Abstract
1. Frequency and duration of summer droughts are predicted to increase in the near future in many parts of the world, with considerable anticipated effects on riparian plant community composition and species richness. Riparian plant communities along lowland streams are characterised by high species richness due to their system-specific environmental gradients. As these streams and their hydrological gradients are mainly rain-fed, they are sensitive to precipitation changes.
2. We conducted a literature survey and meta-analysis to examine the effects of an increase in summer drought on: (i) riparian plant biomass; (ii) riparian seedling survival and (iii) riparian plant species composition and richness. We also aimed to determine whether hydrological thresholds related to drought tolerance can be distinguished for riparian plant species.
3. ISI Web of Knowledge was searched for relevant peer-reviewed studies, and 23 papers were found that met our criteria and contained quantitative study results. To detect overall responses of biomass and seedling survival, a random-effects model was applied using Comprehensive Meta-analysis™ software. Regression curves were then fitted to response ratio data relating the effects on drought-impacted groups to those on control groups.
4. Our results showed that a drought duration of approximately >30 days strongly reduces riparian plant biomass and that a duration of approximately >30–35 days and high drought intensities (starting from 3 to 4 cm water table decline per day) can be detrimental for riparian seedling survival. Especially Populus and Salix seedlings showed a reduced survival in response to drought, in contrast to Tamarix seedlings, which have the ability to rapidly and expansively elongate their roots. The data also revealed that an increase in drought conditions rapidly leads to a decline of riparian species richness and an increased presence of species adjusted to drier conditions.
5. Riparian groundwater level, surface water permanence and certain plant traits, especially plasticity in rooting depth, were mentioned most frequently as factors determining species responses. Very few studies mentioned hydrological thresholds, such as critical values for ground- and/or surface water levels, and so far these results have proved difficult to generalise.
6. Our meta-analysis has shown that the projected increase in the duration and intensity of drought periods, especially intense droughts lasting more than 30 days, can be expected to narrow the riparian wetland zone with typical hydric species and accelerate riparian wetland species losses in the near future. This may require extra efforts in terms of management and restoration of species-rich riparian areas.
Keywords: climate change, hydrological change, literature survey, riparian plant communities, vegetation
Introduction
Climate change is a considerable potential threat to biodiversity in most biomes, especially in vulnerable habitats (MEA, 2005). A warmer and at the same time more variable climate is expected to enhance the probability of extreme events such as droughts and floods (Wetherald & Manabe, 2002). Summer droughts are likely to become more intense in many parts of the world, due to a decrease in precipitation combined with an increase in evaporation in spring and summer (Wetherald & Manabe, 1999; Douville et al., 2002; Wang, 2005). Since the 1970s global aridity has increased substantially in most of Africa, southern Europe, East and South Asia and eastern Australia (Dai, 2011). A likely increase in persistent droughts is projected in the 21st century for most of Africa, southern Europe and the Middle East, most of the Americas, Australia and Southeast Asia (Dai, 2011). Climate change is expected to have a significant impact on lowland streams and their adjacent riparian zones, through local and regional changes in temperature and rainfall, which result in modified river and stream flows and wetland water regimes (Arnell & Reynard, 1996; IPCC, 2007; Dankers & Feyen, 2009).
Lowland streams are usually rain-fed systems and their riparian zones are often regarded as vulnerable due to their sensitivity to changes in precipitation and temperature (Décamps, 1993) and their overall impacted and degraded status around the world (Tockner & Stanford, 2002). However, Catford et al. (2013) suggested that riparian systems are resilient to climate change since they have evolved under conditions of hydrological extremes. Riparian wetlands, the temporarily flooded areas along rivers and streams, are of great ecological importance because they harbour a large number of distinctive plant and animal species (Naiman, Décamps & Pollock, 1993; Naiman & Décamps, 1997; Jansson et al., 2005; Sabo et al., 2005). Their high diversity is caused by multiple environmental gradients, resulting in mosaics of different habitats (Naiman et al., 1993). Moreover, riparian wetlands provide several important ecosystem services by storing and purifying water, preventing erosion, providing spawning habitat and nurseries for fish species and serve as corridors for plant dispersal (Naiman et al., 1993; Goodson et al., 2004; Sabo et al., 2005; Soons, 2006; Verhoeven et al., 2006, 2008; Richardson et al., 2007; Capon et al., 2013).
The projected decrease in summer precipitation and increase in evaporation can quickly lead to a reduction in soil moisture in summer (Manabe & Wetherald, 1987; Gregory, Mitchell & Brady, 1997; Wetherald & Manabe, 1999). Lower soil moisture can have adverse effects on plant life and may also decrease the supply of ground water by restricting capillary processes (Gregory et al.,1997). Several abiotic factors control soil moisture content, which is a very important habitat prerequisite for plants. Sediment type and particle size are important factors for the water-holding capacity of soils and determine the thickness of the capillary fringe, which can compensate for a deeper water table (Gonzalez, Comin & Muller, 2010). The lower the water-holding capacity of soils, the greater the sensitivity of plants to drought.
Documented responses of riparian plant species to drought conditions vary. These responses are influenced by both the duration and intensity of the drought period, as well as by specific plant species traits. Individuals may use plastic response mechanisms to cope with drought conditions, while species may undergo a range shift or adapt to the drought conditions in the long term. Individual plants can use several response mechanisms to cope with drought conditions. Most individuals will minimise the risk of desiccation by maintaining a favourable internal water content (Kozlowski & Pallardy, 2002) and use specific mechanisms to either increase water uptake or decrease water loss (Pallardy, 2008). Internal mechanisms, such as osmoregulation, can keep relative water content of the plant high to use water efficiently (Kozlowski & Pallardy, 2002). At times of water stress, plants can decrease their above-ground surface area and eventually biomass, thereby reducing water loss, or increase their rooting depth, thereby increasing water uptake. The seedling stage is considered vital in the rejuvenation and/or colonisation phase of a plant species, but is especially sensitive as seedlings are known to respond rapidly to changing abiotic conditions (Rood et al., 2008; Stella & Battles, 2010). Life-history strategies of plants (annual versus perennial life cycle for example) and mechanisms such as vegetative quiescence and seed dormancy also play a role in the resilience with respect to drought. In cases of severe drought when plant mechanisms are not sufficiently effective to tolerate drought, species are expected to be replaced by other, more drought-tolerant species, but the rate at which this occurs will be limited also by species' dispersal capacities (Brederveld et al., 2011).
