Abstract
Introduction
We previously reported that PFS may be a candidate surrogate endpoint for OS in first-line ES-SCLC using data from 3 randomized trials (Foster, Cancer 2011). In this validation study (N0424-Alliance), we assessed the patient- and trial-level surrogacy of PFS using data from 7 new first-line phase II/III ES-SCLC trials and across all 10 trials as well (7 new, 3 previous).
Methods
Individual patient data were utilized across the 7 new trials (2259 patients) and all 10 trials (2855 patients). Patient-level surrogacy (Kendall’s τ) was assessed using the Clayton copula bivariate survival model. Trial-level surrogacy was assessed via association of the log hazard ratios on OS and PFS across trials, including weighted (by trial size) least squares regression of Cox model effects (WLS R2) and correlation of the copula effects (Copula R2). The minimum effect on the surrogate (MES) needed to detect a non-zero treatment effect on OS was also calculated.
Results
The median OS and PFS across all 10 trials were 9.8 and 5.9 months, respectively. PFS showed strong surrogacy within the 7 new trials (Copula R2 = 0.90 (SE=0.27); WLS R2 = 0.83 (95% CI: 0.43, 0.95); MES=0.67; Kendall’s τ = 0.58) and across all 10 trials (Copula R2 =0.81 (SE=0.25); WLS R2=0.77 (95% CI: 0.47–0.91); MES=0.70; Kendall’s τ =0.57).
Conclusions
PFS demonstrated strong surrogacy for OS in first-line ES-SCLC based on this external validation study of individual patient data. PFS is a good alternative endpoint to OS and should be considered when resource constraints (time or patient) might make it useful or desirable in place of OS. Additional analyses are needed to assess its appropriateness for targeted agents in this disease setting.
INTRODUCTION
Lung cancer is expected to cause 159,260 deaths within the United States in 20141. About 15% of lung cancer patients have small cell lung cancer (SCLC)2 and about 70% of patients with SCLC have extensive-stage disease (ES-SCLC).2 For patients with ES-SCLC, the current standard treatment in the first-line setting is etoposide and platinum3–6, which generally yields a median overall survival (OS) in the range of 8–12 months. Unfortunately, few dramatic improvements in ES-SCLC therapy have been made in the past 20 years7, leading to a situation where a shorter term, surrogate endpoint could make testing future therapies more efficient.
OS remains the most relevant clinical endpoint within oncology clinical trials, including ES-SCLC. Since the median OS for ES-SCLC patients is relatively short, one may wonder why it would be important to find a valid surrogate endpoint for OS in this disease. The reasons that a valid surrogate endpoint may still be important in this setting include the fact that a valid surrogate would allow a shorter follow-up time requirement for clinical trials of new agents, and the potential that effective subsequent therapies, such as topotecan8, may make it difficult to assess the true treatment effect of an agent in the first-line setting. Moreover, many phase II trials in SCLC continue to use response rate as the primary endpoint, with no supporting evidence of its association to true clinical benefit.9 A surrogate endpoint is one that can substitute for a true clinical endpoint and can predict patient outcome sooner than with the true endpoint.10–11 To demonstrate that an endpoint is a valid surrogate, it must meet two criteria. First, the surrogate endpoint must be associated with the true clinical endpoint (patient-level surrogacy) and second, the treatment effects on the surrogate endpoint must be strongly associated with the treatment effects on the true endpoint (trial-level surrogacy).10–11 If both of these criteria are met, it can be argued that the surrogate endpoint is valid and can be used in place of the true endpoint.
A PubMed literature search for trials reported over a 10 year period (2005–2014) in first-line ES-SCLC in the phase II setting showed that only 8 of the 46 published trials used PFS as the primary endpoint, with OS being used even less often (7 of 46 trials). Nearly all phase II studies over this period used response as the primary endpoint (30/46). Even in the randomized Phase II setting, response was used more often than PFS, where 7 of the 10 randomized Phase II studies used response as the primary endpoint and only 2 used PFS. We previously reported that progression-free survival (PFS) may be a candidate surrogate endpoint for OS in first-line ES-SCLC using data from 3 randomized NCCTG trials (2 – phase III; 1 – phase II).9 This prior study also demonstrated that PFS is a better predictor of OS than tumor response9, however PFS is still not routinely used as the primary endpoint in the phase II setting in ES-SCLC.
PFS is defined as the time from study registration or randomization to the first of either disease progression or death from any cause. Issues with PFS as an endpoint are well documented and discussed elsewhere.12–18 Despite the many issues with PFS, it is considered a possible surrogate endpoint for OS, as it is unaffected by subsequent therapies and could shorten the time to drug approval. Given preliminary promising evidence of PFS as a candidate surrogate endpoint for OS, we sought to formally assess the patient- and trial-level surrogacy of PFS using data from 7 additional first-line randomized phase II/III trials (2259 patients). For this analysis, individual patient data from the 7 new trials and 10 total trials (including the 3 previous trials) were utilized, which included 8 phase III and 2 phase II studies. These 10 trials (2855 patients) consisted of a series of published first-line randomized phase II/III studies conducted by the NCI-funded cooperative groups or JCOG since 1982, which represents the largest individual patient data analysis in this disease setting that includes multi-center cooperative group trials conducted within the USA, Canada and Japan.
