Abstract
Background
About one-third of patients with IBS-diarrhea (IBS-D) have evidence of increased bile acid (BA) synthesis or excretion.
Aims
To assess effects of the BA sequestrant, colesevelam, on fecal excretion of BAs, hepatic BA synthesis and diarrhea in IBS-D; to appraise whether individual or random stool samples accurately reflect 48-hour total fecal BA excretion and proportions of the main BAs excreted; to study the fecal fat excretion in response to colesevelam.
Methods
Study design: A single-center, unblinded, single-dose trial of effects of colesevelam, 1875mg [3 tablets (625mg tablets)] orally, twice daily, for 10 days on total 48-hour fecal BA excretion and fasting serum C4 (surrogate of hepatic BA synthesis). Stool diaries documented bowel functions for 8 days prior and 8 days during colesevelam treatment. Stool 48-hour samples and fasting serum were collected for fecal fat, fecal BA and serum C4.
Results
Colesevelam was associated with significantly increased fecal total BA excretion and deoxycholic acid excretion, increased serum C4, and more solid stool consistency. There was a significant inverse correlation between number of bowel movements per week and the total BA sequestered into stool during the last 48 hours of treatment. Random stool samples did not accurately reflect 48-hour total or individual fecal BA excretion. Sequestration of BAs by colesevelam did not increase fecal fat.
Conclusions
Colesevelam increases delivery of BAs to stool while improving stool consistency, and increases hepatic BA synthesis, avoiding steatorrhea in patients with IBS-D. Overall effects are consistent with luminal BA sequestration by colesevelam.
Keywords: sequestrant, samples, deoxycholic acid, hepatic synthesis
BACKGROUND
The pathophysiology of irritable bowel syndrome (IBS) includes abnormal motility and sensation, and mucosal defense, manifested as altered mucosal structure and function or low grade inflammation.
Bile acids (BAs) are synthesized and secreted from the liver, modulate lipid digestion, are reabsorbed mainly in the terminal ileum and, through the enterohepatic circulation, return to the liver. The secretion and circulation of BAs are mainly stimulated by food intake which releases cholecystokinin from the upper small intestine, inducing gallbladder contraction. Several prior studies in our laboratory have led us to appreciate the role of BAs in diarrhea-predominant IBS (IBS-D). In a study of 119 patients with IBS,1 32% had abnormal colonic transit measured by scintigraphy at 24 or 48 hours, with accelerated transit in 48% of IBS-D patients; the causes of abnormal transit are unclear.
A meta-analysis showed BA malabsorption (BAM) in up to 50% of patients with chronic functional diarrhea or IBS-D.2 About one-third of patients with IBS have alterations in fecal BA excretion and hepatic BA synthesis rates compared to healthy controls.3,4 The effects of BAs on colonic secretion and motility are multifactorial and include stimulation of the G-protein coupled receptor called TGR5 or GPBAR15 which mediates colonic secretory and motor effects of BAs.6 The diagnosis of BAM in the United States is based predominantly on a therapeutic trial7 of BA sequestrants such as colestipol or cholestyramine. Recent open-label therapeutic trials have documented the efficacy of cholestyramine in 13 patients with idiopathic BA diarrhea (BAD)8 and adequate relief of IBS symptoms, with escalating or flexible dosing of colestipol.9 In the cholestyramine trial,8 there was no significant effect on colonic transit, though there were significant prolongations of small bowel transit time and segmental transverse colon transit time.
The mechanism of action of these BA sequestrants to ameliorate bowel function is not clearly demonstrated in most studies. In addition, the BA sequestrants may have other effects (e.g. binding of other substances, bacterial toxins) and, therefore, the assumption that BAs are the cause of diarrhea may be incorrect. Finally, this approach is compromised by poor tolerance of the traditional resin sequestrants like cholestyramine and by inadequate medication compliance.10,11 The novel BA sequestrant, colesevelam, is administered as a tablet and has had higher patient compliance. In a prior study, we showed that colesevelam slowed colonic transit in patients with unselected IBS-D.12
Our hypothesis in this study was that therapy with the BA sequestrant, colesevelam, sequesters luminal BA, increasing fecal BA excretion and reducing diarrhea in patients with IBS-D who have documented increased fecal BA excretion. The study aims were: to assess the effects of colesevelam on fecal excretion of BAs and on bowel movements IBS-D patients; to study ability of the HPLC assay for fecal BAs to demonstrate responsiveness after treatment with colesevelam; to appraise whether individual or random stool samples accurately reflect 48-hour total fecal BA excretion and proportions of the main BAs excreted; and to study the effect of colesevelam on fecal fat excretion.
