likelihood (“hazard”) of resolution of delirium by Time Burst Suppressed.
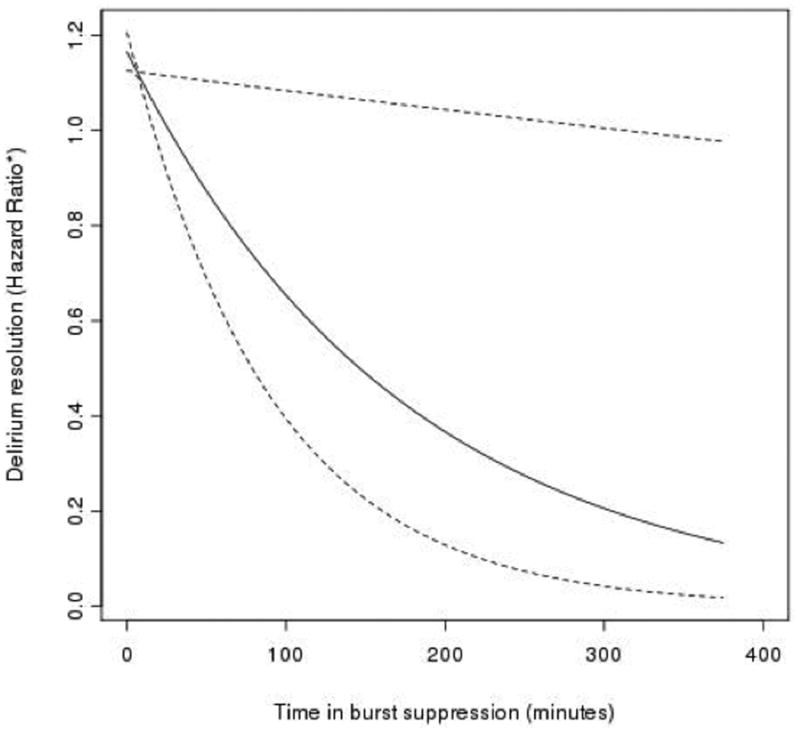
Association between time in burst suppression and the likelihood (“hazard”) of resolution of delirium after adjustment for time at deep (BIS < 40) sedation, severity of illness, and total exposure to benzodiazepines, propofol, and narcotics. Patients with 100 minutes of burst suppression had a 35% lower likelihood of resolution of delirium compared to those with 1 minute of burst suppression. *Hazard ratios are based on results from a survival analysis adjusting for a fixed time at BIS less than forty and the principal component score comprised of the covariates listed above as described in methods.
Discussion
In this observational convenience sample investigation of sedative-exposed comatose ICU patients, we found that time in BIS-detected burst suppression was an independent predictor of the occurrence and duration of delirium. Compared with patients who had little or no burst suppression, those with just one hour of burst suppression during their ICU stay had four-fold increased odds of delirium upon emergence from coma and longer delays in resolution of delirium. These findings suggest that reducing sedation exposure could be an important intervention by which to improve patient outcomes, consistent with previous randomized controlled trials studying spontaneous awakening trials and sedation management without sedation [15, 43, 51].
Additionally, these findings suggest that EEG monitoring could be used in a complementary fashion alongside sedation scales, especially when monitoring those ICU patients for whom a time-limited period of deep sedation is clinically indicated. In these circumstances, avoidance of burst suppression via use of pEEG monitoring might reduce the incidence and duration of post-coma/post deep sedation delirium, a possibility that could be examined in the context of a randomized clinical trial or used on a case-by-case basis (perhaps not as routine but as felt clinically indicated). Non-comatose ICU patients can be directly assessed for delirium using validated assessment tools, such as the CAM-ICU [49, 50] or the Intensive Care Delirium Screening Checklist (ICDSC) [52], and it remains unclear whether pEEG should be used in addition to these delirium assessment tools. Clinical delirium assessment tools cannot be used during coma, however, so pEEG may be especially useful during this period of ICU patient management. Even when burst suppression—which is most likely due to sedation in this population but may also occur due to illness—cannot be avoided, its detection via pEEG could provide important prognostic information and allow patients at high risk for delirium to be identified early during their critical illness.
