Abstract
A series of supramolecular calixarenes efficiently transport distinct molecular species through a liquid membrane when attached to a receptor-complementary choline handle. Calix-[6]arene hexacarboxylic acid was highly effective at transporting different target molecules against a pH gradient. Both carboxylic- and phosphonic-acid-functionalized calix[4]arenes effect transport without requiring a pH or ion gradient. NMR binding studies, two-phase solvent extraction, and three-phase transport experiments reveal the necessary and subtle parameters to effect the transport of molecules attached to a choline “handle”. On the other hand, rescorin[4]arene cavitands, which have similar guest recognition profiles, did not transport guest molecules. These developments reveal new approaches towards attempting synthetic-receptor-mediated selective small-molecule transport in vesicular and cellular systems.
Keywords: Host-guest systems, Molecular recognition, Cavitands, Calixarenes, Liquid-membrane transport, Choline conjugates
Introduction
The machinery of the human organism presents many challenges that must be addressed if the transport of several classes of molecules is to be achieved as part of the effort to treat disease. These challenges have prompted a response in the chemical community to develop new methods of drug delivery[1] The design of effective drug-transport systems hinges on optimizing a daunting array of features such as solubility, directed transport, biocompatibility, and the release of the encapsulated drug. Several successful strategies have relied on the synthesis of drug-polymer conjugates or the embedment of drugs in polymer, vesicle, or micelle carriers.[2] However, these approaches have had limited success in several crucial applications, most notably in transport across the blood–brain barrier.[3]
In 2007, we reported that hydrophobic resorcin[4]arene cavitands could function in an aqueous micellar environment as supramolecular hosts for small organic molecules.[4] This behavior was special, as these types of materials rarely adopt a functional conformation in water – instead they precipitate/dimerize into flat non-functional conformations to avoid unfavorable solvent interactions. Functionalization of the host can circumvent this constraint,[5] and many applications exist for these materials.[6] Since this report, several advances have been made, including embedding cavitands into lipid monolayers to give a surface that can selectively sequester choline conjugates, including a biotin–choline guest. This non-covalent assembly of guest-in-host-in-monolayer could then sequester NeutrAvidin.[7] We have demonstrated that such assemblies also function as pH-selective switches, influencing the orientation of the guest in the host.[8] We know too that fluorescently tagged cavitands distribute regularly in giant unilamellar vesicles (GUVs), and that these vesicles remain viable and non-permeable to their surroundings.[9] New hosts have emerged that are capable of self-micelle formation and compartmentalization of payloads.[10] Resorcinarenes have also formed ion channels in lipid environments.[11] This body of work has encouraged us to apply these results to cellular systems; it has been shown that resorcinarene cavitands can promote the endocytosis of fluorescein, so long as a complementary choline handle is attached.[12] The observation that selective endocytosis is possible is quite remarkable – a non-covalent supramolecular host–guest assembly crosses a cell membrane!
Several different calixarene species have shown successful binding with choline and tetraalkyl ammonium species in a variety of solvents based on cation–π or cation–anion attraction.[13] Alkyl ammoniums (RNH3+) bind with tert-butyl calix[4]arenes, but the larger tetraalkyl ammoniums (NR4+) require a polarizable functional group on the host's upper rim (as seen with sulfonated derivatives),[14] a larger internal cavity (e.g., a calix[5 or 6]arene), or a combination of these two features.[15] New work in the area using sulfonated derivatives that recognize trimethyllysines and disrupt protein–protein interactions is of great relevance and interest to us.[16] Other arrangements have also been successful in recognizing ammoniums, including functionalization of the lower rim with carboxylates. Phosphonate-ester-functionalized cavitands similar to 6 attracted our attention, as they have been used when embedded in lipid membranes for the detection of amino acids.[17] Pillar[n]arenes are an additional class of molecules with ammonium-binding properties.[18]
These phenomena encouraged us to examine the characteristics and properties of host–guest systems that are necessary to effect receptor-mediated transport – something quite distinct from ion-channel formation, or induction of endocytosis. Using liquid-membrane transport experiments, we have revealed some limitations in the ability of resorcinarenes to transport small molecules, and in the process we have also uncovered new leads.
Results and Discussion
We began our work using resorcin[4]arene benzimidazole cavitands 1–3, calixarenes 4–6, and pillar[n]arenes 7 and 8, all of which are known to be hosts for various small-molecule ammonium guests. Our screening partners have a guest–fluorophore conjugate structure (9–12), where a small-molecule handle is attached to a fluorophore payload (Figure 1).
Figure 1.
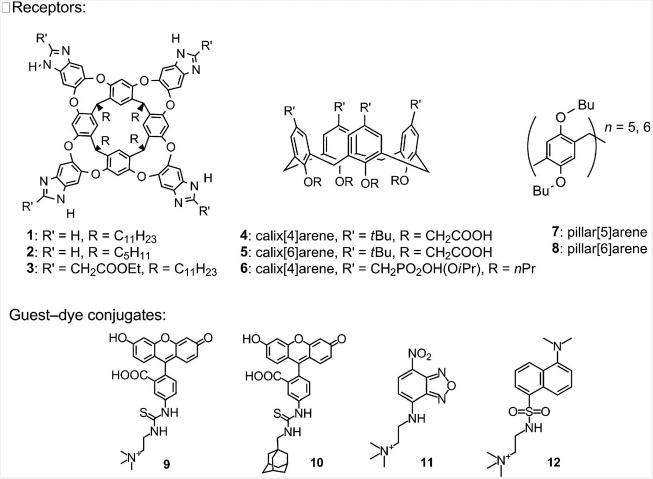
Resorcinarene cavitands 1–3, calixarenes 4–6, and pillar[n]arene hosts 7 and 8, and fluorophore-conjugated guests 9–12 used in this work.