So far, few studies have considered quantitative effects of summer drought on riparian vegetation. However, such information is needed to forecast changes in species composition and diversity of these potentially highly vulnerable ecosystems in the future. To quantify how riparian vegetation responds to summer drought, we addressed the following questions:
What is the relationship between duration of drought and riparian plant biomass? At what duration do negative effects on biomass start to occur?
What are the effects of duration and intensity of drought on riparian seedling survival? Can differences between riparian tree seedling species in drought tolerance be related to relevant plant traits?
What are the effects of an increase in duration and intensity of summer drought on riparian plant species composition and richness?
Can specific hydrological thresholds related to drought be distinguished for riparian plants?
In this study, we used a meta-analysis to assess the quantitative effects of drought on biomass, seedling survival and plant traits related to drought resilience. A literature survey was carried out to evaluate the effects of summer drought on species composition and diversity in riparian habitats along streams, to determine which species are sensitive and whether there are thresholds to be distinguished for the species. We focused on riparian zones along lowland streams outside of the tropics and subtropics.
Methods
We searched ISI Web of Knowledge for scientific peer-reviewed studies on effects of (increased) drought on riparian wetland plant species. To efficiently extract relevant articles, we selected specific keyword strings for our search (see Table S1). Titles and abstracts were all checked for relevance using the following strict study eligibility criteria. We only selected data from field studies carried out in riparian wetlands along streams or rivers, or relevant mesocosm/greenhouse experiments, carried out with riparian wetland plants. Studies from tidal systems, estuaries or lakes were excluded. All selected studies had a before–after (BA), control–impact (CI) or a before–after–control–impact (BACI) design, to be able to quantify the effects of drought. We did not use results from studies on sites with a history of strong disturbance, such as the application of local fertilisation, ditch cleaning, or recent restoration. Studies conducted in the temperate Atlantic, Continental, Boreal and (Semi)-arid biogeographic regions (worldwide) were included. In practice, most of the studies included are of Northern Hemisphere systems, and we acknowledge that our analysis may be less directly applicable to Southern Hemisphere riparian zones with different riparian species, climate, soils, etc. A literature survey was conducted of papers reporting on the response of riparian plant species composition and richness to an increase in summer drought. Since too few quantitative results were provided in the papers regarding the effects of summer drought on species richness, we have undertaken a more classic review of the literature instead of conducting a meta-analysis for this topic. We extracted relevant details on the main trends and observations, responsible mechanisms, biogeographical region, research set-up and thresholds or indicator species. We summarised these details in a descriptive table (see Table S2). In the text below, we focus on the methods used in the meta-analysis that we used for responses of riparian plant biomass and seedling survival.
Plot Digitizer 2.6.1 software (Free Software Foundation, Inc., Boston, MA, U.S.A.) was used to extract data from graphs, in case data were not presented in tables. All available quantitative data were summarised in coding sheets for the species and community responses, as well as for the response variables plant total biomass and seedling survival. Extra information was included concerning the study system, plant communities, relevant plant traits and thresholds (e.g. biogeographical region, vegetation type and groundwater level). With a quantitative research synthesis, we analysed data from all selected study cases. The responses of plant total biomass and seedling survival to drought were calculated as the ratio of the treatment (or after situation; impact) and the control group (or before situation), since this ratio provides a relative quantification of the effect size, which is suitable for comparisons (Borenstein et al., 2005). A response ratio equal to 1 means no change, while a response ratio >1 indicates a positive change (increased biomass or survival), and a value <1 equals a negative change (decreased biomass or survival). To test whether there were any overall, significant effects of drought on biomass or seedling survival, we first used the software program Comprehensive Meta-analysis (CMA version 2.0, Biostat, Inc., Englewood, NJ, U.S.A.; Borenstein et al., 2005), which enabled us to assign weights required for random-effects analysis. For each response variable, the program calculated a two-group comparison for each study case by calculating the effect size. A random-effects model was applied since the true effect size varied from study to study. Study results were calculated in Effect Size metrics with 95% confidence intervals. Standardised mean differences were used, since all effect sizes needed to be transformed into a common metric to calculate an overall effect. A two-tailed Z-test was conducted to examine the null hypothesis (effect size equals zero).
To analyse more specifically the relation between duration and intensity of drought and the response ratio of biomass and seedling survival, we used the statistical package SPSS (IBM SPSS Statistics version 20, IBM, Amsterdam, the Netherlands) to fit linear or logistic weighted regression curves to the response data. We also looked at the relation between duration of drought and response ratio of seedling survival of three plant genera characteristic for riparian zones in the Northern Hemisphere: Salix, Populus and Tamarix. We calculated the R-squared and P-values of all weighted regression curves, to test whether relationships were significant.
Results
Our search query in Web of Science yielded 683 articles, of which only 23 met our thorough eligibility criteria and contained quantitative study results: 12 studies reporting on 32 cases regarding biomass, five studies reporting on 261 cases concerning seedling survival and four studies reporting on species richness.
Plant total biomass
The studies used for our analysis on plant total biomass ranged in drought conditions from very mild drought stress (plants received 400 mL water per day compared with a control of 800 mL water per day, e.g.) to severe drought stress (the plants were not watered at all and the wilting point was reached). The duration of the drought periods varied from 14 to 180 days. Despite this wide range of treatments, our meta-analysis confirms that there is a highly significant overall effect of drought on the amount of total biomass (dry weight) of riparian wetland plants (random-effects model, P < 0.001; Table 1), which becomes critical when droughts last longer than approximately 30 days (Fig.1). Since different species display a different tolerance to drought, a species list is included in Table 1. For shorter periods of drought, some response ratios had values >1, which indicates that there was an initial positive effect of drought on the performance (total biomass) of these species. These cases had a relatively wet control situation (water level 5 cm above substratum), so these particular species showed a more optimal response to dryer conditions. Furthermore, Fig.1 shows that under more severe drought conditions, there is a relatively fast decrease in biomass, while there is a more gradual decrease in biomass when drought conditions are milder.