To our knowledge, our investigation is the first to measure burst suppression during coma in the ICU with the explicit goal of examining burst suppression as a predictor of post-coma/post deep sedation delirium, and the findings may speak to the still poorly understood neuropathophysiology of brain dysfunction. This study builds on prior research which painted an initial picture of the relationship between sedation and delirium. Plaschke and colleagues found that ICU patients with lower BIS values (i.e., deeper levels of sedation) were more likely to be delirious at the time of BIS assessment [53], but they did not examine whether BIS monitoring during coma could predict delirium in the future. Additionally, an earlier study found no association between BIS values and delirium in ICU patients when the two were observed simultaneously, but it did not examine the relationship between burst suppression and the coma-to-delirium transition [31]. Sieber and coworkers did consider subsequent occurrence of delirium in light of preceding delirium and found that patients kept at higher BIS levels during surgery were 50% less likely to develop delirium postoperatively [9]. Chan et al. found that BIS guided operative anesthesia (BIS value 40-60) resulted in less sedatives administered and less post operative delirium [54]. Our results are qualitatively in-line with these findings and show that even relatively short periods of burst suppression during critical illness are associated with subsequent delirium.
Several recent clinical trials reported that protocols prioritizing light sedation significantly improved multiple outcomes [43, 51, 55]. Whereas these trials clearly show that sedation should be minimized in the ICU, they also reveal that an important minority of mechanically ventilated ICU patients (e.g., 18% in the trial by Strom et al.) will require some period of continuous sedation. For these patients, burst suppression might be avoided through the combined use of a clinical sedation scales, pEEG, and protocols such as the ABCDE bundle. The duration of deep sedation, as determined by the sedation scale, should be limited as much as the clinical circumstances allow, and pEEG detection of burst suppression might provide additional information to guide titration of sedatives and avoid over-use of the medications when clinically-induced coma is required for life-support. In our study, delirium was predicted by time in burst suppression even after controlling for duration of deep sedation and cumulative doses of sedatives. Some ICU patients are likely to be more prone than others—despite receiving the same doses of sedatives for the same duration—to develop burst suppression in response to sedatives in combination with their critical illness, and such patients are at higher risk for delirium. Both delirium (especially when persistent for >2 hours after stopping sedatives and analgesics) and burst suppression during critical illness have been shown to predict mortality [1-3, 13, 36], so identification of susceptible patients using pEEG might lead to targeted risk factor modification and therapeutic intervention.
Strengths of this study include continuous BIS monitoring over multiple days early in the period of critical illness requiring mechanical ventilation, which yielded a large amount of EEG data from a heterogeneous population of ICU patients, and prospective assessments of delirium using a validated tool shown in numerous studies to be sensitive and specific when administered by trained research staff, such as those who conducted this investigation [49, 56, 57].
The study also had several limitations. First, the relatively small sample size prevented us from analyzing more complex relationships (e.g., a potential causal pathway involving individual sedatives, burst suppression, and delirium) and did not allow us to adjust for certain covariate for the same reasons (e.g., hypoxia, hypotension, sleep, admitting diagnosis). Second, it was not logistically possible to begin pEEG monitoring as soon as each patient became comatose, so burst suppression may have occurred in some patients prior to onset of BIS monitoring. Such undetected burst suppression would likely bias our results towards the null hypothesis, such that the true association between burst suppression and post-coma delirium may be stronger than that reported. Third, due to limited resources we restricted the duration of pEEG monitoring to 7 days at most, which led to truncated data for some patients and may have biased results to underestimate the association between burst suppression and time to resolution of delirium. Additionally, our use of pEEG to monitor sedation levels is clearly a research tool and not part of routine clinical management. Such pEEG monitoring was not recommended for use in comatose or noncomatose patients by recently published practice guidelines [16]. However, these guidelines did recommend objective measures of brain function (e.g., BIS or sedline) in patients receiving neuromuscular blockade. A number of factors are known to affect the reliability of the numerical BIS scale in critically ill patients [58, 59]. Muscle activity and resulting electromyography (EMG) signals, for example, may cause pEEG artifact that can lead to spuriously high BIS values [60, 61]. Conversely, renal and hepatic encephalopathies can be associated with a slowing of pEEG frequency (though not to the point of burst suppression), which can lower the BIS values even in absence of sedation [62-64]. These factors are less likely to affect the measurement of burst suppression, our primary predictor of interest. Lastly, our observational study design did not allow for exploration of the mechanisms underlying the observed association between burst suppression and post-coma delirium.
