Abstract
Team-based or multidisciplinary care may be a potential way to positively impact outcomes for heart failure (HF) patients by improving clinical outcomes, managing patient symptoms, and reducing health care costs. A multidisciplinary HF team includes a variety of providers in addition to the HF cardiologist—HF nurses, clinical pharmacists, dieticians, exercise specialists, mental health providers, social workers, primary care providers, and additional subspecialty providers. The timing and setting of multidisciplinary care depends on the needs of the patient and the resources available. Multidisciplinary HF teams should be evaluated based on their ability to achieve goals, as well as their potential for sustainability over time.
Keywords: HF, multidisciplinary care, team-based care, quality
INTRODUCTION
Heart failure (HF) is common and costly, affecting more than 5 million Americans with an incidence of 825,000 per year. By 2030, more than 8 million people in the United States are expected to have HF. Annually, HF accounts for more than 1 million hospitalizations in the United States and costs more than $30 billion, with expenses expected to more than double by 2030. HF-related morbidity and mortality remain high despite available treatments. Five-year mortality is approximately 50%, and HF is listed on one in nine death certificates [1, 2].
Advances in treatment options for HF continue to evolve, with new drugs and devices emerging throughout the past decade. Implantable cardioverter defibrillators (ICDs), cardiac resynchronization therapy (CRT), pulmonary artery pressure sensors, and left ventricular assist devices are examples of significant, yet complex therapies which may improve HF outcomes [3–5]. Since there are considerable comorbidities associated with HF, it is important to integrate other strategies into HF care, including behavioral modifications focused on diet, exercise, medication compliance, and self-care as alterable factors driving HF outcomes [6]. Nevertheless, the variety of HF care strategies creates the potential for fragmented care, with multiple disciplines spread across different settings.
