Abstract
The different secondary subunits of the N-methyl-d-aspartate (NMDA) receptor each convey unique biophysical properties to the receptor complex, and may be key in determining the functional role played by NMDA receptors. In the hippocampus, the GluN2A and GluN2B subunits are particularly abundant; however, their exact roles in synaptic plasticity and behavior remain controversial. Here, we show that mice carrying a deletion for the GluN2A subunit (GluN2A−/−) demonstrate a severely compromised NMDA to AMPA receptor current ratio in granule cells from the dentate gyrus (DG), while granule cell morphology is unaltered. This deficit is accompanied by significant impairments in both LTP and LTD in the DG, whereas only minor impairments are observed in the CA1. In accordance with these hippocampal region-specific deficits, GluN2A−/− mice show impaired performance on the DG-associated task of spatial pattern separation. In contrast, GluN2A−/− mice show no deficit in temporal pattern separation, a process associated with CA1 functioning. Thus, our results establish the GluN2A subunit as a significant contributor to both bidirectional synaptic plasticity and spatial pattern separation in the DG.
Keywords: dentate gyrus, long-term depression, long-term potentiation, N-methyl-d-aspartate, NR2A
Introduction
The N-methyl-d-aspartate (NMDA) receptor, an ionotropic glutamate receptor that is expressed throughout the central nervous system, plays a key role in learning and memory (Morris et al. 1986; Staubli et al. 1989; Young et al. 1994; Tsien et al. 1996). The receptor protein is a heteromeric complex composed of 2 obligatory GluN1 (NR1) subunits and 2 regulatory GluN2 (NR2) or GluN3 (NR3) subunits. While variants of the GluN1 subunit impart subtle changes to NMDA receptor function (Traynelis et al. 1998), it is the regulatory subunits that confer dramatically different physiological properties on the receptor (Cull-Candy and Leszkiewicz 2004). In the hippocampus, a structure intimately involved in learning and memory, the GluN2A and GluN2B subunits are the most highly expressed of the known regulatory subunits (Monyer et al. 1994; Wenzel et al. 1997). Differences in the open probabilities, decay rates, and calcium transfer (Chen et al. 1999; Erreger et al. 2005) of these subunits have led to the proposal that the GluN2A and GluN2B subunits play distinct physiological roles (Liu et al. 2004, 2007; Yashiro and Philpot 2008).
The GluN2 subunits have been heavily investigated for their roles in hippocampal synaptic plasticity, specifically in the CA1 region. In the CA1, the threshold for long-term potentiation (LTP) is increased in GluN2A−/− mice (Sakimura et al. 1995; Kiyama et al. 1998), while GluN2B deletion inhibits long-term depression (LTD; Kutsuwada et al. 1996). Because of the poor subunit specificity of GluN2 antagonists, pharmacological studies experiments have thus far failed to elucidate a specific role for these subunits in LTP and LTD (Neyton and Paoletti 2006; Berberich et al. 2007; Morishita et al. 2007) despite some promising early studies (Liu et al. 2004; Massey et al. 2004). Thus, the roles of GluN2 subunits in synaptic plasticity remain unknown.
Closely neighboring the CA1 is the dentate gyrus (DG), a hippocampal region that highly expresses GluN2 subunits (Wenzel et al. 1997; Nacher et al. 2007) and prominently demonstrates NMDA receptor-dependent LTP and LTD (Morris et al. 1986; Fox et al. 2006; Vasuta et al. 2007). The DG has been proposed to play a role in the formation of separable representations of spatial relationships; interestingly, this process, often referred to as spatial pattern separation (Kesner 2007; Deng et al. 2010), has been associated with NMDA receptor expression in the DG (McHugh et al. 2007), although the involvement of specific GluN2 subunits remains unclear.
In this study, we examined how a genetic knockout of the GluN2A subunit affected both synaptic plasticity and behavioral performance on a task thought to require normal DG functioning. Our results suggest a key role for the GluN2A subunit in bidirectional synaptic plasticity and spatial pattern separation in the mouse DG.
Materials and Methods
Animals and Housing Conditions
Male wild-type (WT) and GluN2A knockout (GluN2A−/−) mice with a C57BL/6 background, as characterized previously (Sakimura et al. 1995; Townsend et al. 2003), were housed in standard cages in a colony maintained at 21 °C. Animals were maintained on a 12-h light/dark cycle with access to food and water ad libitum. All procedures were approved by the University of Victoria Animal Care Committee and in accordance with the Canadian Council on Animal Care.