The ethyl-footed benzimidzaole cavitand (i.e., 1, where R = ethyl) was our initial host of choice, as we have previously demonstrated that this host binds aliphatic and cationic guests in aqueous micelles, as well as in [D6]DMSO/D2O mixtures. Additionally, titration with base resulted in enhanced binding in some cases, presumably due to deprotonation of the imidazole rings and build-up of negative charge in the host.[4] Unfortunately, this host was insoluble and non-functional as a binder of 9 in organic solvents or solvent mixtures containing small amounts of [D4]MeOH. We enhanced the solubility and restored the function of the benzimidazole cavity by introducing C5 (2) or C11 (1, 3) feet. For example, host 1[19] bound FITC-choline 9 in CH2Cl2 with 10% [D4]MeOH added, as evident from the upfield trimethylammonium resonance observed around −1.5 ppm (Δδ up to −4 ppm). Resorcinarene cavitands typically bind strongly with choline handles to form slow-exchange complexes. The addition of base changed the nature of the recognition event, as monitored by NMR spectroscopy (see Supporting Information for full details). The binding of 9 with 4–6 was also observable by NMR titration, this time by fast-exchange.
The 1H NMR spectrum of upper-rim tetraphosphonic-acid-functionalized calix[4]arene 6 with FITC-chloline 9 in [D4]MeOH is shown in Figure 2. Guest 9 was sparingly soluble in methanol, but upon the addition of the host, it immediately dissolved. Very slight changes were observed in the chemical shifts of the signals of the ethylene group of 9 when the concentration of the host was varied (seen at δ = 3.7 and 4.2 ppm); the chemical shift of the trimethylammonium group changed more noticeably (δ = 3.27−3.24 ppm). The remaining signals of the host move modestly (see Supporting Information for other host–guest results). Pillar[n]-arenes have little to no solubility in MeOH. Dansyl choline 12 was far more soluble in chloroform/methanol mixtures than FITC-choline 9. Thus we combined pillar[5]arene 7 with dansyl choline 12 in chloroform/methanol, and no binding was observed (Figure 3, a, d, e). The larger internal volume of pillar[6]arene 8 accepts trimethylammoniums; broadening and upfield shift (δ = 3.0 ppm) are observed (Figure 3, a, b). Jobs plot analyses for pillar[6]arene 8 with dansyl choline 12 and calix[4]arene tetraphosphonic acid 6 with FITC-choline 9 are consistent with 1:1 binding models. Tetra acid 6 is probably engaging in ion–ion interactions, while pillarene 8, which lacks acids, binds through cation– π interactions.
Figure 2.
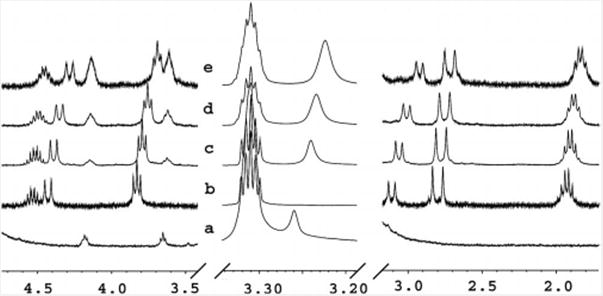
300 MHz 1H NMR spectra in 100% [D4]MeOH (δ = 3.32 ppm) of (a) FITC-choline 9, (b) host 6, (c) 1:0.5 host:guest, (d) 1:1 host:guest, (e) 1:2 host:guest.
Figure 3.
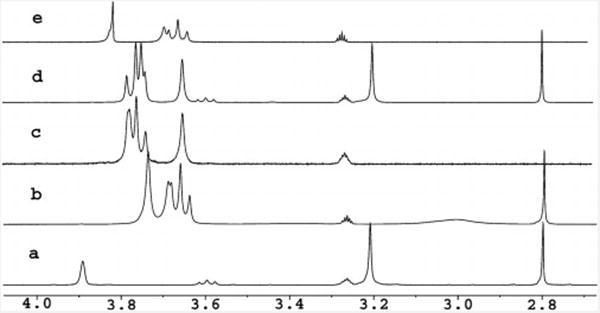
300 MHz 1H NMR spectra in CDCl3 with 20% [D4]-MeOH (δ = 3.25 ppm) of (a) guest dansyl choline 12, (b) 1:1 host 8:guest 12, (c) host 8, (d) 1:1 host 7:guest 12, (e) host 7.
At this stage, it was impossible to evaluate Ka values for hosts 4–8 in either the same solvent system as one another or with the same guest, due to severe solvent incompatibilities. Thus, we used NMR spectroscopy as a simple tool to evaluate host–guest binding in solution for a variety of representative guest–dye conjugates under the best conditions to obtain NMR spectroscopic data for each combination. To better understand the necessary properties for transport, we turned our attention to two-phase extraction experiments.