Team-based or multidisciplinary care may be a potential way to reduce the burden of care and positively impact outcomes for HF patients. Furthermore, team-based care is a cornerstone of the patient-centered medical home model of care for chronic disease [7]. Team-based care requires resources like personnel, funding, infrastructure, and time; therefore, multidisciplinary teams should be evaluated to ensure organized, effective, and worthwhile interventions.
What are the goals for team based care in HF?
Short- and long-term clinical outcomes
Symptom management
Cost reduction
Patient, caregiver, provider satisfaction
The goals of HF care are numerous and diverse depending on perspective. Clinical outcome measures for HF often include mortality and hospital readmissions. While attention has focused on short-term outcomes such as 30-day quality measures enforced by the Centers for Medicare & Medicaid, many patients and providers consider long-term outcomes more important [8]. From a patient perspective, managing symptoms and improving functional capacity is also an important goal. From a societal viewpoint, HF carries substantial public health costs, so managing these costs is a top priority for payers and health care systems.
Utilizing a team of providers may improve the quality of care provided to HF patients. In evaluating multidisciplinary care, teams should be evaluated based on their ability to improve morbidity and mortality, decrease rehospitalizations, and cut costs, as well as their ability to provide patient, caregiver, and provider satisfaction.
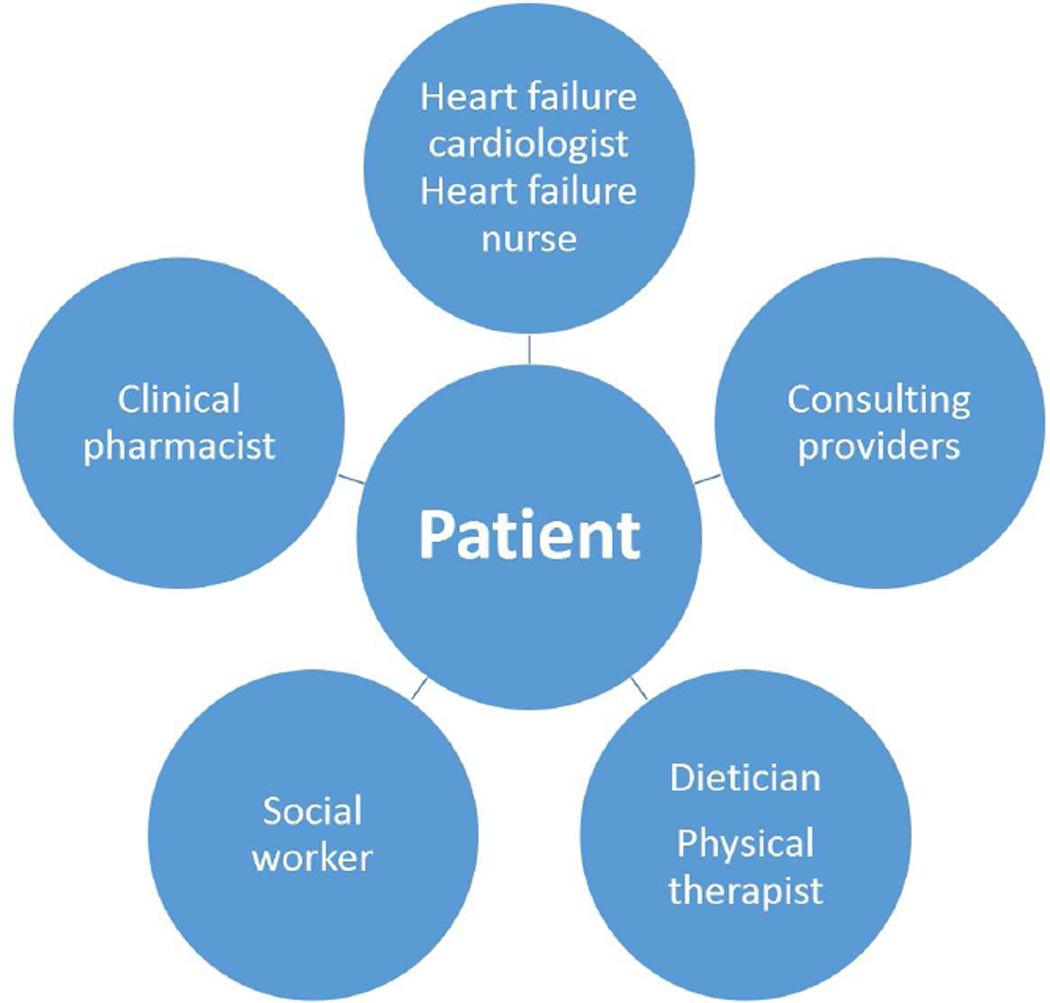
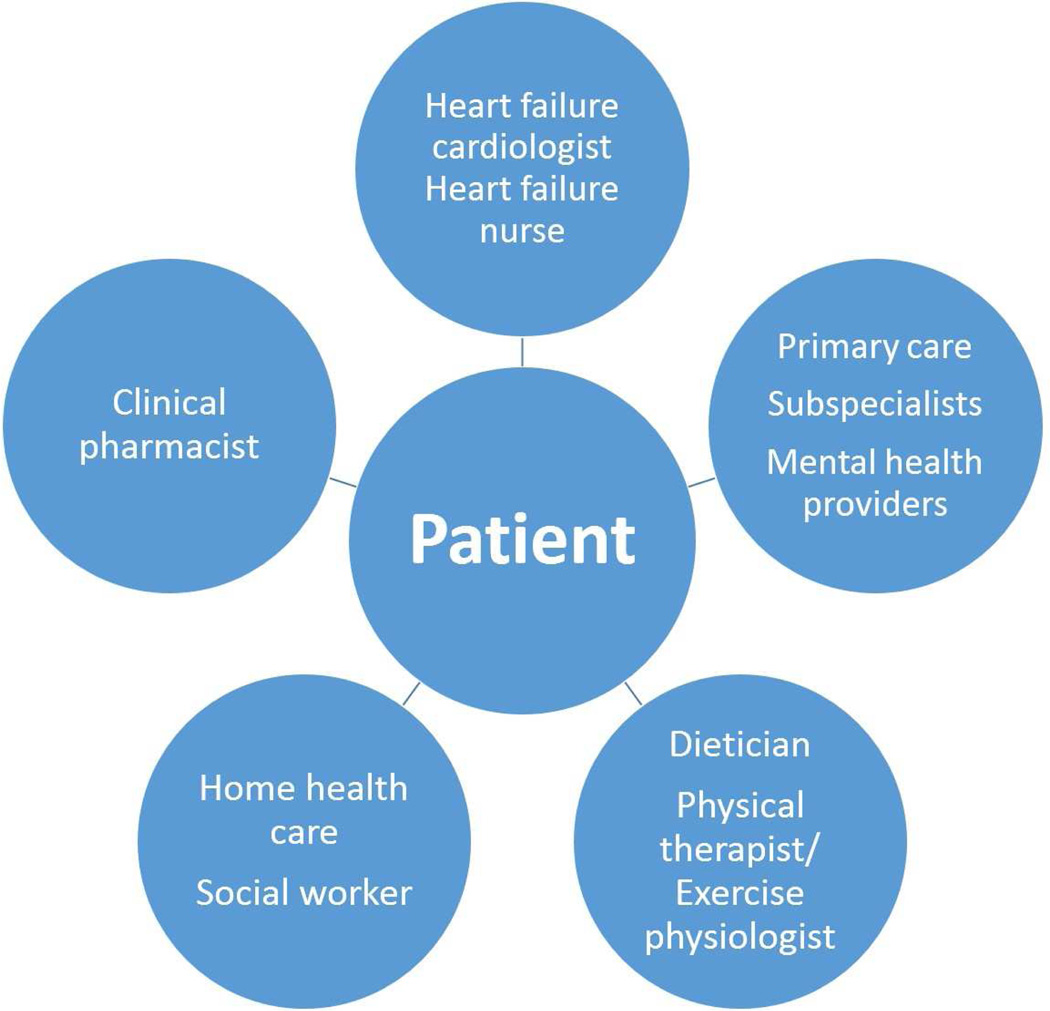
Who are the key players on the HF care team? (Figure 2a, 2b)
Primary care providers
HF cardiologists
HF nurses
Clinical pharmacists
Specialized cardiac providers
Ancillary service providers
Figure 2. HF Multidisciplinary Care Team.