METHODS
Study Design
We conducted a single-center, unblinded, single-dose, controlled, 10-day trial of the effects of colesevelam, 1875mg (3 tablets, 625mg each), taken orally, twice daily, in 12 patients with IBS-D and prior evidence of increased BA synthesis or fecal excretion (Figure 1). Stool 48-hour collections (for BAs) and fasting serum samples (for serum C4) were collected during baseline before treatment, and then during days 9–10 of the ten days of colesevelam dosing for fecal BAs, and on day 11 (the morning following the last dose) for fasting serum C4. The study was approved by Mayo Clinic Institutional Review Board, and the study was registered with ClinicalTrials.gov. #NCT02111603.
Figure 1.
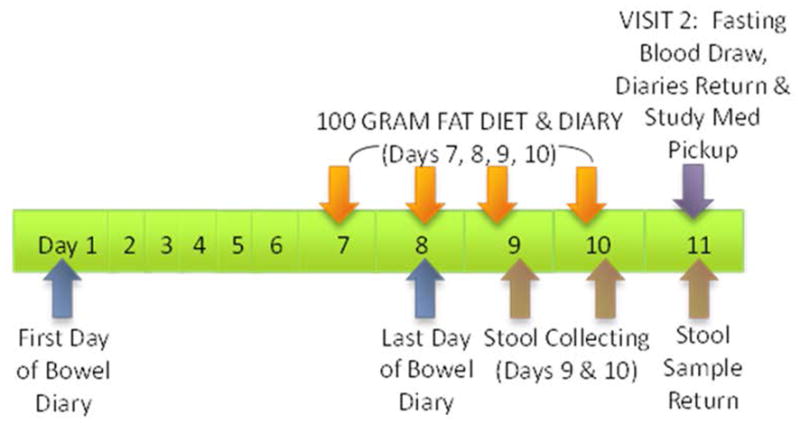
Experimental design of the two phases of the study which were of equal duration: during baseline, no medication was taken; in the treatment phase, patients received colesevelam, 1.875g b.i.d. for 10 days.
Participants
From a database of ~950 patients with functional gastrointestinal diseases who reside within ~150 miles of Mayo Clinic, Rochester, MN, we identified potential participants with prior measurements of BA kinetics and invited those with either increased 48-hour total fecal BAs or increased serum C4 to participate. Two of the patients had prior cholecystectomy and none were on drugs affecting lipid metabolism.
All participants completed a validated bowel disease questionnaire (BDQ, corresponding to Rome criteria)13 and the Hospital Anxiety and Depression Scale (HAD)14 to ensure they had symptoms consistent with IBS-D based on Rome III criteria, and that they had no significant uncontrolled affective disorder based on HAD score. Participants were females or males, 18–65 years of age. Females of childbearing potential had a negative pregnancy test before initiation of medication.
Within the first week prior to the baseline period or during the subsequent 2 weeks during the study period, the following drugs were prohibited: agents that alter gastrointestinal transit [including opioids, narcotics, anticholinergics, tricyclic antidepressants, serotonin and norepinephrine reuptake inhibitors (SNRI) antidepressants], analgesic drugs [including opiates, non-steroidal anti-inflammatory drugs (NSAIDs), COX-2 inhibitors], and medications that could interfere with the interpretation of the study. Birth control pills, estrogen replacement therapy, and thyroxine replacement were permitted. Female subjects who were pregnant or breast-feeding, patients with prior abdominal surgery (except appendectomy), and patients with known chronic liver disease or history of elevated AST/ALT 2.0X upper limit of normal were excluded.
Bowel Function Questionnaires
During the study, participants completed a daily diary to record their bowel functions for each bowel movement including consistency (Bristol Stool Form Scale:15 1-hard lumps, 2-lumpy sausage, 3-cracked sausage, 4-smooth sausage, 5-soft lumps, 6-mushy and 7-watery), ease of passage (1-manual disimpaction, 2-enema needed, 3-straining needed, 4-normal, 5-urgent without pain, 6-urgent with pain, 7-incontinent), and sense of completeness of evacuation (yes=1/no=0). These diaries have been validated for responsiveness.16,17
Colesevelam
Colesevelam was purchased in 625mg tablet form (Welchol®, Daiichi Sankyo, Inc., Parsippany, NJ) through Mayo Clinic Research Pharmacy. Participants took 1875mg (3 tablets, 625mg each) of the medication orally, twice daily, with lunch and supper for 10 days. Because colesevelam can interfere with absorption of other medications, subjects taking hormonal contraceptives for birth control were instructed to use a double barrier method (such as a condom or diaphragm) with a spermicide.
Biological Samples
Blood samples were collected in the morning after overnight fasting before starting the colesevelam and on the morning after the 10-day dosing with colesevelam. The samples were sent to the Mayo Clinic Immunochemical Core Laboratory (ICL) for storage in a −70°C freezer, until batch analysis for serum C4 values in the ICL.