Table 1.
Outcome of random-effects model meta-analysis on effects of drought on biomass (dry weight) of riparian plants. Standard differences (SD) in means with a negative value (‘Favours A’) indicate a negative effect of drought on biomass in the respective study, while positive values (‘Favours B’) indicate a positive effect. Results of 32 cases are shown from the following sources (studies identified by letters in parentheses). Type of study: greenhouse experiments, except Hudon (2004) who performed a field experiment. Asamoah & Bork, 2010 (e); Hudon, 2004 (l); Hussner, Meyer & Busch, 2008 (g); Kleczewski, Herms & Bonello, 2012 (b); Li, Pezeshki & Goodwin, 2004 (i); Nakai, Yurugi & Kisanuki, 2009 (f); Pezeshki, Anderson & Shields, 1998 (j); Romanello et al., 2008 (h); Sletvold & Ågren, 2012 (a); Smith, Wu & Green, 1993 (k); Touchette et al., 2010 (d); Walls, 2010 (c). See reference section for complete references
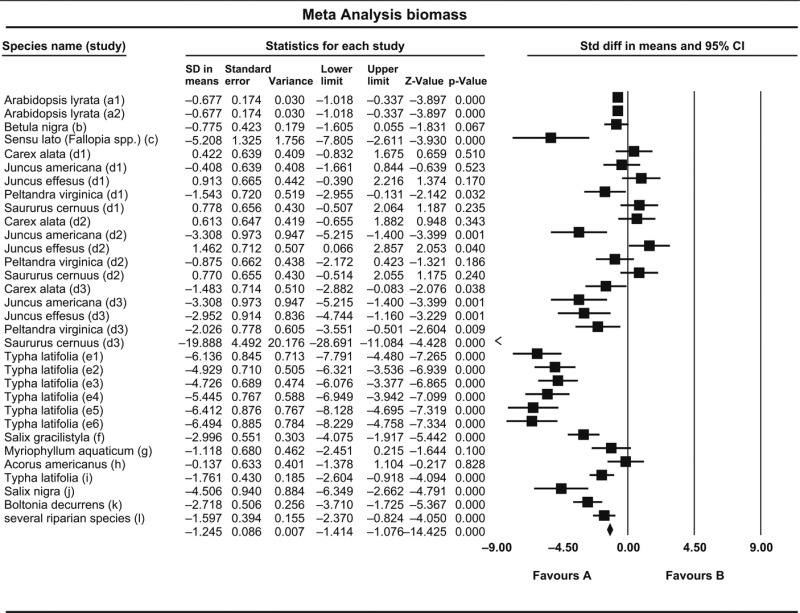 |
Fig. 1.

Effects of duration of drought on riparian plant biomass ratio (mean biomass in drought treatment/mean biomass in control). Studies with intense drought conditions (no water added, or the plants were not watered until the wilting point was reached) and mild drought conditions (water periodically withheld) are indicated by different symbols. A weighted regression analysis is shown. n = 31 cases, from 12 studies.
Seedling survival
The studies concerning seedling survival differed in their degree of drought intensity from very mild drought stress (1 cm water table decline per day) to more severe drought stress (8 cm water table decline per day). The duration of the drought periods ranged from 3 to 90 days. Our meta-analysis confirms the general picture that drought overall has a strong negative effect on seedling survival (Table 2; P < 0.001). Regression analyses on all available cases show a pronounced negative linear response of seedling survival to an increase in the duration of drought (Fig.2a). The negative effect becomes really strong in the case of droughts lasting longer than approximately 30–35 days. When seedling survival is plotted against the duration of drought multiplied by drought intensity (water decline ranging from 1 to 8 cm per day), an even clearer linear effect of drought on seedling survival becomes visible (Fig.2b). Both linear relationships are strong, but show a wide variation among the data points, with no clear cut-off point indicating where response ratios drop below 1.
Table 2.
Outcome of random-effects model meta-analysis on effects of drought on seedling survival. Standard differences (SD) in means with a negative value (‘Favours A’) indicate a negative effect of drought on survival in the respective study, while positive values (‘Favours B’) indicate a positive effect. For two of five studies, including 26 cases, the P-values could be calculated. The three other studies did not show standard deviations or statistical output; hence, meta-analysis results could not be calculated for these studies. Source studies are as follows, identified by letters in parentheses. Type of study: greenhouse experiments, except Stella et al. (2010) who performed a mesocosm experiment. Amlin & Rood, 2002 (a); Horton & Clark, 2000 (b); Mahoney & Rood, 1991; Stella et al., 2010; Van Splunder et al.,1996. See reference section for complete references
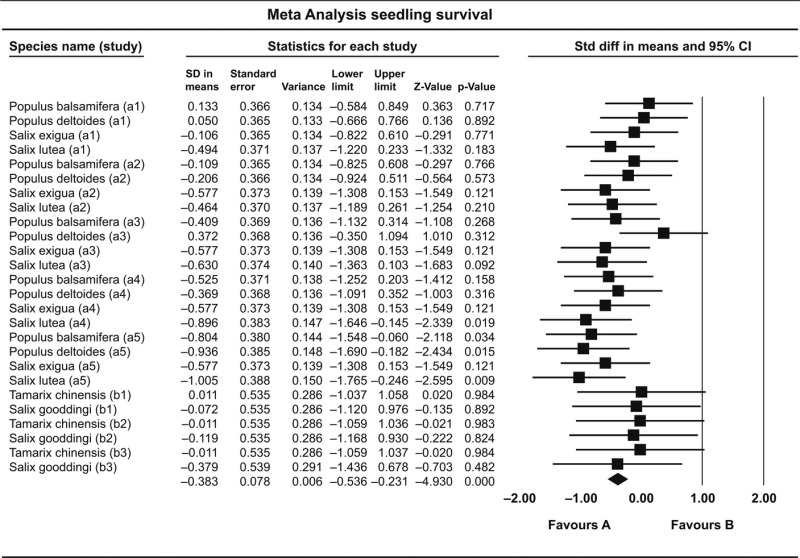 |
Fig. 2.