We began our two-phase extraction experiments with our hosts dissolved in CH2Cl2 and water-soluble guests. We first varied the concentration of NaOH in the aqueous layer, and measured the extraction efficiency between 9 and host 1 (Table 1), using 50 μm of 9 and 100 μm of 1. Enhanced extraction efficiency was observed when NaOH (1 m) was added to the aqueous phase (10% v/v or more).
Table 1.
Extraction of FITC-choline 9 with 1 with variable base concentration.
| Alkali volume [%]a | Extraction [%] | Extraction (control) [%] | Extraction efficiency [%]b |
|---|---|---|---|
| 0 | 0.3 | 1.0 | 0 |
| 2 | 5.2 | 1.0 | 2.1 |
| 5 | 7.4 | 2.7 | 2.3 |
| 10 | 9.9 | 1.9 | 4.0 |
| 15 | 13.1 | 2.0 | 5.6 |
| 20 | 14.9 | 2.1 | 6.4 |
Resorcinarene-based receptor 1 (100 μm) in CH2Cl2, guest 9 (50 μm) was dissolved in water containing the amount stated (% v/v) of NaOH (1 m), stirred for 24 h at 160 rpm. The resulting aqueous phase was compared to a UV/Vis calibration curve after dilution. The results are representative of multiple runs. See Exp. Sect. for full details.
Calculated as (extraction – extraction control) × [guest]/[host].
Under neutral conditions, no guest extraction was observed for this combination of host and guest. We therefore adopted a basified aqueous source phase with 10% (v/v) of NaOH (1.0 m) to compare several resorcinarene cavitands and their ability to extract 9. Tetrabenzimidazole cavitands 1 and 2 showed some extraction of 9, whereas ester 3 gave insignificant extraction (Table 2). It is notable that host 1 showed demonstrable extraction efficiency (7%) with 1 or 2 equiv. of guest present. In a large proportion of literature reports, the concentration of the guest must be of the order of 30–50 times higher than that of the host to effect measurable extraction. As previously reported, base deprotonates the acidic benzimidazole protons (pKa = 12.8), introducing a negative charge and enhancing the complementary electrostatic interaction between the positively charged guest handle and the negatively charged cavitand.[4] In this instance, it seems that the C11 feet of 1 provide a superior platform to extract 9 into an organic phase compared to 2 with its shorter C5 feet. We also carried out these extraction experiments with host 1 and FITC-adamantane 10. Under exactly the same experimental conditions, no extraction of adamantane-handled FITC from the aqueous phase into the organic phase occurred (results not shown), which suggests that the extraction of FITC-choline under alkaline conditions is in part due to an ionic interaction of the choline handle with the negatively charged cavity. This is somewhat surprising as the adamantyl handle is a good guest for cavitands like 1–3, and it is also more hydrophobic. Control experiments revealed that in the absence of a host, 9 and 10 showed similar % extraction into CH2Cl2. Thus, these guests have similar partition coefficients between CH2Cl2 and water, but different affinities for the hosts.
Table 2.
Extraction of FITC-choline 9 with cavitands 1–3 in the presence of base.
| Hosta | Guest 9 concentration [μm] | Extraction [%] | Extraction (control) [%] | Extraction efficiency [%]b |
|---|---|---|---|---|
| 1 | 10 | 14.7 | 2.4 | 1.2 |
| 25 | 13.3 | 0.9 | 3.1 | |
| 50 | 9.9 | 1.7 | 4.0 | |
| 100 | 7.4 | 0.0 | 7.4 | |
| 200 | 3.8 | 0.2 | 7.3 | |
| 2 | 10 | 17.0 | 0.0 | 1.7 |
| 25 | 13.3 | 2.2 | 2.8 | |
| 50 | 6.4 | 1.8 | 2.2 | |
| 100 | 6.9 | 3.0 | 3.9 | |
| 200 | 1.7 | 1.5 | 0.3 | |
| 3 | 10 | 0.0 | 0.0 | 0 |
| 25 | 1.4 | 2.1 | 0 | |
| 50 | 0.7 | 1.8 | 0 | |
| 100 | 2.0 | 3.0 | 0 | |
| 200 | 3.7 | 1.5 | 4.0 |
Resorcinarene-based receptors (100 μm) in CH2Cl2; guest 9 was dissolved in water containing 10% (v/v) NaOH (1 m), stirred for 24 h at 160 rpm. The resulting aqueous phase was measured using a UV/Vis calibration curve after dilution. See Exp. Sect. for full details.
Calculated as (extraction – extraction control) × [guest]/[host].
The extraction of NBD-choline 11 (200 μm) from pH 7 water was best achieved using host 1 (100 μm), which gave a modest extraction efficiency of 5.2%. NBD-choline had a much higher partition into the organic layer under the control conditions (without a host), as its fluorophore is more hydrophobic than that of 9. Hosts 2 and 3 were ineffective at extraction (results not shown), and the addition of base did not resolve this issue. Thus, variation in fluorophore composition did not have a marked effect on extraction by 1 so long as the choline handle remained constant.