Electrophysiology
Electrophysiology Tissue Preparation
Adult male animals (2–4 months) were anesthetized with isoflurane and rapidly decapitated. The brains were removed and immersed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 125 NaCl, 2.50 KCl, 1.25 NaH2PO4, 25.0 NaHCO3, 2.00 CaCl2, 1.30 MgCl2, and 10.0 dextrose, oxygenated with 95%O2/5%CO2. Transverse hippocampal slices (350 μm) were generated using a Vibratome Sectioning System 1500 (Ted Pella, Redding, CA, USA). For whole-cell patch-clamp experiments, transcardial perfusions performed prior to decapitation (Magee et al. 1995), and tissue slicing were conducted using ice-cold choline ACSF containing (in mM): 2.50 KCl, 1.25 NaH2PO4, 25.0 NaHCO3, 0.500 CaCl2, 7.00 MgCl2, 20.0 dextrose, 1.30 ascorbate acid, 2.40 sodium pyruvate, and 110 choline chloride. Slices were gently transferred to an incubation chamber filled with oxygenated ACSF and maintained at 30 °C for a minimum of 1 h postdissection. Slices were transferred to a recording chamber and superfused at a rate of 1–2 mL/min with 30 °C, oxygenated ACSF. A concentric bipolar stimulating electrode (FHC, Bowdoin, ME, USA) was placed under visual guidance into either the medial aspect of the DG molecular layer for DG experiments or the stratum radiatum of the CA1 for CA1 experiments, using an Olympus BX51W1 microscope (Center Valley, PA, USA).
Field Electrophysiology
Borosilicate recording electrodes (0.7–1.5 MΩ) filled with ACSF were placed in either the medial aspect of the DG molecular layer or the stratum radiatum of the CA1. The inputs from the CA3 to CA1 were severed prior to CA1 experiments. Field excitatory postsynaptic potentials (fEPSPs) were evoked using monophasic negative current pulses (120 μs, 10–80 μA) supplied via a digital stimulus isolation unit (Getting Instruments, San Diego, CA, USA). Responses were acquired at 100 kHz using a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA).
For each slice, stimulus intensity was adjusted to yield 50–55% of the maximal response slope (without population spikes). Baseline measurements were collected using individual fEPSPs evoked every 15 s. After a steady baseline of 20 min, a conditioning protocol was used to induce synaptic plasticity, followed by baseline measurements for 1 h. In order to investigate the capacity for LTP in the DG, 2 high-frequency stimulation (HFS) conditioning protocols were used: A weak HFS (wHFS) protocol (2 bursts of 50 pulses, delivered at 100 Hz; 2 × 100 Hz, 30 s intertrain interval), and a strong HFS (sHFS) protocol (4 bursts of 50 pulses, delivered at 100 Hz; 4 × 100 Hz, 30 s intertrain interval). Both HFS protocols were conducted with a doubled pulse width and in the presence of bicuculline methiodide (BMI, 5 µM, Sigma-Aldrich, MO, USA), to block inhibition from γ-aminobutyric acid A (GABAA) receptors and facilitate LTP induction in the DG (Wigstrom and Gustafsson 1983). LTP in the CA1 was evoked using similar protocols, but in the absence of BMI. LTD was induced using a low-frequency stimulation (LFS) conditioning protocol (900 pulses delivered at 1 Hz; Trommer et al. 1996). To verify conditioning protocols-induced NMDA receptor-dependent forms of synaptic plasticity, a series of experiments was conducted using the NMDA receptor antagonist 2-amino-5-phosphovalerate (APV, 50 µM, Sigma-Aldrich). Recordings were analyzed using Clampfit 10.2 (Molecular Devices). Potentiation or depression was quantified by examining the average of the last 10 min of the postconditioning baseline (50–60 min).