This figure provides examples of: (a) inpatient; and (b) outpatient providers of team-based case in HF.
In both the inpatient and outpatient settings, multidisciplinary teams can be organized to care for HF patients. In addition to cardiologists and other physicians, the HF team may include specialized nurses, dieticians, pharmacists, social workers, physical therapists, and psychologists. Importantly, the patients themselves, as well as their families and caregivers, are an integral part of the health care team.
Primary care providers are often the first line of care for HF patients. Primary care providers are responsible for making a timely and accurate diagnosis of HF, initiating therapy, and managing comorbid illnesses. They must be able to recognize when specialized care is required or would benefit their patients, and make the necessary referrals. Even when a patient is referred to a specialized clinic, the primary care provider may continue to provide follow-up and take on the responsibility of coordinating additional care for the patient [9, 10].
Referring patients to specialized outpatient HF clinics, staffed with trained health care providers who are familiar with current guidelines and available resources, has been shown to reduce hospital admissions. [11, 12]. In addition to HF physicians, HF nurses are often included as part of the HF team, and are responsible for a diverse range of interventions. Nursing interventions have been extensively studied and have been shown to positively impact HF patient care. Close follow-up by a HF nurse in the outpatient setting has been shown to improve patient self-care, reduce readmissions, shorten length of stay, and reduce costs [13–16]. Furthermore, when the nurse-led intervention starts during hospitalization and provides assistance with the transition of care to the outpatient setting, there is an additional benefit of improved quality of life [17, 18].
Medical management is at the core of HF therapy, requiring frequent adjustments and dose titrations. Clinical pharmacists can help with education and medication compliance, monitor for drug interactions and intolerances, and promote proper medication reconciliation through transitions of care between different health care providers and different settings. Pharmacist interventions have been shown to reduce medication errors, advance patient knowledge, improve adherence, increase medication titration and optimization, and decrease health care spending [19–24]. As a results of these benefits, the addition of a clinical pharmacist to a HF team has been shown to reduce mortality and HF events [25, 26].
Outside of the core HF providers, patients also benefit from further subspecialized cardiac care by electrophysiologists, cardiac imaging experts, and cardiac surgeons who have experience caring for HF patients. Involving dedicated electrophysiologists in the care of HF patients can increase appropriate use of ICDs and CRT, allowing for timely troubleshooting and optimization of these devices. Multidisciplinary cardiology care for patients with HF has been shown to improve event-free survival [27–29].
In addition to medications, diet plays an important role in the chronic treatment of HF patients. There are many dietary considerations, including restriction of sodium and fluid, management of obesity, prevention of cachexia, and management of comorbidities such as diabetes and hyperlipidemia [30–36]. Additional considerations are present for patients prescribed drugs with potential food interactions, such as warfarin [37]. A dietician may have a positive effect on the many aspects of dietary compliance for HF patients [38].
Growing evidence highlights the importance of exercise and lifestyle modification for stable HF patients. Cardiac rehabilitation provides a structured program for these patients, emphasizing the need for physical therapists or exercise physiologists as part of the HF team. Many HF patients have poor exercise capacity, and regular exercise has been shown to be a safe and effective means of improving functional status and quality of life [39–43]. Furthermore, regular exercise results in a reduction in depressive symptoms in HF patients [44].
Mental health disorders such as depression are common in the HF patient population and add additional complexity, since they often require other services or care approaches [45, 46]. Studies have shown that depression is associated with functional decline, rehospitalization, and death. Furthermore, there is a positive correlation between depression severity and outcomes; worse depression is associated with worse outcomes [47–50]. Cardiologists may overlook depressive symptoms and fail to provide treatment recommendations, so including a psychologist as part of a HF multidisciplinary team can help with diagnosis and management of these often untreated psychological conditions [51].