Stools samples were collected before starting the colesevelam and during days 9–10 of dosing with colesevelam. Samples were collected on both occasions during the last 2 days while patients received a 100-gram fat diet each day for 4 days. The samples were sent to a central laboratory to be frozen immediately for subsequent testing for fecal BAs. Participants received standardized instructions for the 100-gram fat diet (Mayo Clinic brochure #MC1450) and for the stool specimen collection (Mayo Clinic brochure #MC1335-15) used in clinical practice at Mayo Clinic. Fecal collections over a 48-hour period may be considered cumbersome, both for patients and laboratory staff. However, this is presently the only available diagnostic test for BA diarrhea in the United States.7 Moreover, in this research study with dedicated staff and highly cooperative patients, each stool passed was collected and stored separately in order to measure BA content.
Laboratory Measurements
Serum 7α-hydroxy-4-cholesten-3-one (C4) is a measurement of hepatic cholesterol synthesis and is closely related to the fecal loss of BAs. Serum C4 is a validated method for detecting BAM. In head-to-head comparisons with the 75SeHCAT retention test, increased serum C4 had sensitivity of 90% and specificity of 79% in diagnosing BAM,18 where shorter retention half-time of 75SeHCAT is associated with increased level of C4; and it had 98% negative predictive value and 74% positive predictive value for diagnosis of BAM.19
Based on the method published from our laboratory, adapted from Galman et al.,20 we used HPLC/tandem mass spectrometry to measure serum C4 to evaluate BA synthesis rate.21
Fecal bile acid excretion
Using HPLC/tandem mass spectrometry, we have adapted a method used with serum samples22 to measure fecal total and individual BAs2 in a 48-hour collection of stools collected while ingesting a100-gram fat per day diet. Bile acids were measured in each individual stool sample and, finally, all samples were mixed to obtain the total fecal BA excretion over 48 hours. The stool samples were prepared for assay by HPLC/MS by methanol extraction. Approximately 100mg of each stool homogenate was incubated with 2mL of methanol for 30 minutes to extract the BAs.
Daily fecal fat excretion, while ingesting the 100-gram fat diet daily, was measured by nuclear magnetic resonance spectrometry in the Mayo Clinic Department of Laboratory Medicine and Pathology.
In vitro studies
In order to assess the ability of the methanol extraction to separate the bile acids from the sequestrant, colesevelam, we mixed bile acids with colesevelam and performed several extraction experiments. Ten micromoles of each bile acid (CA, CDCA, DCA, LCA, UDCA) were spiked into eight 2-mL aliquots of either water or methanol. Fifty mg of colesevelam was also added to four of the 8 aliquots. Samples were then run on the LC-MS/MS method for detection of free bile acids. The mean concentrations for the four replicates with and without colesevelam were compared for both solvents across all analytes.
Statistical Analysis
The primary analysis compared on-treatment total fecal BA excretion with baseline total fecal BA excretion using the signed rank test. The analyses of secondary endpoints were based on the paired t-test of signed rank test as warranted. The concordance between overall mean stool characteristics and randomly selected individual stool samples was based on Lin’s concordance correlation coefficient23 separately for pre- and post-colesevelam periods.
Endpoints for analysis
The primary endpoint for analysis was change in total 48-hour fecal BAs from baseline in response to treatment with colesevelam. Since the fecal BA measurement included an extraction step that freed the BA into the stool water, the effect of colesevelam was measured as an increase in fecal BA excretion.
The secondary endpoints addressed other effects of colesevelam in patients with IBS-D and BAM; these endpoints were:
Change in fecal bile acid excretion per gram of stool
Change in fasting serum C4
Change in fecal fat excretion
Change in stool consistency on colesevelam compared to baseline
Change in stool frequency on colesevelam compared to baseline
Relative composition of the main individual BAs (CA, CDCA, DCA, LCA and UDCA) in 48-hour stool collection on colesevelam compared to baseline
Comparison of composition of the main individual BAs in individual samples of feces and total fecal 48-hour collection
Sample size assessment
In a prior study of 54 IBS-D patients,24 we identified an IBS-D subgroup (n=23) with high fecal BA excretion (above the 90th percentile in health of 2337μmoles/48h). The average fecal BA excretion in all IBS-D patients was 2495±382 (SEM) μmoles/48h; in patients with IBS-D and evidence of BAM, the mean total fecal BA excretion was 4905μmoles/48h. The estimated standard deviation (SD) in this group was 3706μmoles/48h, and it was the basis for estimating the effect size that could be detected with a study of 12 patients, assuming we could recruit about 50% of the patients known to have IBS-D with BAM in the database.
The effect size is the difference in means (baseline and on colesevelam) of the total fecal BA excretion over 48 hours. Assuming a sample size of 12 subjects, a paired t-test (at two-sided α level of 0.05 and 80% power) would be sufficient to detect a difference in means of 3400μmoles/48h (based on standard deviation of 3706 μmoles/48h). Thus, if colesevelam inactivated or sequestered luminal BA to effectively reduce fecal 48-hour BA to an equivalent of 1500μmoles/48h in the absence of the sequestrants, the drug would have achieved a reduction of luminal BA to bring the latter to be equivalent to the 75th percentile for healthy volunteers. Thus, this effect size would be anticipated to demonstrate a clinically relevant change in total fecal BA and to be associated with improved stool characteristics.