Having completed these initial experiments, we attempted to study the transport of FITC-choline 9 across a liquid membrane using a U-tube apparatus. As some extraction ability had been seen with a 1:1 host:guest ratio, we began with 100 μm of host in the organic phase and 100 μm of guest in the aqueous source phase. Under a variety of conditions, guests never appeared in the receiving phase (Table 3). Under both basic (necessary for extraction efficiency) and neutral conditions, transport was unsuccessful. We next acidified the receiving phase with the hope that this would allow ion exchange and pH-driven cotransport (antiport) and/or protonation of the host, resulting in release of the guest due to a decreased binding affinity. This too was unsuccessful. Trimethylammonium chloride salts were tried, with the hope that they would facilitate release by exchange at the receiving interface, but this did not work. We noted some precipitation in these experiments at the source/organic interface and attributed this to the host. Thus, addition of THF to help redissolve the host was tried. In a last attempt, pH buffering and changing the guest to the more hydrophobic NBD–choline conjugate 11 also failed. Repetition with a higher guest concentration or a longer duration also failed. Host 3, which was unsuccessful in extraction experiments, did not work either.
Table 3.
Transport experiments with cavitands 1 and 3 and fluorophore conjugates in a liquid-membrane U-tube apparatus.
| Hosta | Guest | Source phase | Receiving phase | Transport no |
|---|---|---|---|---|
| 1 | 9 | NaOH (0.1 m) | MQ water | no |
| 1 | 9 | NaOH (0.1 m) | HCl (0.1 m) | no |
| 1 | 9 | NaOH (0.1 m) | NaCl (0.1 mm) | no |
| 1 | 9 | NaOH (0.1 m) | THF (0.1 mm in MQ water) | no |
| 1 | 9 | NaOH (0.1 m) | n-propyltrimethyl-ammonium chloride (0.1 mm in MQ water) | no |
| 1 | 9 | pH 13.26 HEPES | pH 5.94 HEPES | no |
| 1 | 11 | pH 13.26 HEPES | pH 5.94 HEPES | no |
| 1 | 9 (500 μm) | MQ water | MQ water | no |
| 1 | 9 (500 μm) | NaOH (0.1 m) | NaOH (0.1 m) | no |
| 1 | 9 (500 μm) | NaOH (0.1 m) | HCl (0.1 m) | no |
| 3 | 9 | MQ water | MQ water | no |
| 3 | 11 | MQ water | MQ water | no |
Resorcinarene-based receptors (100 μm) in CH2Cl2; guest concentration was 100 μm unless otherwise noted. After stirring for 24 h, aliquots were removed and analyzed for guest concentration by UV/Vis spectroscopy.
Under these conditions, the organic phase was evaporated, the residue was resuspended in water, and the fluorescence was measured. This gave similar results to our extraction experiments, so it is clear that the guest was partitioning into the organic layer, never to escape. The reason for the failure of these experiments to effect any measurable transport, despite the high binding affinity that these hosts show for choline or adamantane as measured by NMR spectroscopy, is unclear. The precipitation of the host at the interface, along with the presence of extracted guest in the organic phase, leaves some unanswered questions. Other guest “handles” might overcome this problem, or perhaps deep cavitands with different upper-rim functionalization might overcome these problems. Good binding does not necessarily lead to transport, so we next examined calixarene hosts 4–6, which NMR spectroscopy showed to be weak binders of choline-handle guests.
We used a host concentration of 500 μm and varied the guest concentration. Table 4 summarizes the results of extraction of FITC-choline. Calix[4]arene phosphonic acid 6 is expected to ionize and bind trimethylammoniums at the upper rim, and calix[4]arene 4, with carboxylates at the lower rim, directs ammonium binding there. Calix[4]arene phosphonic acid 6 showed better extraction efficiency than resorcianarenes in two-phase extraction experiments under neutral aqueous phase conditions (Table 4). Calixarene 4, on the other hand, showed poor extraction efficiency that did not improve with guest concentration under neutral conditions.
Table 4.
Extraction of FITC-choline 9 with different calixarenes 6 and 4 in the absence of base.
| Hosta | Guest 9 concentration [μm] | Extraction [%] | Extraction (control) [%] | Extraction efficiency[%]b |
|---|---|---|---|---|
| 6 | 10 | 37.4 ± 2.3 | 0.1 | 0.7 |
| 25 | 20.1 ± 1.8 | 0.1 | 1.0 | |
| 50 | 24.9 ± 3.8 | 1.0 | 2.4 | |
| 100 | 33.2 ± 4.5 | 1.2 | 6.4 | |
| 200 | 43.4 ± 4.1 | 1.1 | 16.9 | |
| 4 | 10 | 21.1 ± 2.5 | 0.1 | 0.4 |
| 25 | 21.6± 2.8 | 0.1 | 1.1 | |
| 50 | 25.7 ± 3.6 | 1.0 | 2.5 | |
| 100 | 10.6 ± 1.4 | 1.2 | 1.9 | |
| 200 | 6.8 ± 1.3 | 1.1 | 2.3 |
Calixarene-based receptors (500 μm) in CH2Cl2; guest 9 dissolved in water, stirred for 24 h at 160 rpm. The resulting aqueous phase was measured using a UV/Vis calibration curve after dilution, and the results are reported as an average of duplicate runs with maximum deviation from the mean. See Exp. Sect. for full details.
Calculated as (extraction – extraction control) × [guest]/[host].
We again used the three-phase experiment to evaluate guest transport mediated by 6. FITC-choline 9 and NBD-choline 11 were selected due to their different hydrophobicities and tendencies to partition into organic layers. Equal volumes from the source and receiving phases were drawn at 24-hour intervals, and were analyzed by UV/Vis spectroscopy. To our delight, the appearance of substrate in the receiving phase increased with time for both substrates (Figure 4). The receptor efficiently transports both substrates across the bulk liquid membrane. The hydrophobic NBD-choline 11 (1.0 mm source phase, 23% transported) was transported more efficiently than FITC-choline (0.5 mm source phase, 11% transported). NBD-choline had some background transport in the absence of the calixarene (5%), whereas FITC-choline had no inherent transport, due to its inability to partition into the organic phase. Thus the transport of FITC-choline is more significant.