Input–output curves and assays to examine presynaptic mechanisms were examined in separate, naive slices. Input–output curves were generated by measuring the slope of fEPSPs in response to increasing stimulus pulse widths (30–300 µs). Paired-pulse plasticity was assayed by stimulating the medial perforant path with paired stimuli at 25, 50, 100, 200, and 500 ms interstimulus intervals. The paired-pulse plasticity ratio was calculated by dividing the second fEPSP slope with the first fEPSP slope. The number of readily releasable pool vesicles was examined by measuring the decay rate of a series of fEPSPs evoked with a brief HFS protocol (50 pulses, delivered at 100 Hz) in the presence of APV (50 µM) and BMI (5 µM) as described (Cabin et al. 2002; Woo et al. 2005). To determine the rate at which the reserve pool of vesicles replenishes the readily releasable pool, a repetitive stimulation protocol (300 pulses, delivered at 14 Hz) was administered in the presence of APV (50 µM) and BMI (5 µM), as previously described (Cabin et al. 2002; Woo et al. 2005).
Whole-Cell Electrophysiology
For whole-cell recordings, recording electrodes (4–8 MΩ) were backfilled with an internal recording solution consisting of (in mM): 120 Cs-gluconate, 17.5 CsCl, 0.100 EGTA, 5.00 QX-314, 10.0 HEPES, 4.00 ATP, 0.300 Tris–GTP, and 14.0 phosphocreatine (pH 7.2). An Olympus BX51W1 microscope with oblique illumination was used to visually target dentate granule cells in the outer region of the dentate granular cell layer (Fig. 1A). Recordings were acquired using an Axopatch 200B (Molecular Devices), filtered at 5 kHz using a four-pole Bessel filter and digitized at 20 kHz using a Digidata 1440A (Molecular Devices).
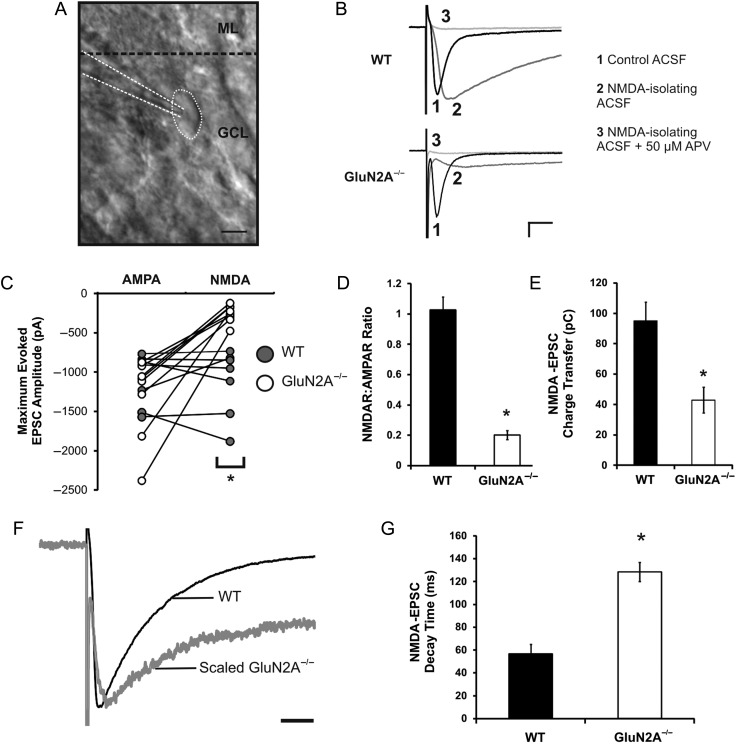
Figure 1.
Reduced NMDA:AMPA ratio and increased NMDA receptor decay rate in GluN2A−/− mice. (A) Image of a dentate granule cell, taken at ×40 magnification using oblique illumination (ML, molecular layer; GCL, granule cell layer). (B) Representative traces of EPSCs from WT and GluN2A−/− synapses, recorded in control ACSF (1), low-Mg2+ (0.1 mM) ACSF with NBQX (5 μM) and glycine (20 µM) (NMDA-isolating ACSF) (2), and low-Mg2+ ACSF with NBQX, glycine, and APV (50 μM) (NMDA-isolating ACSF + APV) (3). (C) Maximum amplitudes of AMPA and NMDA-EPSCs in WT and GluN2A−/− cells revealed a significant reduction in NMDA-EPSCs at GluN2A−/− synapses. (D) The NMDA:AMPA EPSC ratio was severely reduced at GluN2A−/− synapses. (E) Charge transfer via NMDA receptors was significantly reduced at GluN2A−/− synapses. (F) Representative traces of a GluN2A−/− NMDA-EPSC (gray), scaled to a WT NMDA-EPSC (black). (G) Weighted decay time constant of NMDA-EPSCs was significantly increased at GluN2A−/− synapses (constant generated through double exponential fit of decay phase). In all graphs, wild-type (WT): black; GluN2A−/−: white. Data are represented as mean ± SEM. *P < 0.05 denotes statistical difference. Scale bar for A = 10 μm, scale bar for B = 200 pA, 20 ms, scale bar for F = 50 ms.