MATERIALS AND METHODS
Data and Trial Characteristics
Individual patient data were utilized from the 7 new non-NCCTG trials (2259 patients) and all 10 randomized ES-SCLC first-line therapy trials that accrued 2855 patients between 1982 and 2007 (Table I). This included 8 phase III studies and 2 phase II studies. The radiographic scanning interval was similar across all studies, where it was generally from 3 to 6 weeks during treatment. One trial had 4 treatment arms, thus 12 total two-arm comparisons were made. OS was the primary endpoint in all phase III trials. For the phase II studies, the primary endpoint was 1-year OS rate (CALGB 30103) or response rate (NCCTG 932053), with none powered for OS (i.e. time-to-death). The randomized phase II studies were included because of the low number of available randomized trials in this disease setting. Three phase III trials and one randomized phase II trial showed a significant OS benefit for the experimental treatment vs. the control treatment (NCIC CTG BR4, JCOG 9511, NCCTG 862051, NCCTG 932053; Table II). In addition, one trial demonstrated significantly worse OS for the experimental treatment vs. the control treatment (CALGB 30103; Table II).
Table I.
Trial and Patient Characteristics
| Trial | New Trial? |
N1 | # of Arms (% exp2) |
Treatments | Phase | Period of Accrual |
Patient Characteristics | Outcomes pooled (across all arms) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| ECOG PS (%) |
Age Median (range) |
Gender (% Male) |
Median PFS (95% CI) in months |
Median OS (95% CI) in months |
||||||||
|
| ||||||||||||
| CALGB 3010338 | Yes | 54 | 2 (72.2%) | carboplatin+etoposide (+/− bcl-2) | II | 2003–04 | 0: 39% 1: 48% 2: 13% |
69 (41–81) | 50% | 6.0 (5.4–6.6) | 9.0 (7.3–10.6) | |
| CALGB 973239 | Yes | 562 | 2 (40.2%) | etoposide+cisplatin (+/− paclitaxel+G-CSF) | III | 1998–01 | 0: 30% 1: 65% 2: 5% |
62 (24–82) | 54% | 6.3 (5.9–6.5) | 10.2 (9.7–10.7) | |
| CALGB 903340 | Yes | 308 | 2 (49.4%) | etoposide (21 vs. 3-day) + cisplatin | III | 1991–93 | 0: 21% 1: 47% 2: 31% |
63 (35–82) | 69% | 6.8 (6.3–7.4) | 9.8 (8.8–10.7) | |
| NCIC CTG BR441 | Yes | 305 | 2 (50.2%) | Alternating vs. Standard therapy3 | III | 1982–85 | Not collected | 61 (34–79) | 67% | 5.6 (5.0–6.0) | 8.9 (8.1–9.7) | |
| NCIC CTG BR842 | Yes | 220 | 2 (50.0%) | CODE vs. Alternating CAV/EP4 | III | 1993–96 | 0: 29% 1: 61% 2: 10% |
59 (31–69) | 66% | 7.4 (6.7–8.0) | 11.6 (10.5–12.3) | |
| SWOG S012443 | Yes | 656 | 2 (49.7%) | cisplatin+irinotecan vs. cisplatin+etoposide | III | 2002–07 | 0: 33% 1: 67% 2: 0.5% |
63 (22–86) | 57% | 5.4 (5.0–5.7) | 9.6 (8.9–10.1) | |
| JCOG 951144 | Yes | 154 | 2 (50.0%) | cisplatin+irinotecan vs. cisplatin+etoposide | III | 1995–98 | 0: 12% 1: 77% 2: 10% |
63 (30–70) | 86% | 5.8 (5.0–6.3) | 11.5 (9.8–12.8) | |
| NCCTG 86205145 | No | 299 | 4 (73.6%) | etoposide and cisplatin (4 schedules) | III | 1987–90 | 0: 24% 1: 50% 2: 26% |
64 (34–80) | 65% | 5.7 (5.3–6.3) | 9.9 (9.4–11.0) | |
| NCCTG 89205146 | No | 237 | 2 (50.2%) | cisplatin + etoposide (+/−megestrol acetate) | III | 1990–93 | 0: 17% 1: 54% 2: 29% |
65 (30–81) | 60% | 5.9 (5.1–6.8) | 8.9 (7.9–9.6) | |
| NCCTG 93205347 | No | 60 | 2 (66.7%) | Daily vs. continuous-infusion topotecan | II | 1994–98 | 0: 32% 1: 42% 2: 27% |
66 (48–82) | 55% | 4.5 (2.7–5.9) | 10.8 (8.0–13.0) | |
Number of patients;
exp=experimental arm(s);
standard therapy: (cyclophosphamide, doxorubicin, vincristine); alternating chemo: (standard therapy alternating with etoposide and cisplatin).
CODE: cisplatin, vincristine, doxorubicin, and etoposide; Alternating CAV/EP: alternating cyclophosphamide, doxorubicin, vincristine/etoposide and cisplatin.
Table II.