Future interventional studies comparing different approaches to sedation may be able to determine whether sedatives vs. patient-related factors — such as increased sensitivity to sedatives, low cardiac output, hypotension, hypothermia, metabolic abnormalities, or an interaction with severe sepsis — are the primary driver of burst suppression preceding delirium in the ICU. Because this was a preliminary investigation, limited in size and scope, no firm conclusions can be drawn until larger studies are performed to confirm these findings.
Conclusion
Though many mechanically ventilated ICU patients can and should be managed with light or no sedation whenever possible in accordance with the new SCCM guidelines [16], some individuals must be sedated for a limited time to a depth at which clinical sedation scales lose their utility and the ICU team is “blind” to aspects of the patient's neurological status. In this exploratory study, we found that comatose ICU patients who spent more time in burst suppression, as detected by pEEG, were most likely to have post-coma delirium and had longer delays in resolution of delirium. Avoidance of burst suppression may be an important safety goal for ICU teams managing patients undergoing iatrogenic, sedative-induced coma in working towards decreasing delirium and its associated poor outcomes. Additional research is needed to confirm these findings and to determine whether clinical use of pEEG can improve the outcomes of ICU patients.
Acknowledgments
Funding/Support: Dr. Watson received support from the National Institutes of Health (MO1 RR-00095) and the Vanderbilt CTSA grant UL1 RR024975-01 from NCRR/NIH, Dr. Girard is supported by the National Institutes of Health (NIH) (AG034257) and the Veterans Affairs (VA) Tennessee Valley Geriatric Research, Education, and Clinical Center, Dr. Pandharipande is supported by the VA Clinical Science Research and Development Service (VA Career Development Award), and Dr. Ely is supported by the NIH (AG027472 and AG035117) and the VA Tennessee Valley GRECC. Additional funding for this research project was provided by Covidien, which provided the BIS™ monitors used for this investigation. These sponsors had no role in study design; data collection, analysis, and interpretation; or publication of results.
Disclosure Statement: Dr. Watson has received an unrestricted research grant for an investigator initiated study from Aspect Medical Systems, Inc. Drs. Watson, Girard, Pandharipande, and Ely have received honoraria from Hospira Inc. Dr. Pandharipande has received honoraria from Orion Corporation. Drs. Pandharipande and Ely have received honoraria from Hospira Inc. Dr. Ely has also received honoraria from Orion and Abbott. All other authors have no disclosures.
Copyright form disclosures: Dr. Watson consulted for the First International Meeting of Critical Care, Bogota, Columbia (speaker's fee); received support for travel (speaker at the Critical Care Congress); and received support for article research from NIH. Her institution received grant support. Dr. Girard lectured for Hospira, Inc. and received support for article research from NIH. His institution received grant support from NIH (AG034257). Dr. Pandharipande lectured for Hospira Inc and Orion Pharma. His institution received grant support from NIH and Hospira Inc.
Footnotes
The remaining authors disclosed that they do not have any potential conflicts of interest.
References
- 1.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 2.Pisani MA, Kong SY, Kasl SV, et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shehabi Y, Riker RR, Bokesch PM, et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38:2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 4.Thomason JW, Shintani A, Peterson JF, et al. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9:R375–R381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 7.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Sieber FE, Zakriya KJ, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33:66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 11.Skrobik Y, Leger C, Cossette M, et al. Factors Predisposing to Coma and Delirium: Fentanyl and Midazolam Exposure; CYP3A5, ABCB1, and ABCG2 Genetic Polymorphisms; and Inflammatory Factors*. Crit Care Med. 2013;41:999–1008. doi: 10.1097/CCM.0b013e318275d014. [DOI] [PubMed] [Google Scholar]