Figure 4.
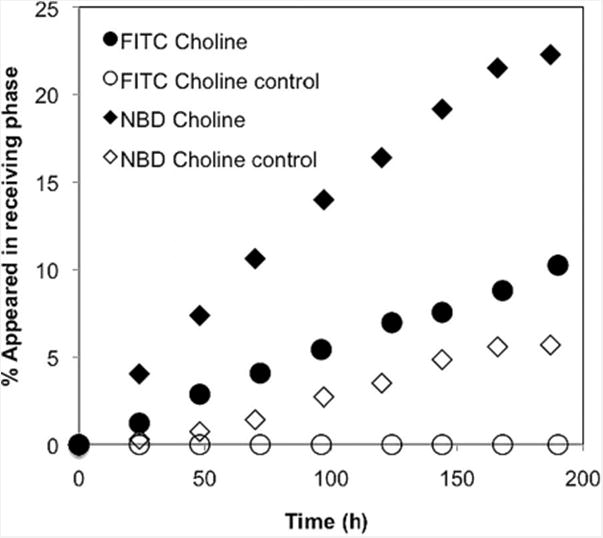
Transport of different substrates with calix[4]arene tetraphosphonic acid receptor 6 in a U-tube experiment. Source phase: MQ water (4mL), NBD-choline 11 (1 mm) and FITC-choline 9* (0.5 mm); organic phase: CH2Cl2 (10 mL), 6 (0.5 mm); receiving phase: MQ water; stirring speed = 400 rpm, room temperature, results are reported as the average of duplicate trials. For FITC-choline, the maximum deviation from the mean was ±0.3% (not shown), and for NBD-choline, it was ±0.8% (not shown). (* guest precipitation occurred at the interface when 1 mm solution was used.).
U-tube transport experiments were repeated using different substrates, and transport flux was measured (Table 5). The efficiency depends on the type of complementary guest handle and on the nature of the fluorophore. As can be seen, the rate of transport (flux/control) is FITC-choline 9 >>> NBD-choline 11 > FITC-adamantane 10 > dansyl choline 12. Comparing FITC-choline and FITC-adamantane, the substrate with the quaternary ammonium guest handle was transported more than 12 times faster than the substrate with the adamantane handle. The ionic phosphate–ammonium interaction plays a key role for transport in this system.
Table 5.
Transport flux for different guests mediated by 6 at neutral pH.
| Guesta | Transport flux [μmolcm−2min−1] | Control [μmolcm−2 min−1] |
|---|---|---|
| FITC-choline 9 | 8.9 +0.2 × 10−6 | 0.00 |
| FITC-adamantane 10 | 7.3 ± 0.2 × 10−7 | 4.36 × 10−7 |
| NBD-choline 11 | 5.6 ± 0.2 × 10−5 | 9.61 × 10−6 |
| Dansyl choline 12 | 4.6 ±0.1 × 10−5b | 5.20 × 10−5b |
| carboxyfluoresceinc | 1.6 ±0.1 × 10−5 | 1.54 × 10−5 |
| NBD chloride | 2.0 + 0.1 × 10−8 | not measuredd |
6 (500 μm) in CH2Cl2; guests (500 μm) in water, stirred for 144 h at 400 rpm. The aqueous phase was measured by UV/Vis spec-troscopy and compared to standard calibration curves after appropriate dilution. The results are reported as an average of duplicate runs with maximum deviation from the mean. See Exp. Sect. and Supporting Information for full details.
For guest 12, the flux was less than the control experiment; we determined that 6 extracts and retains the difference in the organic layer.
Carboxyfluorescein was used at 250 μm due to solubility issues at higher concentrations.
The transport rate with host 6 was so low that the control experiment was superfluous.
FITC-choline 9 had the most significant enhancement in transport based on a host–guest interaction. The thesis that a common choline handle can be used to transport hydrophilic and hydrophobic payloads has been demonstrated. Dansyl choline 12 had significant flux in the absence of 6, and we determined that the decrease in flux when calixarene 6 was present was due to 6 acting more strongly as an extraction agent than a transporter. The difference in flux was resolved by evaporation of the organic layer, resuspension in water, and UV/Vis measurement where 12 was found. Neither carboxyfluorescein nor NBD chloride was transported more in the presence of 6 than in its absence (i.e., in the control experiment), confirming that transport is a function the specific host–guest interaction of the choline handle.
The transport efficiency of calixarene carboxylic acids 4 and 5 towards FITC-choline and NBD-choline differed significantly from that of phosphonic acid 6. The ion–ion interaction now takes place at the lower rim of the calixarene. Calixarene tetracarboxylic acid 4 transported 9 into the receiving phase at similar rates, independent of the pH of the receiving phase (Table 6). NBD-choline 11 had an accelerated flux when the receiving phase was acidified. Transport requires the presence of ionizable groups, because the corresponding tetraester of 4 gave no transport of 9 (data not shown). Calixarene 4 exists in a well-defined conformation, and it appears that this has some advantages for transport because when calix[6]arene hexacarboxylic acid 5 was evaluated for the transport of 9 under neutral conditions, no transport was observed. Acidification of the receiving phase restored modest transport.