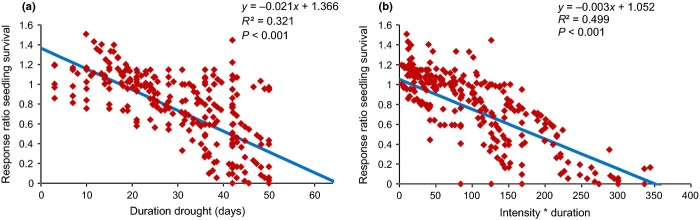
Riparian seedling survival (mean number of seedlings treatment/seedlings control) in relation to (a) the duration of drought and (b) the intensity of drought (cm water decline per day) * duration (days of drought). Weighted regression analyses are shown. (a) n = 261, from five studies. (b) n = 257, from five studies.
The response ratio of seedling survival in relation to the water table decline per day gives a clear picture of the effect of drought intensity (Fig.3). The cases with a 1–2 cm water table decline per day reveal a relatively mild negative relationship, which is almost significant. A decline of 3–4 cm per day shows a significant negative relationship with a steeper slope and a decline of 6–8 cm per day results in an even stronger negative relationship with the response ratio of seedling survival. All selected studies on seedling survival reported data on tree seedlings of the genera Populus, Salix and Tamarix. If we look at these genera specifically, response ratios of the survival of seedlings of both Populus and Salix show a negative relationship with drought duration, while seedling survival of Tamarix does not show a significant trend and seems hardly affected by drought. The general response of Salix seedling survival is smaller than for Populus. Hence, differences in seedling drought tolerance between these three common riparian tree genera are large (Fig.4).
Fig. 3.

Effects of duration of drought on the response ratio of riparian tree seedling survival (mean number of seedlings treatment/seedlings control), with different declines of water table per day (1–2 cm, 3–4 cm and 6–8 water). Weighted regression analyses are shown. n = 257 from five studies. All data points are related to the riparian tree genera Populus, Salix and Tamarix (comparable data points are shown in Fig.4).
Fig. 4.
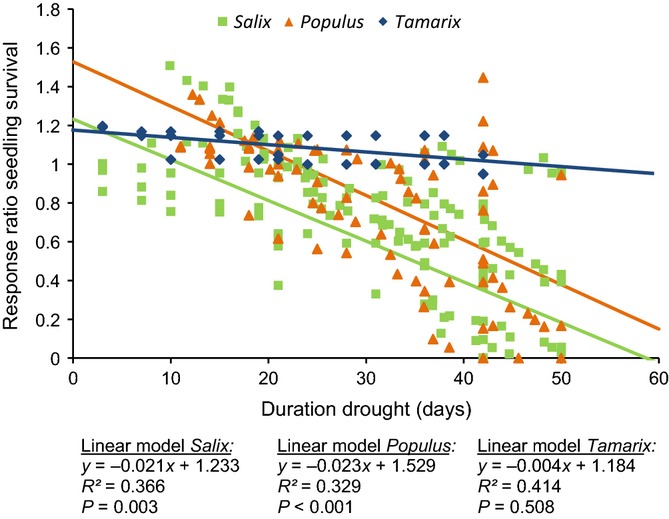
Effects of duration of drought on seedling survival (mean number of seedlings treatment/seedlings control) of the genera Populus, Salix and Tamarix. All data points related to each genus are indicated. Weighted regression analyses are shown. n = 261 from five studies.
Three studies (Mahoney & Rood, 1991; Van Splunder et al., 1996; Horton & Clark, 2000) totalling 14 cases contained quantitative data on the response of root length of seedlings to drought conditions. In these studies, the authors emphasised that species' ability to increase root length is important for tolerating drought conditions. The genera Salix and Populus had on average a lower response ratio of the root length as a reaction to drought (response ratio of 1.27 and 1.53, respectively), compared with the genus Tamarix (response ratio of 2.02). This difference between species of the three common riparian tree genera in root elongating ability most likely explains their capacity to survive increased periods of drought as seedlings. Furthermore, we explored the effects of drought duration on plant height, but found no clear trend.
Species richness
The main findings of 11 selected studies considering drought effects on riparian plant species diversity are described individually (see Table S2). Shifts in species composition and a decrease in riparian species richness were found in almost all studies as a result of periods of more intense, or prolonged summer drought. A decline in riparian plant species richness was found in nine of 11 studies, as a result of a prolonged drought period or increased drought intensity. The studies conducted in desert systems provided quantitative data; water tables ranged from about 4 to 20 m below the ground surface, and stream flow permanence ranged from 71 to <25% in these drought-affected sites. Several studies showed a decline of herbaceous or woody species only (two and three studies, respectively), whereas other studies found declines in both plant categories (four studies). Seven of 11 studies found a shift in species composition from hydric to mesic or xeric species going from the channel upwards, with a relatively high abundance of mesic and xeric species in drought-affected riparian zones. Groundwater table and surface flow permanence are regarded as the main abiotic factors underlying the degree of change in species richness. Plant traits, especially rooting depth and water use efficiency, are mentioned to be important for species to survive dry spells. A strong water table decline, a decrease or absence of surface water flow, in combination with the presence of species with a lack of adaptation to drought, are mentioned to negatively affect species richness. In the articles focussing on dry land or semi-arid systems (six out of 11 articles), surface flow permanence was mentioned to be one of the most important regulating factors for maintaining species diversity (Lite & Stromberg, 2005; Stromberg et al., 2005, 2007; Salinas & Casas, 2007; Stromberg, Hazelton & White, 2009; Stromberg, Lite & Dixon, 2010). In Arizona's Sonoran Desert region for example, streams with flow permanence of <25% have significantly lower riparian species diversity than streams with higher flow permanence (Stromberg et al., 2007). Riparian plant species richness was mentioned to be dependent on groundwater depth in eight of 11 studies.
