Abstract
Leukemia inhibitory factor (LIF), a cytokine that belongs to the interleukin-6 family, regulates multiple important biological functions. Recently, we found that LIF is an important negative regulator of p53 in human colorectal cancer cells. LIF negatively regulates p53 protein levels and functions by activation of the Stat3 signaling pathway, which in turn induces the expression of ID1, the helix-loop-helix (HLH) protein inhibitor of differentiation and DNA binding. ID1 increases MDM2 expression at both mRNA and protein levels to accelerate p53 protein degradation. Overexpression of LIF increases chemoresistance of cultured colorectal cancer cells and colorectal xenograft tumors in a largely p53-dependent manner. Furthermore, LIF is overexpressed in a large percentage of human colorectal cancer specimens and LIF overexpression is associated with a poor prognosis in colorectal cancer patients. Our study revealed a new role of LIF in tumorigenesis through regulation of the p53 signaling pathway.
Keywords: LIF, p53, Stat3, ID1, MDM2, chemoresistance
Leukemia inhibitory factor (LIF) is a member of the interleukin-6 cytokine superfamily. Its name was derived from its ability to induce the differentiation of myeloid leukemia cells and in turn prevent the growth of leukemia cells (1). LIF functions through binding to the LIF receptor complex composed of LIF receptor (LIF-R) and glycoprotein gp130 to activate several signaling pathways in a cell/tissue type specific manner, including the JAK/STAT3, MAPK, and AKT pathways (2). As a cytokine, LIF has been shown to have multiple important functions. LIF can be produced locally in the bone and joint microenvironment to play an essential role for normal bone remodeling and growth through stimulating osteoblast differentiation and bone formation (3). LIF also plays an important role in various aspects of neuronal development, including phenotype determination, survival, and regulating maturation during postnatal neuronal development (4). Ample evidence has shown that LIF regulates the self-renewal function of embryonic stem cells and inhibits their differentiation, thus maintaining their pluripotency (5). Furthermore, LIF plays a crucial role in multiple steps during embryo implantation and placentation (6, 7). LIF is upregulated during peripheral inflammation, and has an anti-inflammatory effect. Exogenous LIF expression can reduce inflammatory hyperalgesia (8). However, the precise role of LIF in tumorigenesis remains largely unknown.
p53 tumor suppressor is a transcription factor and plays a central role in tumor suppression. It regulates many important cellular functions through transcriptional regulation of its target genes. Recently, our group identified LIF as a novel p53 target gene, which mediates p53’s role in embryonic implantation (9). p53 binds to the consensus p53-binding element in the LIF gene and regulates the expressions of LIF under both non-stressed and stressed conditions. It has long been observed that p53−/− female mice have reduced fertility. Our study demonstrated that the decreased fertility in p53−/− female mice is mainly due to the impaired embryonic implantation. At the implantation stage, highest levels of LIF expression are observed in the endometrial tissues, including endometrial glands, where p53 protein levels and activity are selectively increased. We found that loss of p53 decreases the levels and function of uterine LIF, and re-injection of LIF to pregnant p53−/− female mice can rescue the impaired maternal reproduction by improving embryonic implantation. This finding revealed an unexpected role of p53 in reproduction through regulation of LIF expression levels.
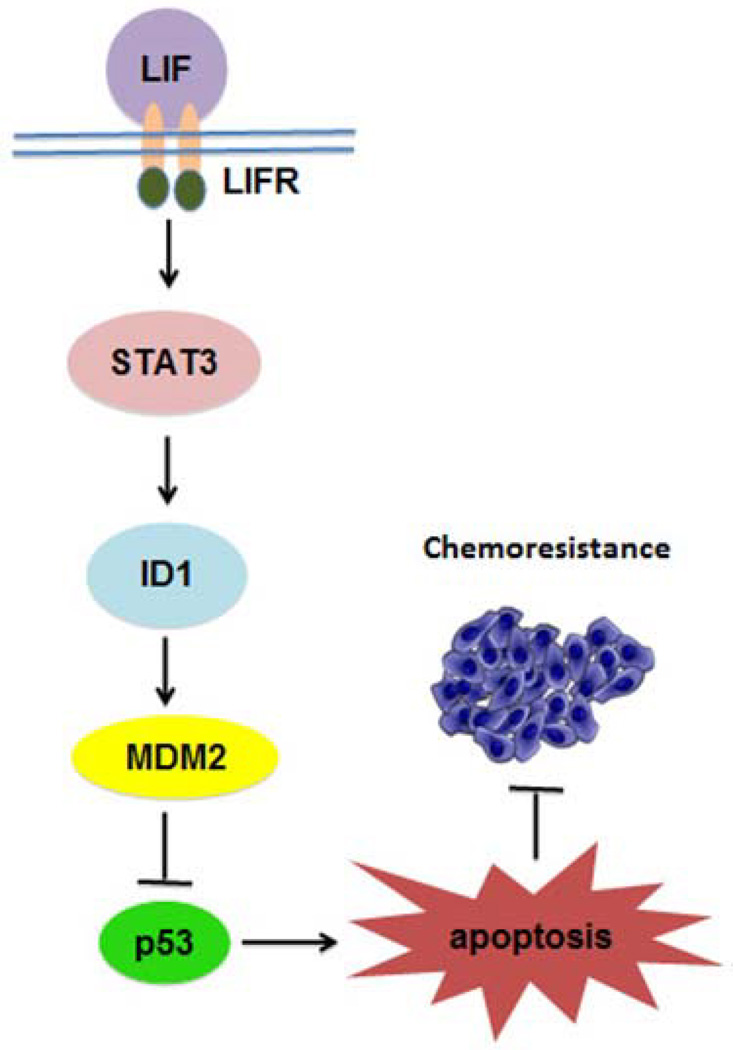
