Abstract
A crude chloroform extract from the Jamaican Ball Moss (Tillandsia recurvata L.) was tested for activity against three human cancer cell lines including; A375 (human melanoma), MCF-7 (human breast) and PC-3 (human prostate cancer) using the WST-1 assay. IC50s obtained against these cell lines; A375, MCF-7 and PC-3 in the presence of the crude extract are; 0.9μg/ml, 40.51μg/ml and 5.97μg/ml respectively indicating the promising anti-cancer activity of the ball moss extract. Further, preliminary phytochemical study was conducted in an attempt to identify and isolate the phytochemicals that could possibly be responsible for the observed bioactivity of the ball moss chloroform extract. As a result, two dicinnamates were isolated; 1,3-di-O-Cinnamoyl-glycerol (1) and (E)-3-(cinnamoyloxy)-2-hydroxypropyl 3-(3,4-dimethoxyphenyl)acrylate (2) and we report for the first time isolation of compound 2. Even though the bioactivity of these two islaotes were fairly weak against the cell lines, the results presented here will prove useful for further research aimed at identifying molecules that maybe effective against melanoma, breast and prostate cancers associated with fewer side-effects.
Keywords: Jamaican Ball Moss, Tillandsia recurvata, dicinnamates, melanoma, breast cancer, prostate cancer
1.0 Introduction
Jamaica is known for its rich biodiversity and its abundance of medicinal plants used in ethno medicines. Medicinal plants continue to play a role in drug discovery and development because of the vast structural diversity of molecules found in the plant kingdom some of which some become new drugs or leads for the development of new drugs (Gordaliza, 2007). Tillandsia recurvata L. (Bromeliaceae) which is commonly called the Jamaican Ball Moss or the Old Man’s beard is one of the several important plants found in Jamaica. The plant which is an angiosperm (not a moss) is distributed throughout the island and is an epiphyte that grows abundantly mainly on Mango trees Oak trees and on power and telephone lines. The plant is characterized by the presence of a rudimentary root system and shoots having 5–8 linear leaves forming a rosette, covered with peltate, absorptive trichomes (D. H. Benzing, 1990). Older plants develop multiple ramets which form a spherical tussock ranging in size from a golf ball up to the size of a basket ball, hence the common name “ball moss” (D. Benzing, 1980; D. H. Benzing, 1990). While there is no official report that the Jamaican Ball Moss is used in Jamaican ethnomedicince, several countries have reports its use in their ethnomedicine. The major reported use is in Brazil where the plant is used against rheumatism, ulcers and hemorrhoids (Agra et al., 2008). Previous phytochemical studies showed the presence of five hydroperoxyclycloartanes, a dicinnamate, a flavanone and a caffeic acid ester from the whole plant extracts (Cabrera & Selde, 1995; de Queiroga et al., 2004). The methanol extract has been shown to possess activity against some histogenic cancer cell lines (Lowe, 2010). The objective of the present study was to confirm the anticancer activity of the Jamaican ball moss including further phytochemical analysis of the plant.
2.0 Results and Discussion
2.1 Chemistry: Structure elucidation of isolated compounds
Compounds 1 and 2 were isolated from the crude extract of T. recurvata L. using a combination of chromatographic separation methods as detailed in the materials and methods sections.
The structures of the two compounds (Figure 1) were elucidated using spectroscopic analysis including MS, IR and NMR methods. On the basis of the spectral data assignments, the two known compounds were identified as compound 1: 1,3-di-O-Cinnamoyl-glycerol (6) and compound 2: (E)-3-(cinnamoyloxy)-2-hydroxypropyl 3-(3,4- dimethoxyphenyl)acrylate (8).
Figure 1.