Leave-one-out Surrogacy Assessment1
| Left Out Trial | Phase | Primary Endpoint | Significantly Positive for Primary Endpoint (based on published findings) | Observed HR (PFS) (experimental vs. control) (95% CI) | Observed HR (OS) (experimental vs. control) (95% CI) | Cox model Predicted HR (OS) (95% CI)2, 3 |
|---|---|---|---|---|---|---|
|
| ||||||
| 7 New Trials: | ||||||
| CALGB 30103 | II | 1-year OS | No4 | 1.93 (1.02, 3.64) | 2.14 (1.12, 4.09) | 1.93 (1.15, 3.23) |
| CALGB 9732 | III | OS | No | 0.96 (0.81, 1.13) | 0.94 (0.79, 1.11) | 0.97 (0.87, 1.09) |
| CALGB 9033 | III | OS | No | 0.85 (0.67, 1.06) | 0.90 (0.72, 1.13) | 0.84 (0.76, 0.94) |
| NCIC CTG BR4 | III | OS | Yes | 0.56 (0.44, 0.71) | 0.72 (0.57, 0.91) | 0.49 (0.41, 0.57) |
| NCIC CTG BR8 | III | OS | No | 0.92 (0.70, 1.20) | 0.90 (0.69, 1.18) | 0.93 (0.83, 1.03) |
| SWOG S0124 | III | OS | No | 0.90 (0.77, 1.05) | 0.94 (0.81, 1.10) | 0.91 (0.81, 1.01) |
| JCOG 9511 | III | OS | Yes | 0.65 (0.47, 0.90) | 0.60 (0.43, 0.83) | 0.65 (0.55, 0.76) |
| 3 Previous Trials: | ||||||
| NCCTG 862051 | III | OS | ||||
| Arm A vs. B | Yes5 | 0.77 (0.55, 1.06) | 0.74 (0.53, 1.02) | 0.76 (0.68, 0.86) | ||
| Arm C vs. B | Not reported6 | 0.79 (0.57, 1.10) | 0.73 (0.53, 1.01) | 0.80 (0.71, 0.89) | ||
| Arm D vs. B | Not reported7 | 0.87 (0.64, 1.20) | 0.99 (0.72, 1.36) | 0.87 (0.78, 0.97) | ||
| NCCTG 892051 | III | OS | No | 1.13 (0.87, 1.46) | 1.20 (0.92, 1.55) | 1.15 (0.99, 1.33) |
| NCCTG 932053 | II | Response8 | Yes8 | 0.72 (0.41, 1.25) | 0.51 (0.29, 0.90) 8 | 0.74 (0.68, 0.81) |
HR: Hazard ratio; CI: Confidence Interval
Bolded prediction intervals didn’t contain observed OS HR.
Unweighted Cox model approach showed 9/12 (75%) comparisons were accurately predicted
Prediction method used other n-1 comparisons.
This study showed a significantly negative result, where the experimental treatment had significantly worsened OS and PFS as compared to the control treatment.
The original publication reported that arm A was significantly improved as compared to Arm B for OS (p=0.04), but using more mature follow-up data showed only a borderline significant result in our study (p=0.06). The result was still very similar overall.
The original publication did not specifically report this finding, but the Kaplan-Meier analyses showed that Arms C and A had overlapping OS curves, which would support that arms C and A were similar and that arm C most likely had significantly improved OS as compared to Arm B. Our finding supports at least a borderline significant result (p=0.06).
The original publication did not specifically report this finding, but the Kaplan-Meier analyses showed that Arms D and B had overlapping OS curves. They were clearly non-significant based on the graph.
The original publication did not report the HRs or p-values for the OS/PFS findings, but our data clearly shows that OS was significantly improved in the experimental arm (vs. control) with a p-value of 0.02.
Institutional Review Boards at the study sites had previously approved these trials, and all participants provided written informed consent. This analysis was conducted under an IRB approved protocol, N0424 (Alliance). See Table I for a detailed listing of the individual trial characteristics, where the 3 NCCTG trials were reported previously.9
Statistical Methods
This study assessed the association between PFS and OS at both the patient and trial-level. First, we assessed the patient- and trial-level surrogacy of PFS using data from 7 new first-line phase II/III ES-SCLC trials to externally validate our previous findings. Subsequently, the patient- and trial-level surrogacy was also assessed across all 10 trials (including the data from the 3 previously reported trials). PFS was defined as the time from randomization to the first of either disease progression or death from any cause, where the progression status was typically based on pre-RECIST criteria (8 of 10 trials). Since we did not have the raw tumor measurement data across all studies, we were unable to convert the progression status into one specific criteria (RECIST vs. pre-RECIST). For this analysis, therefore, we used the progression status information that was collected and reported for each trial. OS was defined as the time from randomization to death due to any cause.
Patient-level surrogacy was assessed using a bivariate survival model constructed from a Clayton copula with Weibull marginal distributions, as developed by Burzykowski T, et al10 and updated by Renfro LA, et al.19 Specifically, the copula association parameter (assumed equal across trials) was transformed onto the scale of Kendall’s τ ∈ [−1,1], where a value of τ equal to 1 would indicate a perfect positive association between OS and PFS and values above 0.50 demonstrate a strong positive association between OS and PFS at the individual patient level. Trial-level surrogacy was assessed via association of the log hazard ratios on OS and PFS across trials, including weighted (by trial size) least squares regression of marginal Cox model effects (WLS R2)20–21 and weighted (by trial size) correlation of the joint copula effects (Copula R2, and associated standard errors (SE)).10,19 Both the WLS R2 and the Copula R2 values range from 0 to 1, with values close to zero suggesting poor surrogacy, and values close to 1 indicating strong surrogacy. Generally, strong trial-level surrogacy is demonstrated with R2 values of around 0.80 or higher as shown in prior studies within colorectal cancer, ovarian cancer, and non-small cell lung cancer.22 In addition, a surrogacy assessment was also conducted based on the leave-one-out prediction of the true endpoint effect (OS) given the surrogate effect (PFS) from the other trials. Specifically, the percentage of trials for which the observed treatment effect (HR) on OS fell within its predicted 95% confidence interval (CI) was calculated, based on PFS.