The resting membrane potential was acquired immediately following successful whole-cell break-in. The current response to a 5-mV, 300-ms step (from −90 to −85 mV) was fit to a double exponential function to calculate series resistance, cell membrane capacitance, and input resistance. Only cells with series resistance values <30 MΩ were used for analysis. Pipette and whole-cell capacitances were compensated. In voltage clamp, cells were maintained at −70 mV using a holding current to measure excitatory postsynaptic currents (EPSCs). α-Amino-3-hydroxyl-5-methyl-isoazole-propionate (AMPA) receptor-mediated currents (AMPA-EPSCs) were determined by stimulating the medial aspect of the DG molecular layer while holding cells at −70 mV. NMDA receptor-mediated currents (NMDA-EPSCs) were isolated in the same cell by exchanging ACSF with low (0.1 mM)-Mg2+ ACSF in the presence of glycine (20 µM), AMPA receptor antagonist NBQX (5 µM), and BMI (5 µM).
Amplitudes and decay time constants for evoked EPSCs were analyzed using Clampfit 10.2 (Molecular Devices). Glutamate receptor amplitudes were measured as the difference between the prestimulation baseline and the evoked EPSC peak. Charge transfer was determined by integrating maximum amplitude NMDA-EPSC waveforms, from the beginning of the downward deflection to 300 ms after stimulus. Decay time constants for NMDA-EPSCs were estimated by using a double exponential fit between cursors placed just beyond the evoked EPSC peak and 300 ms after that point, and the weighted decay time constant was calculated using:
where τ is the fitted time constant and A is the amplitude.
Electrophysiology Drugs
We prepared BMI (Sigma-Aldrich) and APV (Sigma-Aldrich) as concentrated stock solutions in dH2O and diluted them with ACSF to the specified concentrations prior to each experiment. NBQX (Sigma-Aldrich) was dissolved in 0.1% DMSO for stock solutions.
Golgi Staining
Golgi Impregnation and Slice Processing
Male mice (2–4 months) were deeply anesthetized with urethane (10 mg/kg) and transcardially perfused with 0.9% saline. Brains were immediately removed and placed in vials containing 20 mL of modified Golgi–Cox solution (Gibb and Kolb 1998). This solution was replaced with fresh Golgi–Cox solution after 24 h and stored in the dark for 14 days. Brains were then transferred to 30% sucrose (in dH2O) in and stored in the dark for a maximum of 21 days. Coronal sections (200 µm) were generated throughout the length of the hippocampus using a Leica vibratome V1400 (Leica, Nussloch, Germany) in 6% sucrose (in dH2O). Sections were immediately mounted onto 2% gelatin-coated slides and processed as previously described (Redila and Christie 2006). Briefly, sections were placed in: dH2O (1 min), ammonium hydroxide (30 min), dH2O (1 min), Kodafix for film (30 min), dH2O (1 min), 50% ethanol (1 min), 70% ethanol (1 min), 95% ethanol (1 min), 100% ethanol (2 × 5 min), 100% ethanol/Citrisolv/chloroform (1:1:1 for 10 min), and then Citrisolv (2 × 15 min). After processing, all slides were coverslipped using Permount mounting medium (Fisher Scientific, Ottawa, ON, Canada) and stored in the dark.
Dendritic Analysis
All slides were coded prior to analysis, allowing the experimenter to conduct analyses blind to animal identity. Golgi-impregnated dentate granule cells were chosen from the outer 50% of the granular cell layer. Cells were selected if they fulfilled the following criteria: 1) Cells had consistent impregnation throughout the extent of the cell body and dendrites; 2) cells were distinguishable from neighboring impregnated cells; and 3) cells had intact dendritic trees. Each selected cell was traced at ×40 magnification using an Olympus BX51 microscope (Center Valley) and Retiga-2000R camera (QImaging, Surrey, BC, Canada) connected to the Neurolucida and NeuroExplorer morphometry software (MicroBrightField, Williston, VT, USA). Differences between WT and GluN2A−/− cells were assessed by examining mean total dendritic branch length, branch order, and the number of branch nodes. A Sholl analysis (Sholl 1956) was performed by quantifying the number of dendritic processes crossing concentric circles located at 10 µm intervals.