Considering the critical role of p53 in tumor suppression, we then studied the possible role of LIF, an important component in the p53 signaling pathway, in tumorigenesis. We observed that LIF is frequently overexpressed in human tumors, including colorectal cancers. Ectopic expression of LIF promotes chemoresistance of cultured colorectal cancer cells and colorectal xenograft tumors in a largely p53-dependent manner (10). Interestingly, we found that LIF is a negative regulator of p53 (10). In human colorectal cancer cells, p53 protein levels and function are negatively regulated by LIF. The negative regulation of p53 by LIF is mediated by the activation of the Stat3 signaling pathway. Stat3 transcriptionally regulates a set of genes including ID1, the helix-loop-helix (HLH) protein inhibitor of differentiation and DNA binding. ID1 is reported to upregulate MDM2, a major E3 ubiquitin ligase for p53, which binds to p53 and mediates its ubiquitination and protein degradation (11, 12). We found that LIF can clearly increase MDM2 expression at both mRNA and protein levels which is mediated by ID1. The increased MDM2 expression induced by LIF can in turn accelerate p53 protein degradation. Furthermore, we found that LIF overexpression is associated with a poor prognosis of colorectal cancer patients (10). Taken together, our study demonstrates that LIF is an important and novel negative regulator of p53 through the Stat3/ID1/MDM2 pathway, which in turn promotes chemoresistance in colorectal cancer cells and poor prognosis of colorectal cancer patients (Figure 1).
Figure 1. A model illustrating the negative regulation of p53 by LIF that contributes to chemoresistance in human colorectal cancer cells.
LIF binds to LIF receptor complex to activate the Stat3 signaling pathway. Stat3 transcriptionally induces inhibitor of DNA binding 1 (ID1), which can up-regulate MDM2, a key negative regulator of p53. The increased MDM2 levels promote p53 protein degradation, which leads to decreased p53 protein levels and reduced function in apoptosis. In turn, LIF promotes chemoresistance in human colorectal cancer cells.
In addition to the role of LIF as a negative regulator of p53, recent studies including ours have shown that LIF mediates cancer metastasis in multiple tumors. Early studies have shown that LIF is a metastatic factor in rhabdomyosarcomas (13). A recent report showed that LIF can mediate proinvasive activation of stromal fibroblasts in cancer, which contributes to the proinvasive tumor microenvironment (14). Our recent study has shown that LIF promotes the growth and proliferation of breast tumor cells both in vitro and in vivo. Furthermore, LIF promotes metastasis of breast cancer cells as determined by both in vitro and in vivo assays. More importantly, we found that LIF activates the AKT-mTOR signaling pathway in breast cancer cells which plays an important role in mediating the function of LIF in tumorigenesis and metastasis of breast cancer. Clinic data have shown that LIF overexpression is significantly correlated with a poor prognosis in breast cancer patients (15). Consistent with our report, recently, another study has shown that LIF activates the mTORC1/p70S6K signaling pathway to promote tumor growth, and inhibit DNA damage responses, which in turn increases radio-resistance in nasopharyngeal carcinoma (16).
All these evidence indicated that LIF could be a novel biomarker and an important therapeutic target for human cancer, especially for those with LIF overexpression. LIF may promote tumorgenesis and metastasis through multiple mechanisms in different type of tumors. Among them, the finding of the feedback loop of p53-LIF is very intriguing. While our early study revealed LIF as a p53 target gene, our recent work demonstrated that LIF, as a secreted protein, can function through the Stat3/ID1/MDM2 pathway to negatively regulate p53. Therefore, p53 and LIF form a novel negative feedback loop. There are some interesting questions that need to be explored in the future. We found that during implantation, p53 level is increased in uterine tissue transiently and is important for LIF production at the implantation stage. It will be interesting to study whether the high expression of LIF at the implantation stage participates in the down-regulation of p53 after the implantation stage. On the other hand, LIF is found to be overexpressed in many tumors, including colorectal cancer, breast cancer, nasopharyngeal carcinoma and melanoma. It will be important to investigate whether the negative regulation of p53 by LIF can be observed in other types of tumors. This line of work will help us understand the therapeutic potential of targeting LIF in tumors.
Acknowledgement
W.H. is supported by the grants from NIH (1R01CA160558), the Ellison Foundation, NIH-NCI Network on Biobehavioral Pathway in Cancer, and the New Investigator Award of Rutgers Cancer Institute of New Jersey.