Managing HF requires numerous services and resources, and patients may need assistance from social workers in coordinating care in both the inpatient and outpatient settings. Studies that have included social workers as part of multidisciplinary care teams have shown that they make a significant contribution [52]. By anticipating post-discharge needs, arranging home health services, optimizing insurance benefits, and supporting patient caregivers, involvement of social workers can help HF patients better adhere to their treatment plans [12, 53].
Since HF care teams are made up of a variety of providers, many facets of the team require evaluation. A team should be evaluated based on the services they provide, the providers they include, and the patients they treat. In order to establish which providers are the most essential, teams should be evaluated on the types of providers that make up the team. In addition, team evaluations should include both individual assessments and group assessments to determine the quality of each individual provider and the dynamics of the team as a whole. Understanding that different patients will likely require different degrees of attention and intervention, teams should be evaluated on how well they can assess the needs of each patient and utilize the team-based model most efficiently and effectively.
Where should multidisciplinary care take place?
Inpatient hospitalization
Outpatient clinic
Patient home
Remotely via telephone or telemonitoring
Multidisciplinary care can occur in numerous settings—in the hospital or outpatient clinic, at home visits, or via telephone or telemonitoring systems. An early meta-analysis of the impact of the location of team-based care interventions concluded that home visits reduced all-cause readmissions, telephone support improved mortality, and both home visits and telephone interventions reduced HF readmissions. Notably, the interventions that did not include a home component did not affect either readmissions or mortality [54]. A more recent meta-analysis of 47 trials from 2007 to 2013 that assessed transitions of care from the inpatient to the outpatient setting confirmed the benefit of home visits [55]. Home visits were shown to improve 30-day and 3–6 month readmissions; HF clinics and structured telephone support improved 3–6 month all-cause readmissions, but did not impact 30-day outcomes; and home visits, HF clinics, and structured telephone support improved mortality.
Similarly, results of home telemonitoring studies have been mixed. Several small studies have suggested that telemonitoring can improve HF outcomes, whereas larger randomized trials have failed to show a reduction in mortality or hospitalizations compared to standard care [56–58]. One potential reason for these mixed results may be that telemonitoring strategies are often limited by patient compliance. A recent study on automated telemonitoring through implanted devices showed markedly improved outcomes compared to standard care [59]. While self-care is vital for HF patients, these results highlight the benefit of automated follow-up, and the importance of not solely relying on patients to identify early decompensation.
These patient-driven factors may explain why the intervention’s location impacts the intervention’s effect. Home-based interventions allow providers to perform more in-depth assessments and discover potential barriers to optimal care and disease management. Therefore, providers are able to provide more personalized interventions compared to standardized or uniform interventions that occur over the phone or in the hospital or clinic setting.
Since multidisciplinary care takes place in multiple settings, the setting must be individualized to a patient’s needs. Sometimes team members will be the same across different settings, but often teams may be comprised of different individuals and different types of providers. As a result, multidisciplinary care teams should be evaluated based on the care provided and the setting in which this care occurs. Furthermore, because communication within and between teams is critical, teams and team members should be evaluated based on the quality of communication and continuity of care.
When should multidisciplinary care take place?
Diagnosis/new referral
Change in clinical status
Hospitalization
When to initiate multidisciplinary care varies based on the needs and location of the patient. For patients newly referred to an outpatient HF clinic, implementing a multidisciplinary approach to care has been shown to result in improved functional status and quality of life, as well as decreased hospitalizations [60]. For hospitalized patients, many studies have focused on the transition from the inpatient to the outpatient setting; therefore, the multidisciplinary care initiatives have been structured around discharge planning. Studies in which multidisciplinary care was initiated during the hospitalization showed reduced mortality, decreased rehospitalizations and health care costs, and improved quality of life [12, 21, 61–63]. Similar outcomes were achieved when multidisciplinary care was initiated within two weeks post-hospitalization [64–66].
In clinical practice, systems must be secured to ensure sustainability of the multidisciplinary model. Ensuring sustainability requires appropriate resource allocation, with the most resources going to patients at highest risk for adverse outcomes and to those for whom interventions will be most successful. Since HF patients often cycle between clinical stability and decompensation, patients may need more intense care at certain time points and less intense care at others. The team-based care model should be able to adapt according to an individual patient’s needs at any given time.
How should multidisciplinary care be evaluated?
Ability to achieve goals of care
Sustainability over time
As individuals and as part of a team
Given the diversity of HF care teams, which encompass different care providers in different settings at different time points with different goals, there is not a universal way by which to assess and compare the effectiveness of team-based care. Teams should be evaluated based on their ability to achieve certain goals and metrics, including reducing morbidity, mortality, readmissions, and health care costs. Equally important is a team’s ability to improve patient quality of life, and maintain patient, caregiver, and provider satisfaction.