While the above-mentioned studies all dealt with an increase in drought and a reduction in groundwater tables and soil moisture at the drier end of the riparian zone, one study in the Boreal region (Ström et al., 2011), focussed on the impact of a reduction in flooding at the wetter end of the riparian zone. Their study demonstrated that a reduction in summer flooding duration positively affects species richness. This increase in species richness was mainly related to a decrease in biomass of two dominant species: Carex canescens and Carex acuta. This particular study demonstrates that in Boreal continental-climate regions, reduction in summer flooding may increase local species richness at the wetter (lower) end of the riparian zone, while summer drought reduces the width of the riparian zone and its species richness in general.
Hydrological thresholds
Only a few studies that evaluated species responses to drought identified hydrological thresholds for changes in species composition. Lite & Stromberg (2005) clearly indicate that the riparian species Populus fremontii and Salix gooddingii were dominant over Tamarix ramosissima at sites where surface flow was present more than 76% of the time, inter-annual groundwater fluctuation was <0.5 m, and average maximum depth to groundwater was <2.6 m. In this system along the San Pedro river in Arizona, stream flow permanence ranged from 29 to 100%, and maximum depth to groundwater fluctuated from 5.3 to 1.3 m. For each system, there are individual values for ranges in water table fluctuations, which determine the vegetation composition and species responses (see Table S2, final column). While we summarised all available information on (potential) hydrological thresholds, our review analysis clearly shows that the presented values are study specific thresholds, which are hard to generalise, since they are system-specific. This demonstrates that, while badly needed to help estimate future effects, there is currently no general framework for hydrological thresholds, to indicate when changes in riparian species composition occur. However, the results derived from our meta-analysis, indicate that a drought period lasting longer than approximately 30–35 days, combined with a strong drought intensity (a 3–4 cm water table decline per day or more) can have a detrimental effect on both biomass and seedling survival.
Discussion
Our meta-analysis confirms that a longer duration and greater intensity of drought negatively affect both riparian plant total biomass (dry weight) and seedling survival, starting from a drought duration of approximately 30–35 days, although an exact critical threshold is hard to determine. A detailed analysis of the intensity effect shows that seedling mortality clearly increases with a more rapid desiccation of the habitat (starting from a decline in water table of 3–4 cm per day). The differences found between seedlings of three common riparian tree genera are consistent with their general habitat preferences, with both Populus and Salix showing a negative relationship of survival in response to increasing duration of drought, while Tamarix is hardly affected. Tamarix seedlings can cope relatively well with drought conditions, mainly due to their ability to rapidly and expansively elongate their roots. The declines in biomass and seedling survival due to increased drought duration and intensity are expected to lead to a high species turnover, thereby affecting species composition. Plant communities are expected to change towards more drought-tolerant species. Indeed, our review shows that an increase in the duration and intensity of drought generally results in an eventual decline in riparian plant species richness, due to plant mortality, and a shift in species composition from hydric species to mesic and/or xeric species. According to this shift, the riparian zone with hydric species is reduced or narrowed, and part of it is replaced by a zone with mesic or even xeric terrestrial species. While dispersal limits plant species colonisation, and hence replacement rates in riparian zones (Brederveld et al., 2011), the rapid replacement of riparian wetland species by mesic or xeric terrestrial species is likely facilitated by the latter species having nearby source populations in the uplands adjacent to the riparian zone.
We have to stress here that, in addition to an increase in the duration and intensity of drought, climate change is also expected to lead to prolonged or more intense periods of increased flooding (IPCC, 2007; Bates et al., 2008). Therefore, in future riparian plants may have to show a combination of improved tolerance to drought and flooding, to survive. Also, abiotic factors that affect riparian plant communities, such as changes in nutrient availability and soil type, are important to consider, as well as interaction effects between different drivers of riparian plant dynamics.