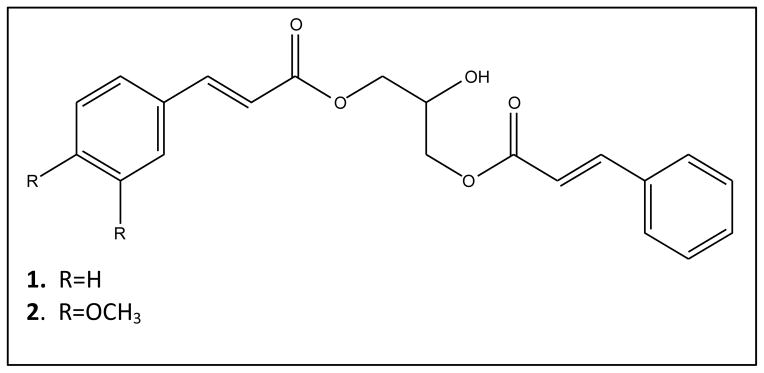
2.2 Biological assays
The potential anticancer activity of the crude extract of Ball Moss and isolated dicinnamates are presented in Table 1. The results indicate that the crude extract is more active against the 3 cell lines than compounds 1 and 2. Previous research conducted on compound 2 against PC-3 and MCF-7 cell lines reported IC50 values of 3.73μg/ml and 35.08μg/ml respectively (MacGregor, 2010) which differs from our values. In an attempt to ensure that the integrity of the cell lines were not compromised and also that laboratory techniques were aligned with literature, the experiments were repeated and a control, paclitaxel was also used against the PC-3 cell line. The obtained IC50 against PC-3 cell lines in the presence of paclitaxel compared well with literature and our repeated IC50s against the cell lines in the presence of compound 2 were fairly precise. It was not possible to draw any conclusions on structure activity relationship between compounds 1 and 2 since none of them was active at the highest concentration tested.
Table 1.
Comparative in vitro inhibitory activity of ball moss extract and compounds 1 and 2 against selected carcinomas
| IC50 (μg/mL) | ||||
|---|---|---|---|---|
| Cell line | Ball Moss CHCl3 extract | Compound 1 | Compound 2 | Paclitaxel* |
| A375 (Human melanoma) | 0.9 ± 0.40 | >35 | >41 | NT |
| MCF-7 (Human breast) | 40.51 ± 10.66 | >35 | >41 | NT |
| PC-3 (Human prostate) | 5.97 ± 4.99 | >35 | >41 | 0.0034 |
NT= Not tested
3.0 Experimental
3.1 Plant collection and preparation
The whole T. recurvata plant was collected from trees and electricity poles in Kingston, Jamaica. A voucher specimen of the plant was identified at the Institute of Jamaica Herbarium where it is deposited with Accession Number: IJ 3411. The collected plant material was air dried under shade and pulverized into a powder.
3.2 Extraction and isolation
2.3 kg of Ball Moss biomass was extracted twice with 5L of Chloroform. The filtrate was dried in a rotavapor to obtain a dark green residue (87.6g). The dark greenish residue was tested in the anticancer assay and showed activity against the PC-3 prostate cancer cell line. The crude extract was suspended in 500mL of distilled water and methanol (1:1). Aliquots of the mixture were flushed through a C18 Sep Pak flash column (10g) to get rid of the chlorophyll and other aliphatic material in the extract. The resulting solution was dried in a speedvac yielding 51.3g of a chlorophyll free fraction (F1).
A sample of F1 (40g) was subjected to flash chromatography (Varian 971-FP Intelliflash) using normal phase silica gel column (600g). The solvent gradient used was n-Hexane and ethyl acetate (10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 2:8 and 0:10). Two hundred and forty fractions of 20mL were collected and eventually pooled to 64 fractions. The 64 fractions were further pooled to 26 fractions based on their thin layer chromatography (TLC) profiles. Following the anticancer screening of the 26 fractions, fractions 7–10 (1.3g) showed similar activity and were pooled and rechromatographed on the Varian Flash Chromatography system using a reverse phase C18 column (16 g) with a Methanol/water gradient (4:6, 5:5, 6:4, 7:3, 8:2, 9:1 and 10:0) to obtain 86 fractions which were pooled to 14 fractions based on TLC analysis. Fractions 3 – 5 (34mg) and fractions 6–9 (26mg) showed major single spots on TLC analysis. Fractions 3–5 thus yielded compound 1 while fractions 6–9 yielded compound 2.
Compound 1: 1,3-di-O-Cinnamoyl-glycerol. Viscous syrup; IR (thin film, NaCl) 3456, 1713, 1168 cm−1. 1H NMR (400 MHz, CD3OD)δ 7.72 (d, J=16.04, 2H), 7.58 (m, 4H), 7.39 (m, 6H), 6.5 (d, J=16.05, 2H), 4.31 (dd, J=1.18, 3.91, 4H), 4.17 (m, 1H). 13C NMR (101 MHz, CD3OD)δ 168.5, 146.9, 135.9, 131.7, 130.2, 129.4, 118.7, 68.7, 66.7 ppm
LC/MS: Product eluted at 8.12 min, C21H20O5, exact mass 352.13, found [M+Na] = 375.0.