Table 6.
Transport flux for 9 and 11, mediated by calixarenes 4 and 5, as a function of pH.
| Hosta | Guest | Receiving phase | Transport flux [μmolcm−2min−1] | Control [μmolcm−2 min−1] |
|---|---|---|---|---|
| 4 | 9 | MQ water | 1.6 × 10−5 | 0 |
| 9 | 0.1 m HCl | 1.2 × 10−5b | 0 | |
| 11 | MQ water | 3.9 × 10−5 | 1.3 × 10−5 | |
| 11 | 0.1 m HCl | 9.4 × 10−5 | 1.3 × 10−5 | |
| 5 | 9 | MQ water | 0 | 0 |
| 9 | 0.1 m HCl | 1.9 × 10−6b | 0 | |
| 11 | MQ water | 3.1 × 10−6 | 1.3 × 10−5 | |
| 11 | 0.1 m HCl | 2.6 × 10−4 | 1.3 × 10–5 |
Calixarene-based receptors (500 μm) in CH2Cl2; guests were dissolved in water, stirred for 96 h at 400 rpm. The receiving phase was measured using a UV/Vis calibration curve after dilution.
A separate calibration curve was used for the acidified receiving phase due to changes in the absorbance of 9.
When guest 11 was studied with calix[6]arene hexacarboxylic acid 5 using a neutral receiving phase, less of the guest was evident in the receiving phase than under control conditions – the organic phase became highly colored, indicating efficient extraction without transport. Over time, precipitation occurred at the source phase interface. Measuring as percent transported, 17% of source substrate 11 was extracted into the organic phase under neutral conditions, but only 8% was transported to the receiving phase. The remaining 9% was found in the organic layer upon evaporation and resuspension in water. Upon acidification of the receiving phase, the flux improved 84-fold. Compared to a control experiment without 5, the flux was 20 times higher. Additionally, the decrease in source-phase absorbance resulted in a quantitative appearance of 11 in the receiving phase (21%), so guest partitioning into the organic phase under acidic conditions became negligible.
We examined pillar[6]arene 8, a neutral host that demonstrated binding affinity for the choline handle with guest 12, but showed no transporting ability under a variety of conditions. In light of the NMR binding experiments (Figure 3), it is clear that ion–ion interactions are the key feature of hosts 4–6 that facilitate transport.
A complete picture of transport by calixarene 6 first involves extraction of the cationic guest into the organic layer. This must be accompanied by charge balance – either choline is cotransported with Cl–, or ionization of one of the phosphonic acids takes place, resulting in an ion–ion interaction and sequestration into the organic phase (Figure 5). It is also possible that the guest ionizes if it contains a FITC moiety (i.e., 9, 10) – but since NBD, which lacks an ionizable group, is transported, this is not a necessary condition, and the host is probably playing the dominant role. Delivery into a receiving phase would require a similar charge balance, and one possibility is that phosphonate anion becomes protonated, and the choline handle is balanced by hydroxide. We estimate the pKa of this type of phosphonate monoester to be 2–3, thus ionization at the source phase is assured. We have not ruled out the alternative cotransport of Cl–, but our results with 4 and 5 strongly support the hypothesis of host ionization. Charge neutrality is maintained by host ionization with the ammonium group, and the inability of the corresponding esters to transport guests is consistent with this analysis.
Figure 5.
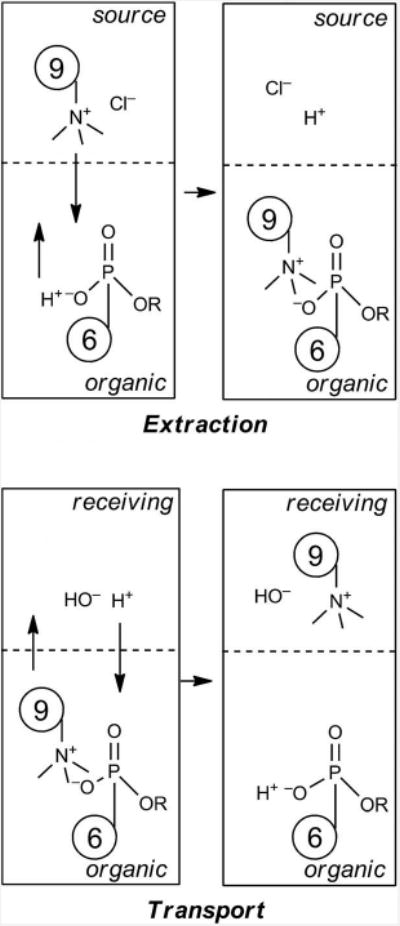
Charge balance for the extraction of guest 9 and transport by 6.
When calix[6]arenecarboxylic acid 5 is used with guest 11 extraction is possible under neutral conditions, but not transport, unless there is a pH gradient large enough to protonate the corresponding acetate anion (Figure 6). It appears that the conformational differences play a significant role in the need for a proton gradient. Efficient transport for calix[4]arene tetraphosphonic acid 6 occurred at neutral pH. The pH had a small effect for calix[4]arenecarboxylic acid 4. Calix[6]arene 5, however, required a pH gradient, the introduction of which resulted in a complete change in behavior of the system. Indeed, ion–ion interactions must be at work as the corresponding hexaester did not show extraction or transport of NBD-choline 11. Thus, it is unlikely that Cl– cotransport is operating, and that this can resolve the issue of charge balance – ionizable groups are essential here too.