We also calculated the minimum treatment effect on PFS (surrogate) needed to predict a statistically significant improvement on OS (true endpoint), called the minimum effect on the surrogate (MES). This was calculated by using an un-weighted least squares regression model for the Cox model treatment effects (log HRs for PFS and OS from each trial), where the PFS treatment effects were used to predict the OS treatment effects on the log scale. For this calculation, we assumed a future trial with a size equal to the median of our trial sizes (n=187), and found the smallest effect on PFS corresponding to a 95% prediction interval for OS that excluded 0 on the negative side (e.g. upper limit of interval on the log HR scale < 0). The MES results are reported on the HR scale. Statistical analyses were performed by Alliance statisticians using SAS v9.2 (SAS Institute, Cary, NC) and R v3.0.2 software (R Foundation for Statistical Computing), where the data was locked for analysis on 9/30/2013.
RESULTS
Patient Characteristics and Outcomes
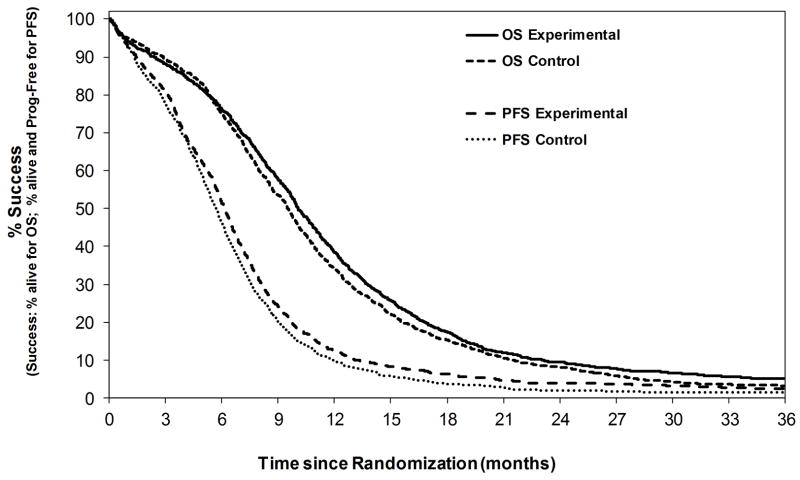
Data included a total of 2855 first-line treated patients with ES-SCLC across all 10 trials (2259 patients from 7 new trials, and 596 from 3 previous trials). The median age was 62 years (range: 22–86). Sixty-two percent were male, and 87% had a PS of 0 or 1. The median follow-up for the 94 patients still alive was 40.2 months (range, 0.3–143.8). The overall median OS and PFS was 9.8 months (95% CI, 9.6–10.1) and 5.9 months (95% CI, 5.8–6.1), respectively. Experimental treatments did slightly better overall as compared to control treatments for both OS and PFS (Figure I). Of the 2803 PFS events, there were 2287 disease progressions (82%) and 516 deaths without progression (18%). The median time from progression to death was 3.9 months (95% CI: 3.6–4.1).
Figure I.
Kaplan-Meier curves for overall survival (OS) and progression-free survival (PFS) by experimental vs. control treated patients (N=2855).
Surrogacy Results
External Validation using 7 new trials
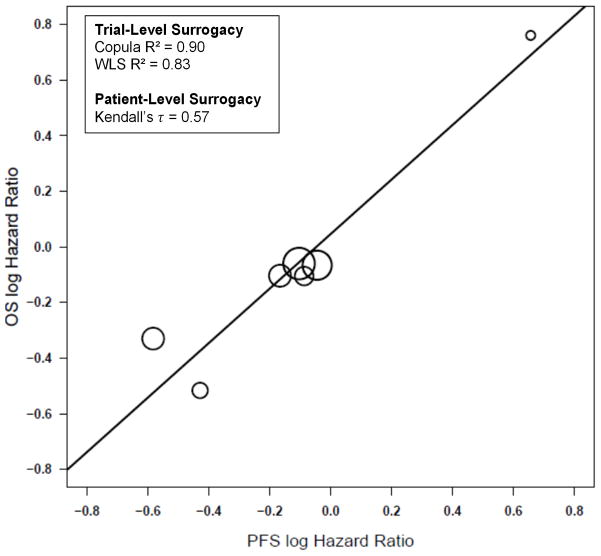
Across the 7 new trials, trial-level surrogacy results were strong, with Copula R2 =0.90 (SE=0.27) and WLS R2 = 0.83 (95% CI: 0.43, 0.95) (Figure II), with strong levels of patient-level surrogacy as well (Kendall’s τ = 0.57). The MES HR across all new trials was 0.67, which indicates that the PFS HR would need to be 0.67 or less in favor of the experimental treatment in order to show a potential benefit for OS. These 7 new trials all included a platinum/etoposide treatment combination in the first-line setting.
Figure II.
Plot of the log of the hazard ratios (HRs) for each experimental arm vs. control arm for each trial for the endpoints of progression-free survival and overall survival across the 7 new trials.