Species vary extensively in their internal mechanisms to limit mortality and thereby tolerate drought. Herbaceous species generally are more sensitive to subsurface moisture and less to groundwater table decline compared with tree species (Higler, 1993). For this reason, it can be expected that herbaceous species show more sensitivity to the direct effects of rainfall declines, while tree species are more sensitive to prolonged, more severe stream discharge declines, which also affect groundwater tables. This may lead to a decline in herbaceous species in the short term, and a decrease in tree species in the long run due to an increase in mortality and reduction in seedling recruitment. Groundwater level, surface flow permanence and the presence of plant traits conducive to drought tolerance, most notably plasticity in root elongation, were mentioned as the most important factors determining the changes in species richness and composition. These results were found across (semi-)arid, Atlantic and Mediterranean systems. The time-scale of changes in species richness differs between these systems, with a drought impact on species richness already occurring during dry seasons in (semi-)arid systems (Lite & Stromberg, 2005; Stromberg et al., 2005, 2007, 2009) and a more gradual response in more temperate regions, where changes in species richness may take several to many years. Full adjustments of plant communities to a new hydrological regime can take more than 10 years in Boreal systems (Ström et al., 2011). In the warm Mediterranean area of Western Australia, a progressive change in riparian community composition towards drought-tolerant riparian species was observed under water table drawdown rates of 9 cm per year over a 33-year period (Froend & Sommer, 2010). Climate models predict the most profound increase in drought frequency and duration to take place in areas where droughts already regularly occur; examples are southern Europe and the Middle-East (Dai, 2011). The most abrupt changes in plant communities are to be expected for regions that are influenced most severely by intense drought events. However, sites that were historically less affected by drought might be adapted to a lesser degree to such conditions, so that extreme summer drought events may have more pronounced effects on riparian plant communities there. Our meta-analysis confirms that the longer a drought lasts, the stronger will be its negative effect on riparian plant biomass and seedling survival, mainly triggered by critical levels of soil water potential for the respective species. A relatively long drought period leads to water stress and the closure of the plants' stomata, to save water from evaporation (Amlin & Rood, 2002). Closed stomata will in turn lead to reduced CO2 availability and consequently reduced photosynthetic activity, which will lead to an extra reduction in water use (Elcan & Pezeshki, 2002). The differences among plant species in seedling survival can be explained by several underlying traits. Root elongation of the seedling is an important factor mentioned in four of five studies explaining the decrease in seedling survival due to drought. Reduction in plant height and leaf area is also mentioned as important factors in the majority of studies (three of five studies), reducing the transpiration area of the plant and thereby saving water. In general, Salix was slightly more sensitive to drought than Populus along (semi)-arid or Mediterranean streams (Van Splunder et al., 1996; Amlin & Rood, 2002; Stella et al., 2010). According to Amlin & Rood (2002), these differences in seedling responses might be caused by the smaller seed size and correspondingly reduced respiratory reserves of Salix seedlings, leading to a slower growth of willow seedling roots compared with cottonwood roots. As a consequence, the willow seedlings were not able to maintain contact with the receding water table and capillary fringe, and hence survival decreased. The natural niches are also important to consider here; Salix often grows closer to the stream than Populus, which usually grows at higher locations. Seedlings of the species Tamarix chinensis are hardly susceptible to drought (Horton & Clark, 2000; Lite & Stromberg, 2005; Salinas & Casas, 2007; Stromberg et al., 2007, 2010). Increased drought periods in semi-arid or Mediterranean systems and the invasive character of Tamarix spp. and its specific response mechanisms to cope with drought may lead to a further domination of Tamarix over Salix and Populus spp. and other typical riparian species in the near future.
To help predict effects of future increased summer drought on riparian vegetation, a general framework for hydrological thresholds is needed. These threshold values may consist of critical values for groundwater or surfacewater levels at which riparian species composition may shift. Since riparian wetlands are dynamic and heterogeneous systems, we suggest that for a general framework the riparian zone should be divided into subzones with different flooding frequencies and groundwater levels for which critical threshold values are determined. Increasing summer drought may initially result in species from drier parts of the riparian zone moving ‘downslope’, with the riparian zone itself narrowing at the upper end. Thresholds, especially for the replacement of wetland species by more common terrestrial species, are critical especially for this upper end. Our results emphasise that a classification into different systems should be made to find such thresholds, based on the biogeographic regions, such as Boreal, Atlantic and Mediterranean systems. A further relevant distinction between systems can be made based on their flow permanence; perennial streams (continuous flow), intermittent streams (absence of flow during a few weeks or months) and ephemeral streams (flow only for hours or days following heavy rainfall). The use of plant functional groups, based on the species' flow response guilds (Merritt et al., 2010), is recommended to allow a generalisation for species responses to changes in hydrological factors. Our meta-analysis on biomass and seedling survival studies makes it possible to identify a drought duration threshold: a drought lasting longer than 30–35 days poses a high risk of detrimental effects on both riparian plant biomass as well as riparian tree seedling survival (results related to the genera Salix, Populus and Tamarix). The latter is especially the case for high drought intensities (starting from 3 to 4 cm water table decline per day). These thresholds give important indications to improve water management to reduce the risks for riparian species during periods of drought.
Our meta-analysis included cases with a study design considering a decline in water table, thereby excluding the primary effects of a temperature increase. In most natural cases, summer drought is stimulated by a temperature increase, as well as decreasing precipitation. An increase in temperature without drought effects is expected to stimulate growth, biomass accumulation and reproduction. Increased temperature in combination with reduced precipitation and thereby increased evaporation will lead to reduced soil moisture and declining groundwater tables. This may lead to a very strong decline in biomass and seedling survival, and, in the long-run, decline in species richness. Further research on the interactive effects of climate-driven changes in temperature and hydrology on riparian plant communities would be useful to help estimate effects of both.
Acknowledgments
This work was supported by the European Union 7th Framework Project REFRESH under contract no. 244121.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Key word strings used for the literature search.
Table S2. Main effects of drought on riparian plant species.