Figure 6.
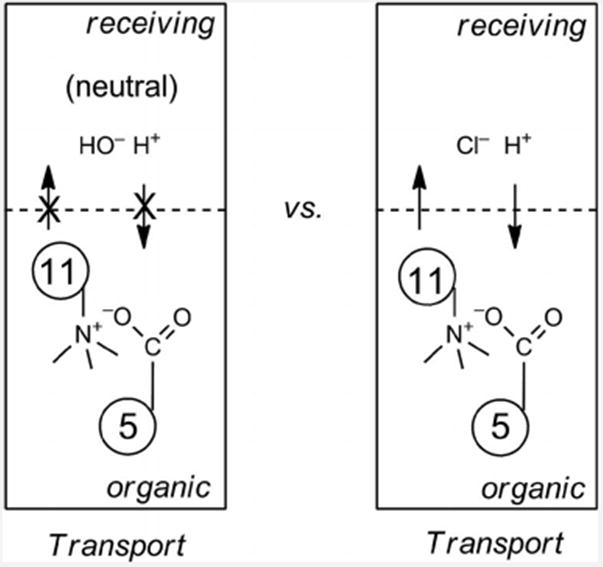
Acidification of the receiving phase with HCl accelerates transport of 11 by 5 over 84-fold.
Conclusions
Calix[6]arenecarboxylic acids have previously been shown to transport cationic amino acids[20] through liquid membranes, and more interestingly multiple calixarenes facilitate the transport of polycationic cytochrome C.[21] Under acidic conditions, calix[6]arene hexacarboxylic acid 5 transports 4.7 times more guest 9 than 6. More interesting to us is the ability of 4 and 6 to carry out transport under neutral conditions. For tetracarboxylic acid 4, perhaps the well-defined conformation imposed by the calix[4]arene ring has some advantages for proton exchange at the receiving phase – or simply the overall energetics of host–guest interaction are just right. Tetraphosphonic acid 6, substituted at the upper rim, also has a well-defined conformation, and may also allow some limited cation–π interactions to take place. In addition, the lower pKa compared to carboxylic acids would clearly result in faster deprotonation at the source interface, but the host is nevertheless likely to be reprotonated at the receiving phase. Alternatively, Cl– cotransport could be at work, or there could be some solubility advantages to a rigid calix[4]arene scaffold or solubility advantages to phosponic– monoesters in the organic phase. These issues remain to be resolved. The conformation of calix[6]arene hexacarboxylic acid 5 is ill defined in organic solvents, yet with an acidified receiving phase, it has a transport flux an order of magnitude higher than 4 or 6. The ease of ionization of these hosts helps us to understand why resorcinarene cavitands 1–3 failed to transport. These cavitands have ionizable protons, as demonstrated in previous reports, but either they are not readily ionized under the current conditions, or perhaps their binding is too strong – giving rise to extraction, but not delivery. The variety of payloads we have reported is small, but the principle of host–guest conjugate transport has been demonstrated. A variety of choline–drug conjugates are currently being examined to better understand the limits to this approach. The application of these results in vesicle and cellular systems will be reported in the sequel.
Experimental Section
Extraction Experiments
These experiments were carried out in 20 mL glass vials. The organic phase consisted of a solution of the receptor in CH2Cl2 (100 μm for resorcinarene-based receptors, and 500 μm for calixarene-based receptors), and the aqueous phase consisted of a solution of guests in MilliQ water. The guest concentration was varied from 10–200 μm. Equal volumes (2 mL each) of organic and aqueous phases were mixed in the vial, and the mixture was stirred at 160 rpm for 24 h. After cessation of stirring and phase separation, samples from aqueous phase were analyzed with a UV/Vis spectrophotometer to determine the fraction transported into the organic phase. The experiments were conducted in duplicate, and the results presented are averages. Control experiments without a host molecule were carried out.
U-tube Transport Experiments
These experiments were carried out using a U-shaped glass tube as shown in Figure 7. The organic phase consisted of a solution of the cavitand in CH2Cl2 (10 mL; 100 μm for resorcinarene-based cavitands, and 500 μm for calixarene-based cavitands). The source phase consisted of an aqueous solution (4 mL) of a guest molecule in either MilliQ water, NaOH (0.1 m), or HEPES buffer solution (pH 13.26). The receiving phase was either pure MilliQ water or HCl (0.1 m). For solutions with a competing guest, MilliQ water or HEPES buffer solution (pH 5.94) was used. The guest concentration in the source phase was 0.5 mm, except in an experiment to determine the percentage transport of NBD-choline as a function of time, in which case the guest concentration was 1.0 mm. The U-tube's upper ends were sealed, and the experiments were started by stirring the organic phase with a magnetic stirring bar at 400 rpm. In experiments to determine the transport flux, samples from the source phase and the receiving phase were drawn after 144 h, and analyzed with a UV/Vis spectrophotometer after adequate dilution with MilliQ water. In experiments to determine the percentage transport of a guest molecule as a function of time, equal volumes of samples (250 μL) were drawn from the source phase and the receiving phase at 24 h intervals, and analyzed with a UV/Vis spectrophotometer after dilution. Two sets of experiments were conducted, and the data presented are the average values. Control experiments using pure CH2Cl2 as the organic phase were carried out.