Overall results
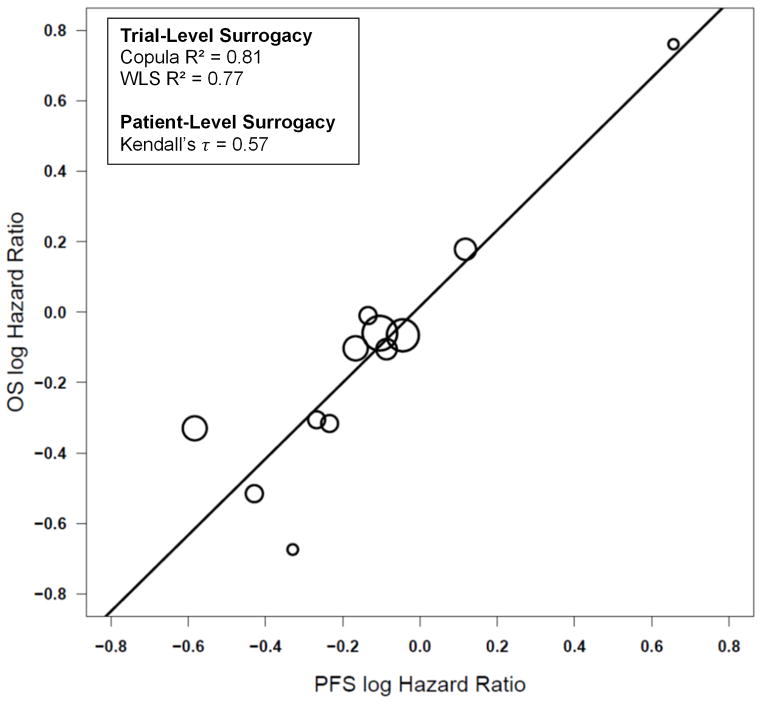
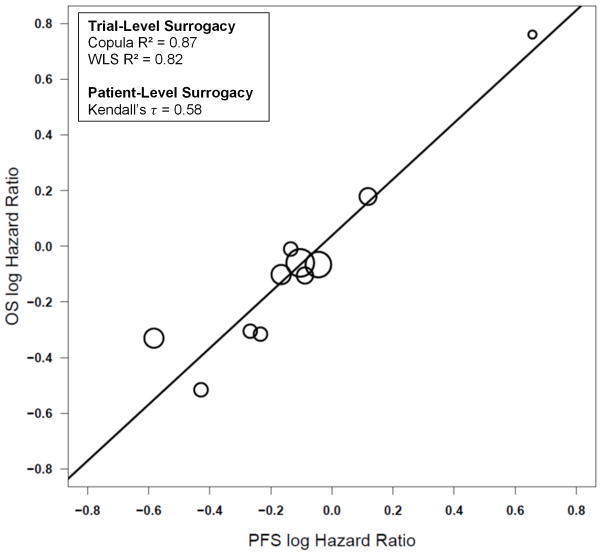
Across all 10 trials, trial-level surrogacy measures were strong, with Copula R2 =0.81 (SE=0.25) and WLS R2=0.77 (95% CI: 0.47, 0.91) (Figure III), with strong levels of patient-level surrogacy as well (Kendall’s τ =0.57). The MES HR across all trials was 0.70, which indicates that the PFS HR would need to be 0.70 or less in favor of the experimental treatment in order to show a potential benefit for OS. Strong surrogacy results were also obtained when assessing the 9 first-line studies that included a platinum/etoposide treatment combination (Copula R2 = 0.87 (SE=0.22); WLS R2 = 0.82 (95% CI: 0.49, 0.92); MES = 0.75; Kendall’s τ =0.58; Figure IV) or when assessing the 8 phase III trials only (Copula R2 = 0.78 (SE=0.30); WLS R2 = 0.76 (95% CI: 0.37, 0.91); MES = 0.73; Kendall’s τ =0.58).
Figure III.
Plot of the log of the hazard ratios (HRs) for each experimental arm vs. control arm for each trial for the endpoints of progression-free survival and overall survival across all 10 trials.
Figure IV.
Plot of the log of the hazard ratios (HRs) for each experimental arm vs. control arm for each trial for the endpoints of progression-free survival and overall survival within the 9 first-line studies that included a platinum/etoposide treatment combination.
The surrogacy assessment, across all 10 trials, using a leave-one-out prediction of each trial’s actual HR for OS based on its observed HR for PFS and joint effects from the other trials yielded a 67% prediction rate for the copula-based approach and a 75% prediction rate for the Cox model based approach, where 9 of the 12 experimental vs. control arm comparisons were accurately predicted (i.e. prediction intervals contained the observed HR for OS) based on the other n-1 comparisons (Table II). Out of the 3 outlier cases for which the predicted HR intervals failed to contain the observed OS HR, one was significant for both OS and PFS and had a predicted OS HR (95% CI) that was significant as well (NCIC CTG BR4). The other 2 outlier cases had observed OS HR confidence limits that overlapped with the predicted OS HR intervals (Table II).
DISCUSSION
In this external validation study, PFS was assessed as a formal surrogate endpoint for OS across the 7 new trials (2259 patients) and across all 10 trials as well (2855 patients). Surrogacy was measured at both the patient and trial level. Strong trial-level surrogacy results were obtained when assessing the 7 new trials only (Copula R2 = 0.90 (SE=0.27); WLS R2 = 0.83), which provided an external validation of our previously reported analysis of the 3 NCCTG studies only (Copula R2 = 0.80).9 Since the results of the 7 new and 3 previous trials were similar, we also pooled the individual patient data across all 10 trials as well. Across all 10 trials, trial-level surrogacy measures were strong (Copula R2 = 0.81 (SE=0.25); WLS R2 = 0.77), and yielded similar results when considering the 9 first-line studies that included a platinum/etoposide treatment combination (Copula R2 = 0.87 (SE=0.22); WLS R2 = 0.82) or when considering the 8 phase III trials only (Copula R2 = 0.78 (SE=0.30); WLS R2 = 0.76). Patient-level surrogacy measures were weaker (Kendall’s τ ranged from 0.57–0.58), but still showed that the correlation between OS and PFS was actually quite strong at the individual patient level, indicating that OS and PFS are definitely positively related.