Both short- and long-term goals should be achievable. Inpatient care teams may focus on helping the patient achieve clinical stability and appropriate discharge, transitional care teams may focus on optimizing care at home following hospitalization, and outpatient care teams may focus on maintaining clinical stability and timely efforts to prevent or halt pending decompensation. Since there may be different team members providing care at different times in different settings, it is essential for team members to have effective communication and handoffs to ensure continuity of care.
The ability to achieve these goals must be maintained over time. Unlike clinical trial settings, team-based care in an actual clinical setting should have no end date. Even though studies have demonstrated that the impact of team-based care may last beyond the team-based intervention, team-based care outside of clinical trials should be continuous and flexible to a patient’s needs.
Teams are made up of individuals, so each person and type of provider on a team must be assessed both individually and as part of a team. Frequent evaluations can ensure that a team is structured appropriately. Additionally, it is important to determine how involved each team member needs to be for each patient at any given time; a patient may need to see some providers frequently and others infrequently. As a patient’s needs change over time, different components of care will become more or less important, fluctuating based on patient status. Effectively incorporating team members and ensuring that their time is used appropriately and efficiently are keys to effective team-based care.
Yet even the right team components at the right time in the right setting may be ineffective if the team is providing ineffective interventions; therefore, in addition to team evaluations, the interventions these teams provide need to be assessed. Multidisciplinary care teams should use validated assessment tools, education methods, and evidence-based recommendations to provide standardized benefits for their patients.
When designing studies to evaluate the efficacy of multidisciplinary care, many factors must be considered. Decisions must be made regarding which outcomes to evaluate and whether the outcomes will be evaluated for individual patients, single centers, or health systems. Furthermore, outcomes can be compared between groups, or groups can act as their own controls, comparing outcomes before and after the implementation of team-based care. While observational studies may help identify which patients benefit from which interventions, given the complexity and diversity of heart failure patients, unmeasured confounders may bias the results. While prospective randomized trials eliminate selection bias, the results may be less generalizable to a broader heart failure population. Pragmatic trial design is important to allow for the flexibility required in team-based care of heart failure patients.
SUMMARY
Comparative effectiveness of multidisciplinary care is limited. Few trials have directly compared one multidisciplinary care structure to another. In trials, most multidisciplinary care interventions are compared with standard of care. However, standards of care are not necessarily uniform across trials, thus the ability to compare interventions across trials is limited. Outside of meta-analyses, there are limited direct comparisons of interventions. Overall, it appears that there are notable benefits of multidisciplinary care, but it is still unknown which interventions provide the most benefit. Different team organizations, follow-up intervals, and interventions need to be compared head-to-head in order to find the optimal team structure to provide the most benefit. While it is likely that the optimal team will be different at different time points or for different patients, further studies are needed to determine which patients will benefit the most from which aspects of team-based care.
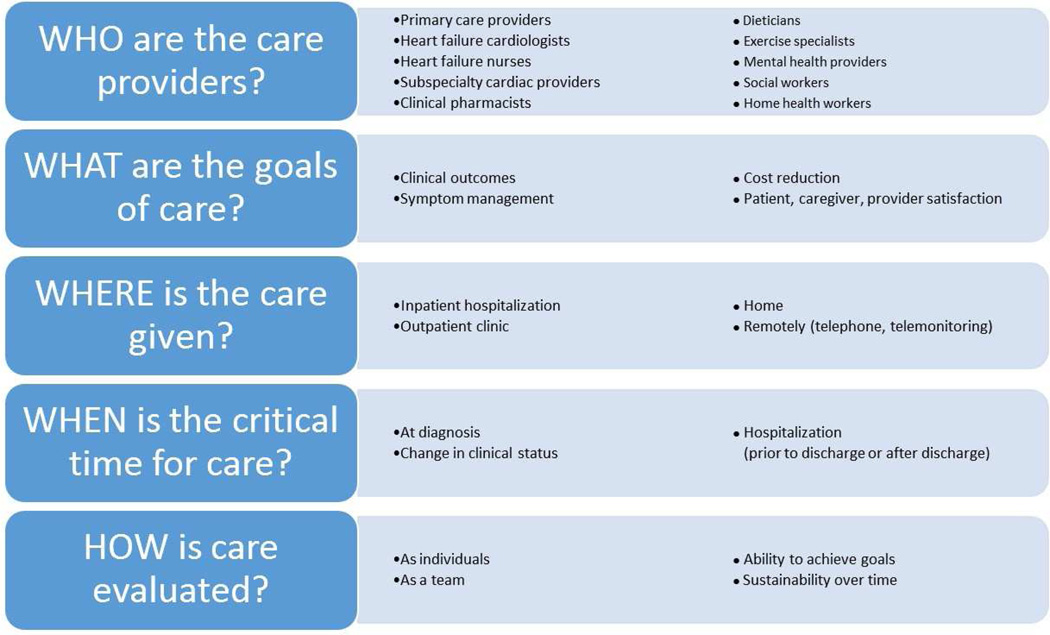
Figure 1. Team-Based Care in HF.