References
- Amlin NM, Rood SB. Comparative tolerances of riparian willows and cottonwoods to water-table decline. Wetlands. 2002;22:338–346. [Google Scholar]
- Arnell NW, Reynard NS. The effects of climate change due to global warming on river flows in Great Britain. Journal of Hydrology. 1996;183:397–424. [Google Scholar]
- Asamoah SA, Bork EW. Drought tolerance thresholds in cattail (Typha latifolia): a test using controlled hydrologic treatments. Wetlands. 2010;30:99–110. [Google Scholar]
- Bates BC, Kundzewicz ZW, Wu S, Palutikof JP. Technical Paper of the Intergovernmental Panel on Climate Change. Geneva: IPCC Secretariat; 2008. Climate change and water; p. 210. [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, U.K: John Whiley & Sons Ltd; 2005. [Google Scholar]
- Brederveld RJ, Jaehnig SC, Lorenz AW, Brunzel S, Soons MB. Dispersal as a limiting factor in the colonization of restored mountain streams by plants and macroinvertebrates. Journal of Applied Ecology. 2011;48:1241–1250. [Google Scholar]
- Capon SJ, Chambers LE, Mac Nally R, Naiman RJ, Davies P, Marshall N, et al. Riparian ecosystems in the 21st Century: hotspots for climate change adaptation? Ecosystems. 2013;16:359–381. [Google Scholar]
- Catford JA, Naiman RJ, Chambers LE, Roberts J, Douglas MM, Davies P. Predicting novel riparian ecosystems in a changing climate. Ecosystems. 2013;16:382–400. [Google Scholar]
- Dai A. Drought under global warming: a review. Wiley Interdisciplinary Reviews – Climate Change. 2011;2:45–65. [Google Scholar]
- Dankers R, Feyen L. Flood hazard in Europe in an ensemble of regional climate scenarios. Journal of Geophysical Research. 2009;114:16. [Google Scholar]
- Décamps H. River margins and environmental change. Ecological Applications. 1993;3:441–445. doi: 10.2307/1941913. [DOI] [PubMed] [Google Scholar]
- Douville H, Chauvin F, Planton S, Royer JF, Salas-Mélia D, Tyteca S. Sensitivity of the hydrological cycle to increasing amounts of greenhouse gases and aerosols. Climate Dynamics. 2002;20:45–68. [Google Scholar]
- Elcan JM, Pezeshki SR. Effects of flooding on susceptibility of Taxodium distichum L. seedlings to drought. Photosynthetica. 2002;40:177–182. [Google Scholar]
- Froend R, Sommer B. Phreatophytic vegetation response to climatic and abstraction-induced groundwater drawdown: examples of long-term spatial and temporal variability in community response. Ecological Engineering. 2010;36:1191–1200. [Google Scholar]
- Gonzalez E, Comin FA, Muller E. Seed dispersal, germination and early seedling establishment of Populus alba L. under simulated water table declines in different substrates. Trees. 2010;24:151–163. [Google Scholar]
- Goodson JM, Davenport A, Gurnell AM, Thompson K. Hydrochory, river flow regime and riparian vegetation. Hydrology: Science & Practice for the 21st Century. 2004;2:99–105. British Hydrological Society International Conference from 12/07/2004 to 16/07/2004. [Google Scholar]
- Gregory J, Mitchell J, Brady A. Summer drought in northern midlatitudes in a time-dependent CO2 climate experiment. Journal of Climate. 1997;10:662–686. [Google Scholar]
- Higler LWG. The riparian community of North-West European lowland streams. Freshwater Biology. 1993;29:1993. [Google Scholar]
- Horton JL, Clark JL. Water table decline alters growth and survival of Salix gooddingii and Tamarix chinensis seedlings. Forest Ecology and Management. 2000;140:239–247. [Google Scholar]
- Hudon C. Shift in wetland plant composition and biomass following low-level episodes in the St. Lawrence River: looking into the future. Canadian Journal of Fisheries and Aquatic Sciences. 2004;61:603–617. [Google Scholar]
- Hussner A, Meyer A, Busch J. The influence of water level and nutrient availability on growth and root system development of Myriophyllum aquaticum. Weed Research. 2008;49:73–80. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) Synthesis report: summary for policymakers. 2007. In: Fourth Assessment Report, IPCC Plenary XXVII. Valencia, Spain.
- Jansson R, Laudon H, Johansson E, Augspurger C. The importance of groundwater discharge for plant species number in riparian zones. Ecology. 2007;88:131–139. doi: 10.1890/0012-9658(2007)88[131:tiogdf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Jansson R, Zinko U, Merritt DM, Nilsson C. Hydrochory increases riparian plant species richness: a comparison between a free-flowing and a regulated river. Journal of Ecology. 2005;93:1094–1103. [Google Scholar]
- Kleczewski NM, Herms DA, Bonello P. Nutrient and water availability alter belowground patterns of biomass allocation, carbon partitioning, and ectomycorrhizal abundance in Betula nigra. Trees. 2012;26:525–533. [Google Scholar]
- Kozlowski TT, Pallardy SG. Acclimation and adaptive responses of woody plants to environmental stresses. The Botanical Reviews. 2002;68:270–334. [Google Scholar]
- Li S, Pezeshki SR, Goodwin S. Effects of soil moisture regimes on photosynthesis and growth in cattail (Typha latifolia. Acta Oecologica. 2004;25:17–22. [Google Scholar]
- Lite SJ, Stromberg JC. Surface water and ground-water thresholds for maintaining Populus - Salix forests, San Pedro River, Arizona. Biological Conservation. 2005;125:153–167. [Google Scholar]
- Liu JZ, Chen YN, Chen YJ, Zhang N, Li WH. Degradation of Populus euphratica community in the lower reaches of the Tarim River, Xinjiang, China. Journal of Environmental Science (China) 2005;17:740–747. [PubMed] [Google Scholar]
- Mahoney JM, Rood SB. A device for studying the influence of declining water table on poplar growth and survival. Tree Physiology. 1991;8:305–314. doi: 10.1093/treephys/8.3.305. [DOI] [PubMed] [Google Scholar]
- Manabe S, Wetherald R. Large-scale changes of soil wetness induced by an increase in atmospheric carbon dioxide. Journal of the Atmospheric Sciences. 1987;44:1211–1235. [Google Scholar]
- Merritt DM, Scott ML, Le Roy Poff N, Auble GT, Lytle DA. Theory, methods and tools for determining environmental flows for riparian vegetation: riparian vegetation-flow response guilds. Freshwater Biology. 2010;55:206–225. [Google Scholar]
- Millennium Ecosystem Assessment (MEA) Ecosystems and Human Well-Being: Biodiversity Synthesis. Washington DC, U.S.A: World Resources Institute; 2005. [Google Scholar]