Compound 2: (E)-3-(cinnamoyloxy)-2-hydroxypropyl 3-(3,4-dimethoxyphenyl)acrylate; White solid, IR (thin film, NaCl) 3485, 1710, 1158, 1140 cm−1.
1H NMR (400 MHz, CD3OD); δ 7.73 (d, 1H, J=16 Hz), 7.67 (d, 1H, J=16 Hz), 7.58 (m, 1H), 7.57 (d, 1H, J=4 Hz), 7.38 (m, 3H), 7.20 (d,1H, J=1.6 Hz), 7.16 (m, 1H), 6.94 (d, 1H, J=8.4 Hz), 6.55 (d, 1H, J=16 Hz), 6.44 (d, 1H, J=16 Hz), 4.29(m, 4H), 4.16 (m, 1H), 3.85 (s, 3H), 3.84 (s, 3H);
13C NMR (101 MHz, CD3OD); δ 168.8, 168.4, 153.0, 150.8, 146.9, 146.8, 135.8, 131.7, 130.1, 129.4, 128.8, 124.2, 118.7, 116.2, 112.7, 111.7, 79.6, 68.7, 66.8 66.5, 56.7, 56.5.
LC/MS: Product eluted at 7.87 min, C23H24O7 exact mass 412.15, found [M+Na] = 435.1.
Following comparison with NMR data in the literature Compound 1 was found to have been isolated from the Ball Moss (6) and compound 2 was reportedly synthesized (MacGregor, 2010).
3.3 Biological assay
3.3.1 Cell lines and culture medium
Three human tumor cell lines (PC-3, A375 and MCF-7) were obtained from American Type Culture Collection (ATCC) (Manassas, VA, USA). The cells were maintained in minimum essential media supplemented with 10% fetal calf serum, 20 mM l-glutamine, 2% penicillin–streptomycin, and 0.2% gentamicin.
3.3.2 Anticancer cell proliferation assay
The WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1, 3-benzene disulfonate) (Roche) colorimetric assay was used (Ngamwongsatit et al., 2008). Briefly, on the day the experiment was initiated, cells were trypsinized and plated into 96 well plates in 50μl of media and incubated overnight. The next day approximately 18 hours after plating, 50μl of media containing the required drug concentration was added per well. Cells were plated at a density so that 72 hours post drug addition, the cells will be in log phase (500–2000 cells/well). The compounds and extracts were solubilized in DMSO. The cells are allowed to proliferate for 72 hours 37°C in humidified atmosphere of 5% CO2. The experiment is terminated using WST-1 (Roche) 10μl per well and absorbance is read at 450 nm/690 nm. The effect of drugs on growth is assessed as percent of cell viability. The IC50 values were determined from the extract dose versus control growth curves using Graph Prism software. All experiments were carried out in duplicate and the mean results determined.
Acknowledgments
The authors are grateful to Mr. Hieu Tran of the Institute of Human Virology for assisting with cell culture during this study and Dr. Rena Lapidus of the Translational Core of the Greenebaum Cancer Center, University of Maryland School of Medicine for validating the results of the anticancer activity of Jamaican Ball Moss.
References
- Agra M, Silva K, Basilio I, Freitas P, Barbosa-Filho J. Survey of medicinal plants used in the region Northeast of Brazil. Brazilian J Pharmacognosy. 2008;18 [Google Scholar]
- Benzing D. The biology of the bromeliads 1980 [Google Scholar]
- Benzing DH. Vascular epiphytes. Cambridge: 1990. [Google Scholar]
- Cabrera G, Selde A. Hydroperoxycycloartanes from Tillandsia recurvata. J Nat Prod. 1995;58:1920–1924. [Google Scholar]
- de Queiroga M, Andrade L, Florencio K, Fatima A, Silva M, Barbosa-Filho J, et al. Chemical constituents from Tillandsia recurvata. Fitoterapia. 2004;75:423–425. doi: 10.1016/j.fitote.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol. 2007;9:767–776. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- Lowe H. Jamaica Patent No: US Patent 2010
- MacGregor A. C. I. P. Office; 2010. [Google Scholar]
- Ngamwongsatit P, Banada PP, Panbangred W, Bhunia AK. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. Journal of Microbiological Methods. 2008;73:211–215. doi: 10.1016/j.mimet.2008.03.002. [DOI] [PubMed] [Google Scholar]