- 12.Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 13.Patel SB, Poston JT, Pohlman A, et al. Rapidly Reversible, Sedation-related Delirium versus Persistent Delirium in the ICU. Am J Respir Crit Care Med. doi: 10.1164/rccm.201310-1815OC. ePub Jan 14 2014. [DOI] [PubMed] [Google Scholar]
- 14.Mehta S, Burry L, Cook D, et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA. 2012;308:1985–1992. doi: 10.1001/jama.2012.13872. [DOI] [PubMed] [Google Scholar]
- 15.Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 16.Barr J, Fraser GL, Puntillo K, et al. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 17.Vasilevskis EE, Ely EW, Speroff T, et al. Reducing Iatrogenic Risks. ICU -Acquired Delirium and Weakness- Crossing the Quality Chasm. Chest. 2010;138:1224–1233. doi: 10.1378/chest.10-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasilevskis EE, Pandharipande PP, Girard TD, Ely EW. A screening, prevention, and restoration model for saving the injured brain in intensive care unit survivors. Crit Care Med. 2010;38:S683–S691. doi: 10.1097/CCM.0b013e3181f245d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balas MC, Vasilevskis EE, Burke WJ, et al. Critical Care Nurses' Role in Implementing the “ABCDE Bundle” Into Practice. Crit Care Nurse. 2012;32:35–47. doi: 10.4037/ccn2012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and Safety of the Awakening and Breathing Coordination, Delirium Monitoring/Management, and Early Exercise/Mobility Bundle. Crit Care Med. 2014 doi: 10.1097/CCM.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kollef MH, Levy NT, Ahrens TS, et al. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 22.Weinert CR, Calvin AD. Epidemiology of sedation and sedation adequacy for mechanically ventilated patients in a medical and surgical intensive care unit. Crit Care Med. 2007;35:393–401. doi: 10.1097/01.CCM.0000254339.18639.1D. [DOI] [PubMed] [Google Scholar]
- 23.Patel SB, Kress JP. Sedation and analgesia in the mechanically ventilated patient. Am J Respir Crit Care Med. 2012;185:486–497. doi: 10.1164/rccm.201102-0273CI. [DOI] [PubMed] [Google Scholar]
- 24.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 25.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 26.Riker RR, Fraser GL, Simmons LE, Wilkins ML. Validating the sedation-agitation scale with the bispectral index and visual analog scale in adult ICU patients after cardiac surgery. Intensive Care Med. 2001;27:853–858. doi: 10.1007/s001340100912. [DOI] [PubMed] [Google Scholar]
- 27.Friedman D, Claassen J, Hirsch LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg. 2009;109:506–523. doi: 10.1213/ane.0b013e3181a9d8b5. [DOI] [PubMed] [Google Scholar]
- 28.Rathakrishnan R, Gotman J, Dubeau F, Angle M. Using continuous electroencephalography in the management of delayed cerebral ischemia following subarachnoid hemorrhage. Neurocrit Care. 2011;14:152–161. doi: 10.1007/s12028-010-9495-2. [DOI] [PubMed] [Google Scholar]
- 29.Newey CR, Sarwal A, Hantus S. Continuous electroencephalography (cEEG) changes precede clinical changes in a case of progressive cerebral edema. Neurocrit Care. 2011 doi: 10.1007/s12028-011-9650-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Oddo M, Carrera E, Claassen J, et al. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37:2051–2056. doi: 10.1097/CCM.0b013e3181a00604. [DOI] [PubMed] [Google Scholar]
- 31.Ely EW, Truman B, Manzi DJ, et al. Consciousness monitoring in ventilated patients: bispectral EEG monitors arousal not delirium. Intensive Care Med. 2004;30:1537–1543. doi: 10.1007/s00134-004-2298-1. [DOI] [PubMed] [Google Scholar]
- 32.Ogilvie MP, Pereira BM, Ryan ML, et al. Bispectral index to monitor propofol sedation in trauma patients. J Trauma. 2011;71:1415–1421. doi: 10.1097/TA.0b013e3182178b8b. [DOI] [PubMed] [Google Scholar]
- 33.Hwang S, Lee SG, Park JI, et al. Continuous peritransplant assessment of consciousness using bispectral index monitoring for patients with fulminant hepatic failure undergoing urgent liver transplantation. Clin Transplant. 2010;24:91–97. doi: 10.1111/j.1399-0012.2009.01148.x. [DOI] [PubMed] [Google Scholar]
- 34.Riker RR, Fraser GL. Altering intensive care sedation paradigms to improve patient outcomes. Anesthesiol Clin. 2011;29:663–674. doi: 10.1016/j.anclin.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Cottenceau V, Petit L, Masson F, et al. The use of bispectral index to monitor barbiturate coma in severely brain-injured patients with refractory intracranial hypertension. Anesth Analg. 2008;107:1676–1682. doi: 10.1213/ane.0b013e318184e9ab. [DOI] [PubMed] [Google Scholar]