All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
Demographics
The mean age of the 12 participants (11 female, 1 male) was 43.1±3.7 years and mean BMI was 31.1±2.4kg/m2.
In Vitro Studies of Extraction of Colesevelam Bound to Colesevelam
To show that our bile acid extraction and detection method is able to measure all bile acids in the methanol extracted sample, in vitro studies were performed in different matrices in the presence and absence of colesevelam (Table 1). When bile acids are mixed with colesevelam in an aqueous environment, no free bile acids are detectable by mass spectrometry. Thus, colesevelam does bind all bile acids in this environment. In contrast, when colesevelam is mixed with bile acids in methanol, all of the bile acids are free and detected by our assay. Owing to the extreme solubility differences in methanol between colesevelam (not soluble) and bile acids (soluble), bile acids do not bind to colesevelam in methanol. Thus, results of methanol extracted bile acids presented in this study include all bile acids found in stool, whether they were free or colesevelam-bound in vivo.
Table 1.
Bile Acids +/− Colesevelam in Water or Methanol (μM +/− SD)
| LCA | DCA | UDCA | CDCA | CA | ||
|---|---|---|---|---|---|---|
| Water | BA | 9.4 (1.3) | 9.2 (1.5) | 9.5 (1.3) | 9.6 (1.5) | 10.1 (1.6) |
| BA + colesevelam | 0.1 (.2) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | |
| Methanol | BA | 10.0 (1.5) | 9.9 (1.7) | 9.8 (1.2) | 10.1 (1.7) | 9.9 (1.7) |
| BA + colesevelam | 10.0 (2.4) | 10.0 (2.3) | 9.9 (2.3) | 10.2 (2.5) | 9.7 (2.3) | |
Effect of Colesevelam on Total Fecal BA Excretion
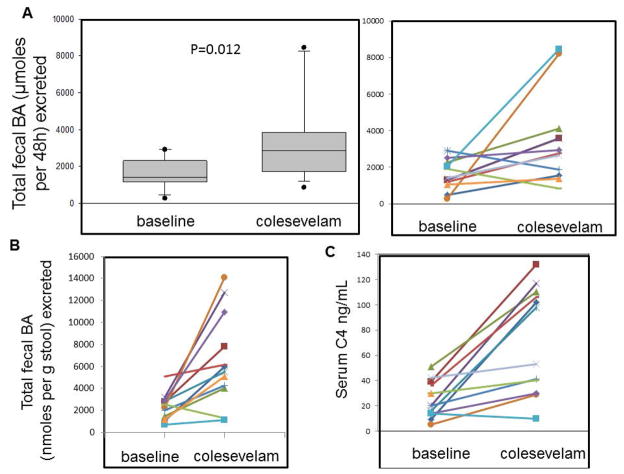
Colesevelam resulted in higher fecal excretion of BAs in stool compared to baseline. The increase in total fecal BAs was observed for the entire group (Figure 2A, left panel) and for 10 of the 12 patients with IBS-D (Figure 2A, right panel). Colesevelam also increased the fecal BA excretion per gram of stool (Figure 2B).
Figure 2.
Comparison of (A) total fecal bile acid excretion over 48 hours during baseline and colesevelam treatment in the 12 patients individually (right panel) and as a group (left panel). Figures 2 (B) and (C) show individual data during baseline and colesevelam treatment for total fecal bile acid excretion per gram of stool, and fasting serum C4 respectively.
Effect of Colesevelam on Fasting Serum C4
Colesevelam significantly increased fasting serum C4 measurement [p<0.01 (signed rank test); Table 2 and individual data shown in Figure 2C].
Effects of Colesevelam on Proportion of Main Primary and Secondary BAs and Fecal Fat
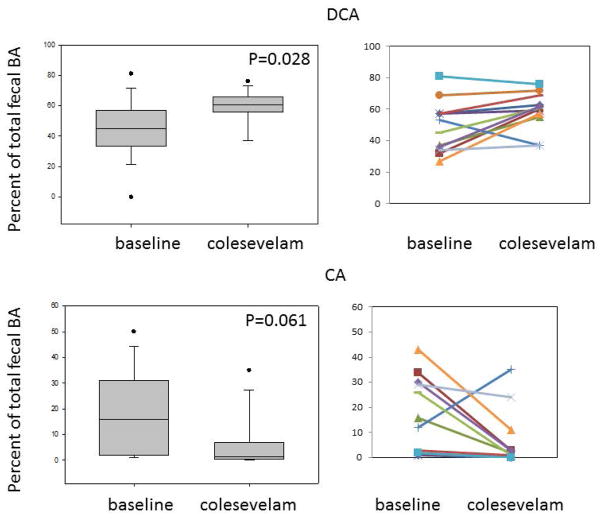
Colesevelam was associated with increased proportion of deoxycholic acid in the stool (Figure 3) and a numerical reduction in the proportion of cholic acid in stool; there were no significant differences in the proportions of CDCA, lithocholic or ursodeoxycholic acids. There was no significant effect of colesevelam on fecal fat excretion (Table 2, Figure 4). There was no major difference in the total fecal BA in the two patients with prior cholecystectomy compared to the other patients. Thus, fecal BA excretions in these two patients were 2916 and 2498 μmoles/48 hours at baseline and 1878 and 2932 μmoles/48 hours on colesevelam treatment.