This figure displays who, what, where, when, and how of team-based care in HF.
KEY POINTS.
Goals of multidisciplinary care in heart failure (HF) include improving clinical outcomes, managing patient symptoms, and reducing health care costs.
Providers in a multidisciplinary HF team include HF cardiologists and nurses, other health care providers, pharmacists, and ancillary support including exercise specialists, dieticians, and social workers.
Multidisciplinary care can take place in an inpatient or outpatient setting, at home, or remotely.
Multidisciplinary HF teams should be evaluated based on their ability to achieve goals, as well as their potential for sustainability over time.
ACKNOWLEDGMENTS
The authors would like to thank Erin Hanley, MS for her editorial contributions to this manuscript. Ms. Hanley did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted.
SOURCES OF FUNDING
Dr. Cooper is supported by grant T32HL069749-11A1 from the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Cooper has no relevant disclosures to report.
Dr. Hernandez reports research grant funding from Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Novartis, Portola Pharmaceuticals; and receiving honoraria from Amgen, GlaxoSmithKline, Janssen, and Novartis.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 4.Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376(9744):875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 5.Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381(9860):29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 6.Colonna P, Sorino M, D’Agostino C, et al. Nonpharmacologic care of heart failure: counseling, dietary restriction, rehabilitation, treatment of sleep apnea, and ultrafiltration. Am J Cardiol. 2003;91(9a):41F–50F. doi: 10.1016/s0002-9149(02)03337-4. [DOI] [PubMed] [Google Scholar]
- 7.Rittenhouse DR, Shortell SM. The patient-centered medical home: will it stand the test of health reform? JAMA. 2009;301(19):2038–2040. doi: 10.1001/jama.2009.691. [DOI] [PubMed] [Google Scholar]
- 8.Department of Health and Human Services. Centers for Medicare & Medicaid Services. [Accessed January 13, 2015];Federal Register, Part II. U.S. Government Publishing Office web site. http://www.gpo.gov/fdsys/pkg/FR-2011-08-18/pdf/2011-19719.pdf. Updated August 18, 2011.
- 9.Hobbs FD. Primary care physicians: champions of or an impediment to optimal care of the patient with heart failure? Eur J Heart Fail. 1999;1(1):11–15. doi: 10.1016/s1388-9842(98)00006-3. [DOI] [PubMed] [Google Scholar]
- 10.Konstam MA, Greenberg B. Transforming health care through the medical home: the example of heart failure. J Card Fail. 2009;15(9):736–738. doi: 10.1016/j.cardfail.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol. 1997;30(3):725–732. doi: 10.1016/s0735-1097(97)00208-8. [DOI] [PubMed] [Google Scholar]
- 12.Rich MW, Beckham V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333(18):1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 13.Cline CM, Israelsson BY, Willenheimer RB, et al. Cost effective management programme for heart failure reduces hospitalisation. Heart. 1998;80(5):442–446. doi: 10.1136/hrt.80.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaarsma T, Halfens R, Huijer Abu-Saad H, et al. Effects of education and support on selfcare and resource utilization in patients with heart failure. Eur Heart J. 1999;20(9):673–682. doi: 10.1053/euhj.1998.1341. [DOI] [PubMed] [Google Scholar]
- 15.Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to preventreadmission of patients with heart failure. J Am Coll Cardiol. 2002;39(1):83–89. doi: 10.1016/s0735-1097(01)01699-0. [DOI] [PubMed] [Google Scholar]
- 16.Strömberg A, Mårtensson J, Fridlund B, et al. Nurse-led heart failure clinics improve survival and self-care behaviour in patients with heart failure: results from a prospective, randomised trial. Eur Heart J. 2003;24(11):1014–1023. doi: 10.1016/s0195-668x(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 17.Blue L, Lang E, McMurray JJ, et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ. 2001;323(7315):715–718. doi: 10.1136/bmj.323.7315.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison MB, Browne GB, Roberts J, et al. Quality of life of individuals with heart failure: a randomized trial of the effectiveness of two models of hospital-to-home transition. Med Care. 2002;40(4):271–282. doi: 10.1097/00005650-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Milfred-Laforest SL, Chow SL, Didomenico RJ, et al. Clinical pharmacy services in heart failure: an opinion paper from the Heart Failure Society of America and American College of Clinical Pharmacy Cardiology Practice and Research Network. J Card Fail. 2013;19(5):354–369. doi: 10.1016/j.cardfail.