- 36.Watson PL, Shintani AK, Tyson R, et al. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med. 2008;36:3171–3177. doi: 10.1097/CCM.0b013e318186b9ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tobias JD. Bispectral index monitoring documents burst suppression during pentobarbital coma. J Intensive Care Med. 2008;23:258–262. doi: 10.1177/0885066608318459. [DOI] [PubMed] [Google Scholar]
- 38.Engel GL, Romano J. Delirium: reversibility of the electroencephalogram with experimental procedures. Arch Neurol Psychiatry. 1944;51:378–392. [Google Scholar]
- 39.Engel GL, Romano J. Delirium, a syndrome of cerebral insufficiency. J Chronic Dis. 1959;9:260–277. doi: 10.1016/0021-9681(59)90165-1. [DOI] [PubMed] [Google Scholar]
- 40.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 41.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 44.AspectMedicalSystems. A-2000 XP Platform Bispectral Index (BIS) Monitoring System Service Information Manual. 2009 [Google Scholar]
- 45.Liu N, Chazot T, Genty A, et al. Titration of propofol for anesthetic induction and maintenance guided by the bispectral index: closed-loop versus manual control: a prospective, randomized, multicenter study. Anesthesiology. 2006;104:686–695. doi: 10.1097/00000542-200604000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 47.Ruokonen E, Parviainen I, Jakob SM, et al. Dexmedetomidine versus propofol/midazolam for long-term sedation during mechanical ventilation. Intensive Care Med. 2009;35:282–290. doi: 10.1007/s00134-008-1296-0. [DOI] [PubMed] [Google Scholar]
- 48.Haenggi M, Blum S, Brechbuehl R, et al. Effect of sedation level on the prevalence of delirium when assessed with CAM-ICU and ICDSC. Intensive Care Med. 2013 doi: 10.1007/s00134-013-3034-5. [DOI] [PubMed] [Google Scholar]
- 49.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 51.Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375:475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 52.Bergeron N, Dubois MJ, Dumont M, et al. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 53.Plaschke K, Fichtenkamm P, Schramm C, et al. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med. 2010;36:2081–2089. doi: 10.1007/s00134-010-2004-4. [DOI] [PubMed] [Google Scholar]
- 54.Chan MT, Cheng BC, Lee TM, et al. BIS-guided Anesthesia Decreases Postoperative Delirium and Cognitive Decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 55.Treggiari MM, Romand JA, Yanez ND, et al. Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med. 2009;37:2527–2534. doi: 10.1097/CCM.0b013e3181a5689f. [DOI] [PubMed] [Google Scholar]
- 56.Mitasova A, Kostalova M, Bednarik J, et al. Poststroke delirium incidence and outcomes: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2012;40:484–490. doi: 10.1097/CCM.0b013e318232da12. [DOI] [PubMed] [Google Scholar]
- 57.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32:2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 58.Riker RR, Fraser GL, Simmons LE, Wilkins ML. Validating the Sedation-Agitation Scale with the Bispectral Index and Visual Analog Scale in adult ICU patients after cardiac surgery. Intensive Care Med. 2001;27:853–858. doi: 10.1007/s001340100912. [DOI] [PubMed] [Google Scholar]
- 59.Tonner PH, Paris A, Scholz J. Monitoring consciousness in intensive care medicine. Best Pract Res Clin Anaesthesiol. 2006;20:191–200. doi: 10.1016/j.bpa.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Vivien B, Di Maria S, Ouattara A, et al. Overestimation of Bispectral Index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology. 2003;99:9–17. doi: 10.1097/00000542-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Nasraway SA, Wu EC, Kelleher RM, et al. How reliable is the Bispectral Index in critically ill patients? A prospective, comparative, single-blinded observer study. Crit Care Med. 2002;30:1483–1487. doi: 10.1097/00003246-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 62.Young GB, Bolton CF, Archibald YM, et al. The electroencephalogram in sepsis-associated encephalopathy. J Clin Neurophysiol. 1992;9:145–152. doi: 10.1097/00004691-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 63.Kaplan PW. The EEG in metabolic encephalopathy and coma. J Clin Neurophysiol. 2004;21:307–318. [PubMed] [Google Scholar]
- 64.Brenner RP. The interpretation of the EEG in stupor and coma. Neurologist. 2005;11:271–284. doi: 10.1097/01.nrl.0000178756.44055.f6. [DOI] [PubMed] [Google Scholar]