Figure 3.
Changes in percentage of deoxycholic acid (DCA) and cholic acid (CA) in stool with colesevelam, suggesting that the medication sequesters the secretory, dehydroxylated secondary bile acid, DCA, which is derived from the primary bile acid, CA. There were no significant differences in lithocholic acid or chenodeoxycholic acid in stool.
Table 2.
Effects of colesevelam on bowel function endpoints and fecal fat (mean ± SEM). There were significant differences in stool form, fecal total BA excretion, fecal total BA excretion per gram of stool, and serum C4 (shown in figures).
| Baseline | Colesevelam | |
|---|---|---|
| # stools per week | 17.6 ± 0.4 | 15.1 ± 1.9 |
| Stool form (BSFS) | 4.8 ± 0.3 | 4.4 ± 0.3 |
| Ease of stool passage | 4.7 ± 0.2 | 4.2 ± 0.1 |
| Total fecal BA excretion (μmoles/48h) | 1558 ± 229 | 3496 ± 709 |
| Total fecal BA excretion per gram of stool (nmoles/g stool) | 2332 ± 344 | 6585 ± 1196 |
| Fecal fat (g/day) | 6.4 ± 1.2 | 6.8 ± 0.9 |
| Serum C4 (ng/mL) | 24.7 ± 4.2 | 72.3 ± 12.2 |
BSFS=Bristol Stool Form Scale; BA=bile acid
Figure 4.
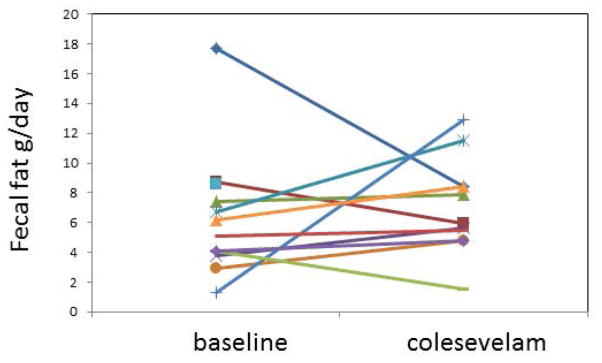
Effect of colesevelam on fecal fat in patients with diarrhea-predominant irritable bowel syndrome and bile acid malabsorption.
Effects of Colesevelam on Stool Form and Bowel Function
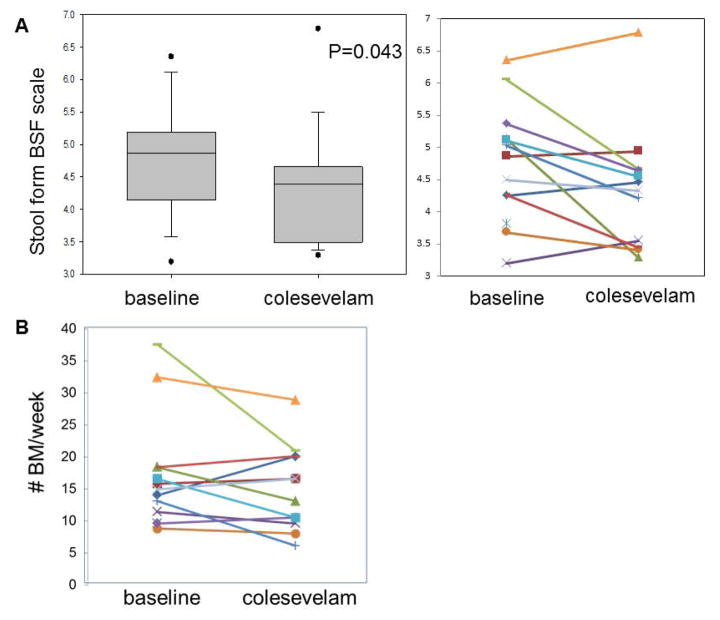
Colesevelam resulted in a significant decrease in the average score of stool consistency on the Bristol Stool Form Scale (Figure 5A). There were numerical reductions in number of bowel movements per week (Figure 5B) and improvements in ease of passage, but these were not statistically significant (Table 1).
Figure 5.
Effects of colesevelam on (A) stool form [Bristol Stool Form Scale (BSF scale)] and (B) stool frequency per week in patients with diarrhea-predominant irritable bowel syndrome and bile acid malabsorption.
Correlation of Fecal BA Excretion and Number of Stools per Week
There was a significant inverse correlation between the fecal excretion of total BAs and number of bowel movements per week, suggesting that sequestration of the BAs by colesevelam reduced the diarrhea (Figure 6).