Surrogacy analyses of PFS versus OS have been performed across many disease sites with mixed results.14 PFS has been shown to be a valid surrogate endpoint for OS in advanced ovarian and advanced colorectal cancer. 10, 23–25 Other disease sites, including advanced breast cancer,26–29 advanced prostate cancer30–31, advanced gastric cancer32, and advanced non–small-cell lung cancer (NSCLC)33 have not supported PFS as a surrogate endpoint for OS. Within NSCLC, PFS has been assessed as a possible surrogate endpoint for OS with conflicting conclusions. One study reported that PFS may be an acceptable surrogate for OS in future trials in advanced NSCLC. In this pooled analysis study of 2,838 patients from 7 randomized trials, the Copula R2 was 0.8334 In another meta-analysis study of 2334 NSCLC patients from 5 randomized trials, only moderate surrogacy was found with a Copula R2 of 0.62.35
In this study, the survival post-progression was short with a median of only 3.9 months and the trial-level surrogacy was found to be strong (Copula R2 = 0.90 (7 new trials); Copula R2 = 0.81 (all 10 trials)). This result is in-line with prior research that has shown that the level of surrogacy tends to be related to the survival post-progression.36 For diseases with longer survival times post-progression, the surrogacy tends to be lower (e.g., breast cancer), whereas for diseases with shorter survival times post-progression (e.g., colon cancer prior to the availability of effective multi-agent therapy) the surrogacy tends to be stronger.
Within the randomized phase II setting, PFS is definitely appropriate to use as the primary endpoint in first-line ES-SCLC for two major reasons: 1) PFS has been shown to be a better predictor of OS compared to tumor response within ES-SCLC9 and 2) most randomized phase II trials are powered to detect a large treatment effect (i.e. HR ≤ 0.70), which is similar to the MES values we observed in this study (MES = 0.67 (7 new trials); MES = 0.70 (all 10 trials)). The MES of 0.70 from this pooled analysis demonstrates that if the observed PFS hazard ratio (HR) for a study was found to be 0.70 or less in favor of the experimental treatment (vs. control), PFS would still potentially predict for a significant improvement in OS for the experimental treatment. If a strong PFS effect is observed in the phase II setting (HR ≤ 0.70), it would provide the study team with confidence to launch a larger phase III study with OS or PFS as the primary endpoint.
Despite the many strengths of this study, there are some limitations as well. First, most of the studies included were pre-RECIST (8 of the 10). Second, this study only included a series of 10 (7 new and 3 previous) published first-line trials. As such, the confidence intervals around the surrogacy estimates are wide, leaving uncertainty about the true surrogacy effect. We also stress that the trials included in this analysis, while many, are not comprehensive of all trials performed in this disease to date and that they represent a non-random subset of trials. As the data becomes available from trials utilizing newer agents and targeted therapeutics, further analysis is warranted for assessing the validity of PFS as a surrogate for OS in that setting. Third, we acknowledge that including trials that enrolled patients from 1982 until 2007 is a weakness in the analysis due to changes in workup methods and response assessment recommendations. We note however that 8 of the 10 included trials used the same criteria for assessment of progression (pre-RECIST), while the other 2 trials used RECIST criteria (CALGB 30103, SWOG S0124). In addition, this time period has shown modest gains in OS mainly due to improvements in supportive care, the use of PCI, and changes in imaging modalities (stage migration).37 Finally, this study only included older trials that generally used platinum/etoposide treatment combinations (all 7 new and 2 of 3 previous). The surrogacy results may differ when newer treatment regimens are shown to be more effective than platinum/etoposide.
In conclusion, PFS demonstrated strong potential surrogacy for OS in first-line ES-SCLC based on this individual patient data analysis, which represents the largest surrogacy analysis ever in this disease population. This study supports PFS as a potential alternative to OS as the primary endpoint in randomized trials that use platinum/etoposide regimens in first-line ES-SCLC, especially when resource constraints (time or patient) might make PFS more useful or desirable in place of OS. Given the limited difference between median PFS and OS in this setting (around 4 months), OS may still be preferred, especially in the phase III setting. Additional analyses are needed to assess appropriateness of PFS as a surrogate endpoint for targeted agents in this disease setting.
Acknowledgments
Research Support: This study was supported, in part, by grants from the National Cancer Institute to the North Central Cancer Treatment Group (CA25224) and to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair, CA31946) and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601). NCIC Clinical Trials Group is supported by funding received from the Canadian Cancer Society Research Institute (Grant #021039 and #015469) and the JCOG trial was supported by the Grant of Ministry of Health, Labour and Welfare in Japan.
Source of Funding: Nathan R. Foster M.S., Lindsay A. Renfro Ph.D., Sumithra J. Mandrekar Ph.D., and Keyue Ding Ph. D. received NCI or NIH grant support for the submitted work, Xiaofei F. Wang Ph.D. received Duke University and NIH funding for the submitted work, Suresh S. Ramalingam M.D. received consultancy money from the following companies: Abbrie, Amgen, Celgene, AstraZeneca, Ariad, Areo, Lilly, Genentech, Novartis, and BMS.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute, the National Institute of Health, or the other groups (JCOG, Canadian Cancer Society Research Institute).
Conflicts of Interest: For the remaining authors, none were declared.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014 Jan;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. [Accessed in April 2014];US National Institutes of Health website on small-cell lung cancer for health professionals. Available at http://www.cancer.gov/cancertopics/pdq/treatment/small-cell-lung/healthprofessional/page1.