- Naiman RJ, Décamps H. The ecology of interfaces: riparian zones. Annual review of Ecology and Systematics. 1997;28:621–658. [Google Scholar]
- Naiman RJ, Décamps H, Pollock M. The role of riparian corridors in maintaining regional biodiversity. Ecological Applications. 1993;3:209–212. doi: 10.2307/1941822. [DOI] [PubMed] [Google Scholar]
- Nakai A, Yurugi Y, Kisanuki H. Growth response of Salix gracilistyla cuttings to a range of substrate moisture and oxygen availability. Ecological research. 2009;24:1057–1065. [Google Scholar]
- Pallardy SG. Physiology of Woody Plants. 3rd edn. the Netherlands: Academic press, Elsevier; 2008. http://www.scribd.com/doc/5015386/Physiology-of-woody-plants. [Google Scholar]
- Pezeshki SR, Anderson PH, Shields FD. Effects of soil moisture regimes on growth and survival of black willow (Salix nigra) posts (cuttings) Wetlands. 1998;18:460–470. [Google Scholar]
- Richardson DM, Holmes PM, Esler KJ, Galatowitsch SM, Stromberg JC, Kirkman SP, et al. Riparian vegetation: degradation, alien plant invasions, and restoration prospects. Diversity and Distributions. 2007;13:126–139. [Google Scholar]
- Romanello GA, Chuchra-Zbytniuk KL, Vandermer JL, Touchette BW. Morphological adjustments promote drought avoidance in the wetland plant Acorus americanus. Aquatic Botany. 2008;89:390–396. [Google Scholar]
- Rood SB, Pan J, Gill KM, Franks KM, Samuelson GM, Shepherd A. Declining summer flows of Rocky Mountain rivers: changing seasonal hydrology and probable impacts on floodplain forests. Journal of Hydrology. 2008;349:397–410. [Google Scholar]
- Sabo JL, Sponseller R, Dixon M, Gade K, Harms T, Heffernan J, et al. Riparian zones increase regional species richness by harboring different, not more, species. Ecology. 2005;86:56–62. [Google Scholar]
- Salinas MJ, Casas JJ. Riparian vegetation of two semi-arid Mediterranean rivers: basin-scale responses of woody and herbaceous plants to environmental gradients. Wetlands. 2007;27:831–845. [Google Scholar]
- Sletvold N, Ågren J. Variation in resistance and tolerance to drought among Scandinavian populations of Arabidopsis lyrata. Evolutionary Ecology. 2012;26:559–577. [Google Scholar]
- Smith M, Wu Y, Green O. Effect of light and water-stress on photosynthesis and biomass production in Boltonia decurrens (Asteraceae), a threatened species. American Journal of Botany. 1993;80:859–864. [Google Scholar]
- Soons MB. Wind dispersal in freshwater wetlands: knowledge for conservation and restoration. Applied Vegetation Science. 2006;9:271–278. [Google Scholar]
- Stella JC, Battles JJ. How do riparian woody seedlings survive seasonal drought? Oecologia. 2010;164:579–590. doi: 10.1007/s00442-010-1657-6. [DOI] [PubMed] [Google Scholar]
- Stella JC, Battles JJ, McBride JR, Orr BK. Riparian seedling mortality from simulated water table recession, and the design of sustainable flow regimes on regulated rivers. Restoration Ecology. 2010;18:284–294. [Google Scholar]
- Ström L, Jansson R, Nilsson C, Johansson ME, Xiong S. Hydrologic effects on riparian vegetation in a boreal river: an experiment testing climate change predictions. Global Change Biology. 2011;17:254–267. [Google Scholar]
- Stromberg JC, Bagstad KJ, Leenhouts JM, Lite SJ, Makings E. Effects of stream flow intermittency on riparian vegetation of a semiarid region river (San Pedro River, Arizona) River Research and Applications. 2005;21:925–938. [Google Scholar]
- Stromberg JC, Beauchamp VB, Dixon MD, Lite SJ, Paradzick C. Importance of low-flow and high-flow characteristics to restoration of riparian vegetation along rivers in arid southwestern United States. Freshwater Biology. 2007;52:651–679. [Google Scholar]
- Stromberg JC, Hazelton AF, White MS. Plant species richness in ephemeral and perennial reaches of a dryland river. Biodiversity and Conservation. 2009;18:663–677. [Google Scholar]
- Stromberg JC, Lite SJ, Dixon MD. Effects of stream flow patterns on riparian vegetation of a semiarid river: implications for a changing climate. River Research and Applications. 2010;26:712–729. [Google Scholar]
- Tabacchi E. Structural variability and invasions of pioneer plant communities in riparian habitats of the middle Adour River (SW France) Canadian Journal of Botany. 1995;73:33–44. [Google Scholar]
- Tockner K, Stanford JA. Riverine flood plains: present state and future trends. Environmental Conservation. 2002;29:308–330. [Google Scholar]
- Touchette BW, Iannacone LR, Turner G, Frank A. Ecophysiological responses of five emergent-wetland plants to diminished water supply: an experimental microcosm study. Aquatic Ecology. 2010;44:101–112. [Google Scholar]
- Van Splunder I, Voesenek LACJ, Coops H, DeVries XJA, Blom CWPM. Morphological responses of seedlings of four species of Salicaceae to drought. Canadian Journal of Botany. 1996;74:1988–1995. [Google Scholar]
- Verhoeven JTA, Arheimer B, Yin C, Hefting MM. Regional and global concerns over wetlands and water quality. Trends in Ecology and Evolution. 2006;21:96–103. doi: 10.1016/j.tree.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Verhoeven JTA, Soons MB, Janssen R, Omtzigt N. An Operational Landscape Unit approach for identifying key landscape connections in wetland restoration. Journal of Applied Ecology. 2008;45:1496–1503. [Google Scholar]
- Walls RL. Hybridization and plasticity contribute to divergence among coastal and wetland populations of invasive hybrid Japanese knotweed s.l. (Fallopia spp.) Estuaries and Coasts. 2010;33:902–918. [Google Scholar]
- Wang G. Agricultural drought in a future climate: results from 15 global climate models participating in the IPCC 4th Assessment. Climate Dynamics. 2005;25:739–753. [Google Scholar]
- Westwood CG, Teeuw RM, Wade PM, Holmes NT, Guyard P. Influences of environmental conditions on macrophyte communities in drought-affected headwater streams. River Research and Applications. 2006;22:703–726. [Google Scholar]
- Wetherald RT, Manabe S. Detectability of summer dryness caused by greenhouse warming. Climatic Change. 1999;43:495–511. [Google Scholar]
- Wetherald RT, Manabe S. Simulation of hydrologic changes associated with global warming. Journal of Geophysical Research. 2002;107:15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Key word strings used for the literature search.
Table S2. Main effects of drought on riparian plant species.