Figure 6.
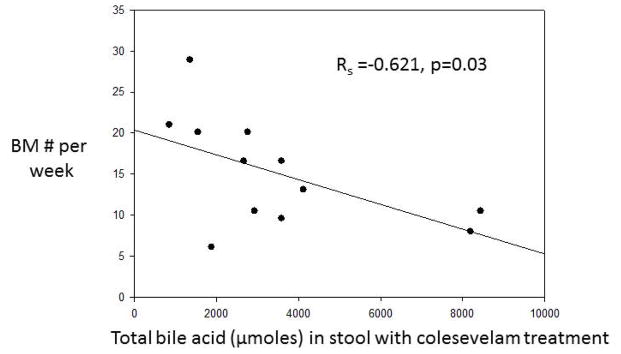
Significant inverse correlation between the fecal excretion of total bile acids and number of bowel movements (BM) per week, suggesting that sequestration of the bile acids by colesevelam reduced the diarrhea.
Comparison of Proportions of Primary and Secondary BAs in Individual and Total Stool Samples in Each Participant
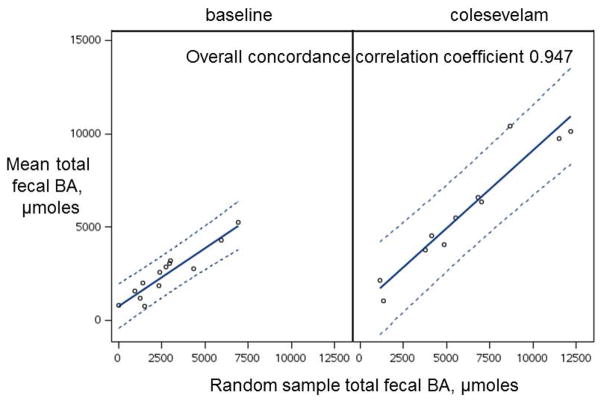
The fecal BA excretion in a random sample of stool was closely correlated (concordance correlation coefficient 0.947) with the mean excretion in all samples from individual patients, both at baseline and after colesevelam treatment (Figure 7).
Figure 7.
Concordance between mean total fecal bile acid (BA) excretion in all samples from an individual patient, and the total fecal BA content in a randomly selected individual sample.
However, individual stool samples could not be used in lieu of the total 48-hour fecal samples to estimate the total or the proportion of the main primary and secondary BAs in stool, as shown by the relatively low concordance correlations for total BAs and moderate coefficients for LCA and DCA, which are the greatest proportions of fecal BAs, and higher coefficients for CDCA, CA and UDCA, which are relatively low proportions of BAs in patients with intact colon (Table 3).
Table 3.
Concordance correlation coefficients (mean ± SEM) of stool total and individual bile acids (BAs) for the 48-hour collection and a randomly selected stool sample.
| Random sample vs total 48h collection | Baseline | Colesevelam |
|---|---|---|
| Total BAs | 0.53 ± 0.13 | 0.54 ± 0.15 |
| cholic acid | 0.84 ± 0.08 | 0.83 ± 0.06 |
| deoxycholic acid | 0.68 ± 0.14 | 0.85 ± 0.07 |
| ursodeoxycholic acid | 0.96 ± 0.20 | 0.54 ± 0.20 |
| chenodeoxycholic acid | 0.88 ± 0.05 | 0.90 ± 0.06 |
| lithocholic acid | 0.44 ± 0.21 | 0.88 ± 0.07 |
DISCUSSION
Our study has shown that, among patients with IBS-D who have prior evidence of BAM, the sequestrant, colesevelam, resulted in increased delivery of BAs to stool. This is accompanied by a significantly more solid stool form, and the stool frequency correlates inversely with the BA sequestered by colesevelam and delivered to the stool. This paradox, that is, the increase in fecal BAs and the more solid stool and reduced number of bowel movements per week, is explained by the binding of the luminal BAs to the sequestrant, colesevelam, and the ability of the preparatory step in the fecal analysis to deliver the BAs from the bound to the free aqueous phase of stool. Specifically, BAs were obtained from stool homogenates using methanol extraction while leaving colesevelam in the insoluble precipitate. Therefore, the extraction contained both free and sequestrant-bound BAs, even though most of the BAs are likely sequestrant-bound in vivo.
Colesevelam also resulted in a significant increase in serum C4, suggesting that the greater loss of BA into stool stimulated hepatic synthesis rate. This increased synthesis of BAs was functionally important in avoiding the development of steatorrhea. However, longer term trials are required to ensure that there are no steatorrhea or malabsorption of fat-soluble vitamins with prolonged treatment.