Figure 7.
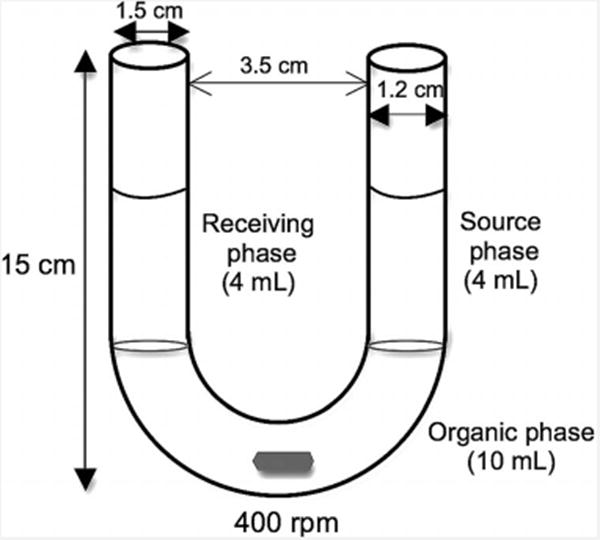
U-shaped glass dimensions and experimental parameters.
Supplementary Material
Acknowledgments
This project was supported by the National Cancer Institute (grant number SC2CA167636). The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/ejoc.201400025.
Supporting Information (see footnote on the first page of this article): Complete details for all experiments. Synthesis and characterization of all new compounds is described with NMR and MS data
References
- 1.Langer R. Science. 1990;249:1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 2.Haag R, Kratz F. Angew Chem Int Ed. 2006;45:1198–1215. doi: 10.1002/anie.200502113. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2006;118:1218–1237. [Google Scholar]
- 3.Pardridge WM. Drug Discovery Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Schramm MP, Hooley RJ, Rebek J. J Am Chem Soc. 2007;129:9773–9779. doi: 10.1021/ja0723378. [DOI] [PubMed] [Google Scholar]
- 5.Hof F, Trembleau L, Ullrich EC, Rebek J. Angew Chem Int Ed. 2003;42:3150–3153. doi: 10.1002/anie.200351174. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2003;115:3258–3261. [Google Scholar]
- 6.Biros SM, Rebek JJ. Chem Soc Rev. 2007;36:93–104. doi: 10.1039/b508530f. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Liao P, Cheng Q, Hooley RJ. J Am Chem Soc. 2010;132:10383–10390. doi: 10.1021/ja102252d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YJ, Lek MT, Schramm MP. Chem Commun. 2011;47:9636–9638. doi: 10.1039/c1cc12901e. [DOI] [PubMed] [Google Scholar]
- 9.Feher KM, Hoang H, Schramm MP. New J Chem. 2012;36:874–876. doi: 10.1039/C2NJ20961F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubitschke J, Javor S, Rebek J. Chem Commun. 2012;48:9251–9253. doi: 10.1039/c2cc34065h. [DOI] [PubMed] [Google Scholar]
- 11.Yoshino N, Satake A, Kobuke Y. Angew Chem Int Ed. 2001;40:457–459. [PubMed] [Google Scholar]; Angew Chem. 2001;113:471–473. [Google Scholar]
- 12.Ghang YJ, Schramm MP, Zhang F, Acey RA, David CN, Wilson EH, Wang Y, Cheng Q, Hooley RJ. J Am Chem Soc. 2013;135:7090–7093. doi: 10.1021/ja401273g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Späth A, König B. Beilstein J Org Chem. 2010;6:32. doi: 10.3762/bjoc.6.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinkai S, Araki K, Matsuda T, Manabe O. Bull Chem Soc Jpn. 1989;62:3856–3862. [Google Scholar]; Shinkai S, Araki K, Manabe O. J Am Chem Soc. 1988;110:7214–7215. [Google Scholar]
- 15.Koh KN, Araki K, Ikeda A, Otsuka H, Shinkai S. J Am Chem Soc. 1996;118:755–758. [Google Scholar]
- 16.Daze KD, Pinter T, Beshara CS, Ibraheem A, Minaker SA, Ma MCF, Courtemanche RJM, Campbell RE, Hof F. Chem Sci. 2012;3:2695–2699. [Google Scholar]
- 17.Zadmard R, Schrader T. J Am Chem Soc. 2005;127:904–915. doi: 10.1021/ja045785d. [DOI] [PubMed] [Google Scholar]
- 18.Ogoshi T, Kanai S, Fujinami S, Yamagishi Ta, Nakamoto Y. J Am Chem Soc. 2008;130:5022–5023. doi: 10.1021/ja711260m. [DOI] [PubMed] [Google Scholar]; Tao H, Cao D, Liu L, Kou Y, Wang L, Meier H. Sci China Chem. 2012;55:223–228. [Google Scholar]
- 19.Choi HJ, Park YS, Song J, Youn SJ, Kim HS, Kim SH, Koh K, Paek K. J Org Chem. 2005;70:5974–5981. doi: 10.1021/jo0506478. [DOI] [PubMed] [Google Scholar]
- 20.Oshima T, Inoue K, Furusaki S, Goto M. J Membr Sci. 2003;217:87–97. [Google Scholar]
- 21.Oshima T, Suetsugu A, Baba Y, Shikaze Y, Ohto K, Inoue K. J Membr Sci. 2008;307:284–291. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