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Eggink RN, Lenderink AW, Widdershoven JW, et al. The effect of a clinical pharmacist discharge service on medication discrepancies in patients with heart failure. Pharm World Sci. 2010;32(6):759–766. doi: 10.1007/s11096-010-9433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwadry-Sridhar FH, Arnold JM, Zhang Y, et al. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J. 2005;150(5):982. doi: 10.1016/j.ahj.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med. 2007;146(10):714–725. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 23.Bouvy ML, Heerdink ER, Urquhart J, et al. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: a randomized controlled study. J Card Fail. 2003;9(5):404–411. doi: 10.1054/s1071-9164(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 24.Luzier AB, Forrest A, Feuerstein SG, et al. Containment of heart failure hospitalizations and cost by angiotensin-converting enzyme inhibitor dosage optimization. Am J Cardiol. 2000;86(5):519–523. doi: 10.1016/s0002-9149(00)01005-5. [DOI] [PubMed] [Google Scholar]
- 25.Gattis WA, Hasselblad V, Whellan DJ, et al. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Arch Intern Med. 1999;159(16):1939–1945. doi: 10.1001/archinte.159.16.1939. [DOI] [PubMed] [Google Scholar]
- 26.Koshman SL, Charrois TL, Simpson SH, et al. Pharmacist care of patients with heart failure: a systematic review of randomized trials. Arch Intern Med. 2008;168(7):687–694. doi: 10.1001/archinte.168.7.687. [DOI] [PubMed] [Google Scholar]
- 27.Swedberg K, Cleland J, Cowie MR, et al. Successful treatment of heart failure with devices requires collaboration. Eur J Heart Fail. 2008;10(12):1229–1235. doi: 10.1016/j.ejheart.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Altman RK, Parks KA, Schlett CL, et al. Multidisciplinary care of patients receiving cardiac resynchronization therapy is associated with improved clinical outcomes. Eur Heart J. 2012;33(17):2181–2188. doi: 10.1093/eurheartj/ehs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang WH, Boehmer J, Gras D. Multispecialty approach: the need for heart failure disease management for refining cardiac resynchronization therapy. Heart Rhythm. 2012;9(8 Suppl):S45–S50. doi: 10.1016/j.hrthm.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 30.Arcand J, Ivanov J, Sasson A, et al. A high-sodium diet is associated with acute decompensated heart failure in ambulatory heart failure patients: a prospective follow-up study. Am J Clin Nutr. 2011;93(2):332–337. doi: 10.3945/ajcn.110.000174. [DOI] [PubMed] [Google Scholar]
- 31.Paterna S, Parrinello G, Cannizzaro S, et al. Medium term effects of different dosage of diuretic, sodium, and fluid administration on neurohormonal and clinical outcome in patients with recently compensated heart failure. Am J Cardiol. 2009;103(1):93–102. doi: 10.1016/j.amjcard.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 32.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 33.Habbu A, Lakkis NM, Dokainish H. The obesity paradox: fact or fiction? Am J Cardiol. 2006;98(7):944–948. doi: 10.1016/j.amjcard.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 34.Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349(9058):1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 35.Dickson VV, Buck H, Riegel B. A qualitative meta-analysis of heart failure self-care practices among individuals with multiple comorbid conditions. J Card Fail. 2011;17(5):413–419. doi: 10.1016/j.cardfail.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Payne-Emerson H, Lennie TA. Nutritional considerations in heart failure. Nurs Clin North Am. 2008;43(1):117–132. doi: 10.1016/j.cnur.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Self TH, Reaves AB, Oliphant CS, et al. Does heart failure exacerbation increase response to warfarin? A critical review of the literature. Curr Med Res Opin. 2006;22(11):2089–2094. doi: 10.1185/030079906X132479. [DOI] [PubMed] [Google Scholar]
- 38.Kuehneman T, Saulsbury D, Splett P, et al. Demonstrating the impact of nutrition intervention in a heart failure program. J Am Diet Assoc. 2002;102(12):1790–1794. doi: 10.1016/s0002-8223(02)90384-6. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keteyian SJ, Leifer ES, Houston-Miller N, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2012;60(19):1899–1905. doi: 10.1016/j.jacc.2012.08.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belardinelli R, Georgiou D, Cianci G, et al. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99(9):1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 42.McKelvie RS. Exercise training in patients with heart failure: clinical outcomes, safety, and indications. Heart Fail Rev. 2008;13(1):3–11. doi: 10.1007/s10741-007-9052-z. [DOI] [PubMed] [Google Scholar]