We had previously observed that colesevelam treatment in unselected patients with IBS-D was associated with slower emptying of the ascending colon (average 4 hours longer) compared with placebo.12 In these unselected patients, we noted that the treatment effect was associated with baseline serum C4 levels (P=0.0025) suggesting that patients with IBS-D and increased BA synthesis were more likely to respond to colesevelam. Overall, colesevelam was associated in the prior study with greater ease of stool passage (P=0.048) and somewhat firmer stool consistency (P=0.12). The current study extends the previous findings and demonstrates that the significantly improved stool consistency is associated with sequestration of the BAs in the colon, preventing their diarrheagenic effects. This is also supported by the inverse correlation between number of stools per week and the total fecal BAs measured in stool after extraction of the BAs from their bound state.
The diagnosis of BAM has been a clinical challenge in the United States since the 75SeHCAT retention test is not approved. A definitive diagnosis is desirable before committing patients to ingesting the relatively unpalatable resins in powder form or the relatively expensive tablet forms such as colesevelam. With the availability of tests such as serum C4, serum fibroblast growth factor-19 (FGF-19)25 and fecal BA excretion at reference laboratories, we anticipate that there will be greater use of these diagnostic tests after their fuller validation.
One of the steps of validation of the diagnostic is demonstration of responsiveness to treatment and concordance with clinical benefit. The current study has shown that the HPLC/MS method used by Mayo Clinic Department of Laboratory Medicine and Pathology to measure total and individual BAs is responsive to treatment with the BA sequestrant, colesevelam, and that this biochemical effect is associated with clinical improvement in the diarrhea. We have set up the method to be run together with a measurement of fecal fats, which is commonly investigated in patients with chronic diarrhea. This combination would also be useful in patients with diarrhea due to ileal resection (type 1 BA diarrhea) and cholecystectomy or pancreatic exocrine insufficiency (type 3 BA diarrhea). Thus, in such patients, knowing both the fecal fat and fecal BA excretion would help clinicians determine the degree of dietary fat restriction in addition to the sequestration of BAs to relieve the diarrhea.
Our study also addressed the clinically relevant question: Does a random measurement of stool BA (total content and proportion of main primary and secondary BAs) provide sufficient information to avoid the need for a 48-hour collection? Unfortunately, we came to the same conclusion as with random fecal fat estimates relative to 48-hour fat excretion that the random sample provides insufficient information on BA excretion. Variation in BA excretion per gram fecal weight in each bowel movement, and in the fecal pellet and supernatant were reported in 3 patients with ileal resection diarrhea and suggested that individual stool samples were not representative of the total fecal BA excretion.26
The main limitations of our current study are the absence of a placebo arm and the small sample size; these limitations reduce our confidence in the clinical benefit of colesevelam in patients with IBS-D and BAM. However, the major objective of our study was to demonstrate responsiveness of fecal BAs with the HPLC/MS assay, and future, appropriately powered studies will be designed to address the clinical efficacy of this therapeutic approach. A recent systematic review also endorsed the potential use of colesevelam in the management of chronic diarrhea due to bile acid malabsorption.27
In conclusion, the current study demonstrates the utility of measurement of fecal BAs and serum C4 to characterize either increased synthesis or excretion of BAs as targets for treatment with a BA sequestrant. We have demonstrated the responsiveness to colesevelam at the biochemical level and in the bowel dysfunction of such patients, particularly stool consistency and number of bowel movements. We have also shown that, as with fecal fat estimation, the 48-hour stool collection is necessary to measure fecal BA excretion and prove BAM in 12 such patients with IBS-D, confirming the experience published over 40 years ago of Mitchell et al. in studies performed on 3 patients with prior ileal resection.26 These studies also add to the plethora of data suggesting that there is an opportunity to diagnose and specifically treat the cause of symptoms in patients who carry a label of IBS-D.
Acknowledgments
Guarantor of the article: Dr. Michael Camilleri is the guarantor of this article; he takes responsibility for the integrity of the work as a whole, from inception to published article.
Specific author contributions:
Michael Camilleri: principal investigator; author of manuscript
Andres Acosta: fellow investigator, screening of patients; co-author
Irene Busciglio: recruitment of participants, conduct of study
Amy Boldingh: recruitment of participants
Roy B. Dyer: measurement of serum C4
Alan R. Zinsmeister: biostatistician
Alan Lueke: measurement of fecal fat and bile acids
Amber Gray: measurement of fecal fat and bile acids
Leslie J. Donato: supervision of measurement of fecal fat and bile acids, co-author
All authors approved the final version of the manuscript.