- 3.Okamoto H, Watanabe K, Kunikane H, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer. 2007;97(2):162–9. doi: 10.1038/sj.bjc.6603810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol. 1992;10(2):282–91. doi: 10.1200/JCO.1992.10.2.282. [DOI] [PubMed] [Google Scholar]
- 5.Pujol JL, Carestia L, Daurès JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer. 2000;83(1):8–15. doi: 10.1054/bjoc.2000.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna N, Bunn PA, Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24(13):2038–43. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 7.Ferraldeschi R, Lorigan P. Extensive-stage small-cell lung cancer—moving beyond response rate? Ann Oncol. 2009;20 (5):801–802. doi: 10.1093/annonc/mdp247. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24(34):5441–7. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 9.Foster NR, Qi Y, Shi Q, et al. Tumor response and progression-free survival as potential surrogate endpoints for overall survival in extensive stage small-cell lung cancer: findings on the basis of North Central Cancer Treatment Group trials. Cancer. 2011 Mar 15;117(6):1262–71. doi: 10.1002/cncr.25526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burzykowski T, Molenberghs G, Buyse M, et al. Validation of surrogate endpoints in multiple randomized clinical trials with failure time endpoints. Appl Stat. 2001;50:405–22. [Google Scholar]
- 11.Buyse M, Burzykowski T, Michiels S, et al. Individual- and trial-level surrogacy in colorectal cancer. Stat Methods Med Res. 2008;17:467. doi: 10.1177/0962280207081864. [DOI] [PubMed] [Google Scholar]
- 12.Sridhara R, Mandrekar SJ, Dodd LE. Missing data and measurement variability in assessing progression-free survival endpoint in randomized clinical trials. Clin Cancer Res. 2013 May 15;19(10):2613–20. doi: 10.1158/1078-0432.CCR-12-2938. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharya S, Fyfe G, Gray RJ, et al. Role of sensitivity analyses in assessing progression-free survival in late-stage oncology trials. J Clin Oncol. 2009;27(35):5958–5964. doi: 10.1200/JCO.2009.22.4329. [DOI] [PubMed] [Google Scholar]
- 14.Booth CM, Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol. 2012;30(10):1030–1033. doi: 10.1200/JCO.2011.38.7571. [DOI] [PubMed] [Google Scholar]
- 15.Freidlin B, Korn EL, Hunsberger S, et al. Proposal for the use of progression-free survival in unblinded randomized trials. J Clin Oncol. 2007;25:2122–2126. doi: 10.1200/JCO.2006.09.6198. [DOI] [PubMed] [Google Scholar]
- 16.Panageas KS, Ben-Porat L, Dickler MN, et al. When you look matters: The effect of assessment schedule on progression-free survival. J Natl Cancer Inst. 2007;99:428–432. doi: 10.1093/jnci/djk091. [DOI] [PubMed] [Google Scholar]
- 17.Dancey JE, Dodd LE, Ford R, et al. Recommendations for the assessment of progression in randomised cancer treatment trials. Eur J Cancer. 2009;45:281–289. doi: 10.1016/j.ejca.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 18.Qi Y, Allen Ziegler KL, Hillman SL, et al. Impact of disease progression date determination on progression-free survival estimates in advanced lung cancer. Cancer. 2012 Nov 1;118(21):5358–65. doi: 10.1002/cncr.27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renfro LA, Shang H, Sargent DJ. Impact of copula directional specification on multi-trial evaluation of surrogate endpoints. J Biopharm Stat. 2014 Jun 6; doi: 10.1080/10543406.2014.920870. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23(34):8664–70. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 21.Sargent DJ, Patiyil S, Yothers G, et al. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol. 2007;25(29):4569–74. doi: 10.1200/JCO.2006.10.4323. [DOI] [PubMed] [Google Scholar]
- 22.Mauguen A, Pignon JP, Burdett S, et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: a re-analysis of meta-analyses of individual patients’ data. Lancet Oncol. 2013 Jun;14(7):619–26. doi: 10.1016/S1470-2045(13)70158-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buyse M, Burzykowski T, Carroll K, et al. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J Clin Oncol. 2007;25:5218–5224. doi: 10.1200/JCO.2007.11.8836. [DOI] [PubMed] [Google Scholar]
- 24.Tang PA, Bentzen SM, Chen EX, et al. Surrogate end points for median overall survival in metastatic colorectal cancer: Literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J Clin Oncol. 2007;25:4562–4568. doi: 10.1200/JCO.2006.08.1935. [DOI] [PubMed] [Google Scholar]
- 25.Bast RC, Thigpen JT, Arbuck SG, et al. Clinical trial endpoints in ovarian cancer: Report of an FDA/ASCO/AACR Public Workshop. Gynecol Oncol. 2007;107:173–176. doi: 10.1016/j.ygyno.2007.08.092. [DOI] [PubMed] [Google Scholar]
- 26.Sherrill B, Amonkar M, Wu Y, et al. Relationship between effects on time-to-disease progression and overall survival in studies of metastatic breast cancer. Br J Cancer. 2008;99:1572–1578. doi: 10.1038/sj.bjc.6604759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackshaw A, Knight A, Barrett-Lee P, et al. Surrogate markers and survival in women receiving first-line combination anthracycline chemotherapy for advanced breast cancer. Br J Cancer. 2005;93:1215–1221. doi: 10.1038/sj.bjc.6602858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26:1987–1992. doi: 10.1200/JCO.2007.10.8407. [DOI] [PubMed] [Google Scholar]