- 43.Flynn KE, Piña IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blumenthal JA, Babyak MA, O’Connor C, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA. 2012;308(5):465–474. doi: 10.1001/jama.2012.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guck TP, Elsasser GN, Kavan MG, et al. Depression and congestive heart failure. Congest Heart Fail. 2003;9(3):163–169. doi: 10.1111/j.1527-5299.2003.01356.x. [DOI] [PubMed] [Google Scholar]
- 46.Koenig HG. Depression in hospitalized older patients with congestive heart failure. Gen Hosp Psychiatry. 1998;20(1):29–43. doi: 10.1016/s0163-8343(98)80001-7. [DOI] [PubMed] [Google Scholar]
- 47.Vaccarino V, Kasl SV, Abramson J, et al. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Cardiol. 2001;38(1):199–205. doi: 10.1016/s0735-1097(01)01334-1. [DOI] [PubMed] [Google Scholar]
- 48.Sherwood A, Blumenthal JA, Trivedi R, et al. Relationship of depression to death or hospitalization in patients with heart failure. Arch Intern Med. 2007;167(4):367–373. doi: 10.1001/archinte.167.4.367. [DOI] [PubMed] [Google Scholar]
- 49.Jiang W, Kuchibhatla M, Clary GL, et al. Relationship between depressive symptoms and long-term mortality in patients with heart failure. Am Heart J. 2007;154(1):102–108. doi: 10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 50.Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–1856. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 51.Moser DK. Psychosocial factors and their association with clinical outcomes in patients with heart failure: why clinicians do not seem to care. Eur J Cardiovasc Nurs. 2002;1(3):183–188. doi: 10.1016/S1474-5151(02)00033-6. [DOI] [PubMed] [Google Scholar]
- 52.Takeda A, Taylor SJ, Taylor RS, et al. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;9:CD002752. doi: 10.1002/14651858.CD002752.pub3. [DOI] [PubMed] [Google Scholar]
- 53.Ric MW, Gray DB, Beckham V, et al. Effect of a multidisciplinary intervention on medication compliance in elderly patients with congestive heart failure. Am J Med. 1996;101(3):270–276. doi: 10.1016/s0002-9343(96)00172-6. [DOI] [PubMed] [Google Scholar]
- 54.Holland R, Battersby J, Harvey I, et al. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005;91(7):899–906. doi: 10.1136/hrt.2004.048389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160(11):774–784. doi: 10.7326/M14-0083. [DOI] [PubMed] [Google Scholar]
- 56.Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: Abridged Cochrane Review. Eur J Heart Fail. 2011;13(9):1028–1040. doi: 10.1093/eurjhf/hfr039. [DOI] [PubMed] [Google Scholar]
- 57.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363(24):2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123(17):1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 59.Hindricks G, Taborsky M, Glikson M, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384(9943):583–590. doi: 10.1016/S0140-6736(14)61176-4. [DOI] [PubMed] [Google Scholar]
- 60.Feldman DE, Ducharme A, Giannetti N, et al. Outcomes for women and men who attend a heart failure clinic: results of a 12-month longitudinal study. J Card Fail. 2011;17(7):540–546. doi: 10.1016/j.cardfail.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Naylor MD, Brooten DA, Campbell RL, et al. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675–684. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]
- 62.McDonald K, Ledwidge M, Cahill J, et al. Heart failure management: multidisciplinary care has intrinsic benefit above the optimization of medical care. J Card Fail. 2002;8(3):142–148. doi: 10.1054/jcaf.2002.124340. [DOI] [PubMed] [Google Scholar]
- 63.Angermann CE, Störk S, Gelbrich G, et al. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circ Heart Fail. 2012;5(1):25–35. doi: 10.1161/CIRCHEARTFAILURE.111.962969. [DOI] [PubMed] [Google Scholar]
- 64.Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354(9184):1077–1083. doi: 10.1016/s0140-6736(99)03428-5. [DOI] [PubMed] [Google Scholar]
- 65.Inglis SC, Pearson S, Treen S, et al. Extending the horizon in chronic heart failure: effects of multidisciplinary, home-based intervention relative to usual care. Circulation. 2006;114(23):2466–2473. doi: 10.1161/CIRCULATIONAHA.106.638122. [DOI] [PubMed] [Google Scholar]
- 66.Ducharme A, Doyon O, White M, et al. Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial. CMAJ. 2005;173(1):40–45. doi: 10.1503/cmaj.1041137. [DOI] [PMC free article] [PubMed] [Google Scholar]