The authors thank the Mayo Clinic Department of Laboratory Medicine and Pathology for waiving charges for measurements of fecal bile acids and fats, and we thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Abbreviations
- BA
bile acid
- BDQ
bowel disease questionnaire
- C4
7α-hydroxy-4-cholesten-3-one
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- DCA
deoxycholic acid
- FGF-19
Fibroblast growth factor 19
- GPBAR1
G protein-coupled bile acid receptor 1
- HAD
Hospital Anxiety and Depression Scale
- IBS
irritable bowel syndrome
- IBS-D
diarrhea-predominant irritable bowel syndrome
- LCA
lithocholic acid
- NSAIDs
non-steroidal anti-inflammatory drugs
- SNRIs
serotonin and norepinephrine reuptake inhibitors
- UDCA
ursodeoxycholic acid
Footnotes
ClinicalTrials.Gov registration number: NCT02111603
Statement of Interests
Declaration of funding interests: Dr. Camilleri’s research on bile acid diarrhea is supported by NIH R01 DK92179 and by Mayo Foundation (Atherton and Winifred W. Bean Endowed Professorship). The study was conducted with the help of the Nursing Core of Mayo Clinic CCaTS (grant #UL1-TR000135 from National Institutes of Health).
Authors’ declaration of personal interests: Please see attached.
References
- 1.Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychological and autonomic functions in 119 patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wedlake L, A’Hern R, Russell D, et al. Systematic Review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707–717. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 3.Wong BS, Camilleri M, Carlson P, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10:1009–1015. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin A, Camilleri M, Vijayvargiya P, et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:1270–1275. doi: 10.1016/j.cgh.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole DP, Godfrey C, Cattaruzza F, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22:814–825. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alemi F, Poole DP, Chiu J, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijayvargiya P, Camilleri M, Shin A, et al. Methods for diagnosis of bile acid malabsorption in clinical practice. Clin Gastroenterol Hepatol. 2013;11:1232–1239. doi: 10.1016/j.cgh.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stotzer P-O, Abrahamsson H, Bajor A, Sadik R. Effect of cholestyramine on gastrointestinal transit in patients with idiopathic bile acid diarrhea: a prospective, open-label study. Neuroenterology. 2013;2:Article ID 235657, 5. doi: 10.4303/ne/235657. [DOI] [Google Scholar]
- 9.Bajor A, Törnblom H, Rudling M, et al. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut. 2014 Apr 12; doi: 10.1136/gutjnl-2013-305965. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson M, Hådell K, Holme I, et al. Compliance with and efficacy of treatment with pravastatin and cholestyramine: a randomized study on lipid-lowering in primary care. J Intern Med. 1998;243:373–380. doi: 10.1046/j.1365-2796.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- 11.Bays HE, Maki KC, Schmitz K. Colesevelam hydrochloride powder for oral suspension versus cholestyramine powder for oral suspension: comparison of acceptability and tolerability. Endocr Pract. 2011;17:218–225. doi: 10.4158/EP10251.OR. [DOI] [PubMed] [Google Scholar]
- 12.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159–165. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talley NJ, Phillips SF, Wiltgen CM, et al. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–1479. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 14.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 15.Heaton KW, Radvan J, Cripps H, et al. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992;33:818–824. doi: 10.1136/gut.33.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andresen V, Camilleri M, Busciglio I, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761–768. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 17.Manini ML, Camilleri M, Goldberg M, et al. Effects of velusetrag (TD-5108) on gastrointestinal transit and bowel function in health and pharmacokinetics in health and constipation. Neurogastroenterol Motil. 2010;22:42–49. e7–8. doi: 10.1111/j.1365-2982.2009.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sauter GH, Münzing W, von Ritter C, et al. Bile acid malabsorption as a cause of chronic diarrhea: diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci. 1999;44:14–19. doi: 10.1023/a:1026681512303. [DOI] [PubMed] [Google Scholar]
- 19.Brydon WG, Nyhlin H, Eastwood MA, et al. Serum 7 alpha-hydroxy-4-cholesten-3-one and seleno-homocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea. Eur J Gastroenterol Hepatol. 1996;8:117–123. doi: 10.1097/00042737-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Gälman C, Arvidsson I, Angelin B, et al. Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res. 2003;44:859–866. doi: 10.1194/jlr.D200043-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri M, Nadeau A, Tremaine WJ, et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alpha C4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009;21:734–e43. doi: 10.1111/j.1365-2982.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tagliacozzi D, Mozzi AF, Casetta B, et al. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chem Lab Med. 2003;41:1633–1641. doi: 10.1515/CCLM.2003.247. [DOI] [PubMed] [Google Scholar]
- 23.Lin LI-K. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 24.Camilleri M, Busciglio I, Acosta A, et al. Effect of increased bile acid synthesis or fecal excretion in irritable bowel syndrome-diarrhea. Am J Gastroenterol. 2014 Jul 29; doi: 10.1038/ajg.2014.215. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pattni SS, Brydon WG, Dew T, et al. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther. 2013;38:967–976. doi: 10.1111/apt.12466. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell WD, Findlay JM, Prescott RJ, et al. Bile acids in the diarrhoea of ileal resection. Gut. 1973;14:348–353. doi: 10.1136/gut.14.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilcox C, Turner J, Green J. Systematic review: the management of chronic diarrhoea due to bile acid malabsorption. Aliment Pharmacol Ther. 2014;39:923–939. doi: 10.1111/apt.12684. [DOI] [PubMed] [Google Scholar]