- 29.Miksad RA, Zietemann V, Gothe R, et al. Progression-free survival as a surrogate endpoint in advanced breast cancer. Int J Technol Assess Health Care. 2008;24:371–383. doi: 10.1017/S0266462308080495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buyse M, Vangeneugden T, Bijnens L, et al. Validation of biomarkers as surrogates for clinical endpoints. In: Bloom JC, Dean RA, editors. Biomarkers in clinical drug development. Marcel Dekker; New York, NY: 2003. pp. 149–168. [Google Scholar]
- 31.Collette L, Burzykowski T, Carroll KJ, et al. Is prostate-specific antigen a valid surrogate end point for survival in hormonally treated patients with metastatic prostate cancer? Joint research of the European Organisation for Research and Treatment of Cancer, the Limburgs Universitair Centrum, and AstraZeneca Pharmaceuticals. J Clin Oncol. 2005;23:6139–6148. doi: 10.1200/JCO.2005.08.156. [DOI] [PubMed] [Google Scholar]
- 32.Paoletti X, Oba K, Bang YJ, et al. Progression-free survival as a surrogate for overall survival in advanced/recurrent gastric cancer trials: a meta-analysis. J Natl Cancer Inst. 2013 Nov 6;105(21):1667–70. doi: 10.1093/jnci/djt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.JC, Massard C, Le CT. Should progression-free survival be the primary measure of efficacy for advanced NSCLC therapy? Ann Oncol. 2010;21:2324–2332. doi: 10.1093/annonc/mdq204. [DOI] [PubMed] [Google Scholar]
- 34.Buyse M, Squifflet P, Laporte S, et al. Prediction of survival benefits from progression-free survival in patients with advanced non-small cell lung cancer: Evidence from a pooled analysis of 2,838 patients randomized in 7 trials. J Clin Oncol. 2008 May 20;26:abstr 8019. [Google Scholar]
- 35.Laporte S, Squifflet P, Baroux N, et al. Prediction of survival benefits from progression-free survival benefits in advanced non-small-cell lung cancer: evidence from a meta-analysis of 2334 patients from 5 randomised trials. BMJ Open. 2013 Mar 13;3(3) doi: 10.1136/bmjopen-2012-001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broglio KR, Berry DA. Detecting an Overall Survival Benefit that Is Derived From Progression-Free Survival. J Natl Cancer Inst. 2009 Dec 2;101(23):1642–9. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schabath MB, Nguyen A, Wilson P, et al. Temporal trends from 1986 to 2008 in overall survival of small cell lung cancer patients. Lung Cancer. 2014 Oct;86(1):14–21. doi: 10.1016/j.lungcan.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudin CM, Salgia R, Wang X, et al. Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J Clin Oncol. 2008 Feb 20;26(6):870–6. doi: 10.1200/JCO.2007.14.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niell HB, Herndon JE, 2nd, Miller AA, et al. Randomized phase III intergroup trial of etoposide and cisplatin with or without paclitaxel and granulocyte colony-stimulating factor in patients with extensive-stage small-cell lung cancer: Cancer and Leukemia Group B Trial 9732. J Clin Oncol. 2005 Jun 1;23(16):3752–9. doi: 10.1200/JCO.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 40.Miller AA, Herndon JE, II, Hollis DR, et al. Schedule dependency of 21-day oral versus 3-day intravenous etoposide in combination with intravenous cisplatin in extensive-stage small-cell lung cancer: A randomized phase III study of the Cancer and Leukemia Group B. J Clin Oncol. 1995;13:1871–1879. doi: 10.1200/JCO.1995.13.8.1871. [DOI] [PubMed] [Google Scholar]
- 41.Evans WK, Feld R, Murray N, et al. Superiority of Alternating Non-Cross-Resistant Chemotherapy in Extensive Small Cell Lung Cancer. A Multicenter, Randomized Clinical Trial by the National Cancer Institute of Canada. Annals of Internal Medicine. 1987;107:451–458. doi: 10.7326/0003-4819-107-4-451. [DOI] [PubMed] [Google Scholar]
- 42.Murray N, Livingston RB, Shepherd FA, et al. Randomized Study of CODE Versus Alternating CAV/EP for Extensive-Stage Small-Cell Lung Cancer: An Intergroup Study of the National Cancer Institute of Canada Clinical Trials Group and the Southwest Oncology Group. J Clin Oncol. 1999 Aug;17(8):2300–8. doi: 10.1200/JCO.1999.17.8.2300. [DOI] [PubMed] [Google Scholar]
- 43.Lara PN, Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27(15):2530–5. doi: 10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus Cisplatin Compared with Etoposide plus Cisplatin for Extensive Small-Cell Lung Cancer. N Engl J Med. 2002;346(2):85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 45.Maksymiuk AW, Jett JR, Earle JD, et al. Sequencing and schedule effects of cisplatin plus etoposide in small-cell lung cancer: results of a North Central Cancer Treatment Group randomized clinical trial. J Clin Oncol. 1994;12:70–76. doi: 10.1200/JCO.1994.12.1.70. [DOI] [PubMed] [Google Scholar]
- 46.Rowland KM, Jr, Loprinzi CL, Shaw EG, et al. Randomized double-blind placebo-controlled trial of cisplatin and etoposide plus megestrol acetate/placebo in extensive-stage small-cell lung cancer: a North Central Cancer Treatment Group study. J Clin Oncol. 1996;14:135–141. doi: 10.1200/JCO.1996.14.1.135. [DOI] [PubMed] [Google Scholar]
- 47.Schaefer PL, Marks RS, Mahoney MR, et al. Randomized phase II study of daily versus continuous-infusion schedules of topotecan in the treatment of extensive-stage small cell lung cancers. Am J Clin Oncol. 2003;26:236–240. doi: 10.1097/01.COC.0000018038.28645.46. [DOI] [PubMed] [Google Scholar]