Footnotes
Conflict of interest: No conflicts declared.
References
- 1.Moreau JF, Donaldson DD, Bennett F, Witek-Giannotti J, Clark SC, Wong GG. Leukaemia inhibitory factor is identical to the myeloid growth factor human interleukin for DA cells. Nature. 1988;336(6200):690–692. doi: 10.1038/336690a0. Epub 1988/12/15. PubMed PMID: 3143918. [DOI] [PubMed] [Google Scholar]
- 2.Gearing DP. The leukemia inhibitory factor and its receptor. Advances in immunology. 1993;53:31–58. doi: 10.1016/s0065-2776(08)60497-6. PubMed PMID: 8512038. [DOI] [PubMed] [Google Scholar]
- 3.Sims NA, Johnson RW. Leukemia inhibitory factor: a paracrine mediator of bone metabolism. Growth factors. 2012;30(2):76–87. doi: 10.3109/08977194.2012.656760. Epub 2012/02/07. PubMed PMID: 22304408. [DOI] [PubMed] [Google Scholar]
- 4.Moon C, Yoo JY, Matarazzo V, Sung YK, Kim EJ, Ronnett GV. Leukemia inhibitory factor inhibits neuronal terminal differentiation through STAT3 activation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):9015–9020. doi: 10.1073/pnas.132131699. Epub 2002/06/27. PubMed PMID: 12084939; PubMed Central PMCID: PMC124415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirai H, Karian P, Kikyo N. Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor. The Biochemical journal. 2011;438(1):11–23. doi: 10.1042/BJ20102152. Epub 2011/07/29. PubMed PMID: 21793804; PubMed Central PMCID: PMC3418323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fry RC. The effect of leukaemia inhibitory factor (LIF) on embryogenesis. Reproduction, fertility, and development. 1992;4(4):449–458. doi: 10.1071/rd9920449. Epub 1992/01/01. PubMed PMID: 1461995. [DOI] [PubMed] [Google Scholar]
- 7.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359(6390):76–79. doi: 10.1038/359076a0. PubMed PMID: 1522892. [DOI] [PubMed] [Google Scholar]
- 8.Banner LR, Patterson PH, Allchorne A, Poole S, Woolf CJ. Leukemia inhibitory factor is an anti-inflammatory and analgesic cytokine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(14):5456–5462. doi: 10.1523/JNEUROSCI.18-14-05456.1998. Epub 1998/07/03. PubMed PMID: 9651226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450(7170):721–724. doi: 10.1038/nature05993. Epub 2007/11/30. doi: nature05993 [pii] 10.1038/nature05993. PubMed PMID: 18046411. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Yue X, Zhao Y, Li X, Wu L, Zhang C, et al. LIF negatively regulates tumour-suppressor p53 through Stat3/ID1/MDM2 in colorectal cancers. Nature communications. 2014;5:5218. doi: 10.1038/ncomms6218. PubMed PMID: 25323535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui CM, Cheung PY, Ling MT, Tsao SW, Wang X, Wong YC, et al. Id-1 promotes proliferation of p53-deficient esophageal cancer cells. [doi: 10.1002/ijc.21874];Int J Cancer. 2006 119(3):508–514. doi: 10.1002/ijc.21874. PubMed PMID: 16506209. [DOI] [PubMed] [Google Scholar]
- 12.Bond GL, Hu W, Levine AJ. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug Targets. 2005;5(1):3–8. doi: 10.2174/1568009053332627. Epub 2005/02/22. PubMed PMID: 15720184. [DOI] [PubMed] [Google Scholar]
- 13.Wysoczynski M, Miekus K, Jankowski K, Wanzeck J, Bertolone S, Janowska-Wieczorek A, et al. Leukemia inhibitory factor: a newly identified metastatic factor in rhabdomyosarcomas. Cancer Res. 2007;67(5):2131–2140. doi: 10.1158/0008-5472.CAN-06-1021. PubMed PMID: 17332343. [DOI] [PubMed] [Google Scholar]
- 14.Albrengues J, Bourget I, Pons C, Butet V, Hofman P, Tartare-Deckert S, et al. LIF mediates proinvasive activation of stromal fibroblasts in cancer. Cell reports. 2014;7(5):1664–1678. doi: 10.1016/j.celrep.2014.04.036. PubMed PMID: 24857661. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Yang Q, Yu H, Wu L, Zhao Y, Zhang C, et al. LIF promotes tumorigenesis and metastasis of breast cancer through the AKT-mTOR pathway. Oncotarget. 2014;5(3):788–801. doi: 10.18632/oncotarget.1772. PubMed PMID: 24553191; PubMed Central PMCID: PMC3996668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu SC, Tsang NM, Chiang WC, Chang KP, Hsueh C, Liang Y, et al. Leukemia inhibitory factor promotes nasopharyngeal carcinoma progression and radioresistance. J Clin Invest. 2013;123(12):5269–5283. doi: 10.1172/JCI63428. PubMed PMID: 24270418; PubMed Central PMCID: PMC3859424. [DOI] [PMC free article] [PubMed] [Google Scholar]