Hippocampal-Dependent Behavioral Tasks
Behavioral Apparatus
The behavioral apparatus used for the metric and temporal ordering of visual objects consisted of a rectangular box measuring 60 cm in diameter. Eight objects (a set of small glass bottles, 2 different sets of LEGO pieces, and a set of plastic bottle water lids) measuring between 2.5 and 5 cm at the base and between 5 and 15 cm tall were used as stimuli in these tasks. These objects were chosen as they are texturally and visually unique, and easily distinguishable for the mice. Between the habituation and test sessions, the mice were placed in a clean cage. The experimental box was wiped down with 70% ethanol after testing of each mouse to remove olfactory cues that could influence object exploration by subsequent mice. The metric spatial change and temporal ordering tasks have been used in transgenic mice previously (Hunsaker et al. 2009, 2010).
Metric Spatial Change Task
The metric spatial change task was composed of 1 exploration session and 1 test session. Male mice (2–4 months) were placed in the experimental box with 2 objects placed 40 cm apart. The mouse was allowed 15 min to freely explore the square and stimulus objects. After the 15-min exploration session, the mouse was removed to a clean cage for 5 min. During this intersession interval, the objects were moved closer to each other so that the objects were 10 cm apart. The mouse was then placed again in the experimental box and given 5 min to re-explore the objects during this test session.
For the 15-min exploration session, total object exploration time (s) was calculated individually for the first, middle, and last 5 min epochs to facilitate comparison between the last 5 min of the exploration session and the 5-min test session. An experimenter blind to the experimental conditions recorded active object exploration and general locomotor activity as dependent measures.
Temporal Ordering Task
The temporal ordering task was composed of 3 exploration sessions and 1 test session. Prior to exploration session 1, 2 identical copies of a first object (object 1) were placed in the extremities of the experimental box, 2.5 cm from the end walls and centered between the long walls. For exploration session 1, the mouse was placed in the center of the experimental box facing away from both objects. The mouse was given 5 min to freely explore the objects. After 5 min, the mouse was removed to a clean cage for 5 min. During this time, the first objects were replaced with 2 duplicates of a second object (object 2). The procedure for exploration session 1 was then repeated twice, with 2 duplicates of new objects each time (i.e., 2 copies of object 2 for exploration session 2 and 2 copies of object 3 for exploration session 3). After exploration session 3, the mouse was removed into a clean cage for 5 min and an unused copy of the first object and an unused copy of the third object were placed into the box. The mouse was again placed into the box and allowed to explore the 2 objects (i.e., object 1 and object 3) during a 5-min test session. For the temporal ordering task, object exploration was defined as active physical contact with the object with the forepaws, whiskers, or nose. Data were collected across all 3 object exploration sessions as well as during the test session by an experimenter blind to the mouse genotype.
Behavioral Analysis
An exploration ratio for the metric spatial change task was calculated to facilitate the comparison between the test session and the last 5 min of the exploration session, as previously described (Goodrich-Hunsaker et al. 2005). Briefly, the ratio was calculated as: [(exploration time during the 5-min test session)/(exploration time during the 5-min test session + exploration time during the last 5 min of the exploration session)]. This constrained all the values between 0 and 1. With this ratio, increased exploration during the 5-min test session compared with the last 5 min of the habituation session is reflected as a ratio >0.5, while decreased exploration (or continued habituation) is reflected as a ratio <0.5. Exploration during the temporal ordering test sessions was also converted into a ratio score to constrain the values between −1 and 1. The ratio was calculated as follows: [(exploration of object 1 − exploration of object 3)/(exploration of object 1 + exploration of object 3)].
Statistical Analysis
Computed results were processed for statistical analysis using Excel 2007 (Microsoft, Richmond, VA, USA) and Statistica 7.0 analytical software (Statsoft, Inc., Tulsa, OK, USA). For all studies, data were presented as mean ± standard error of the mean (SEM). For all electrophysiology experiments, individual Student's t-tests were performed. For behavioral experiments, exploration data that were converted to ratio values were also analyzed using individual Student's t-tests. In addition, a two-way repeated-measures ANOVA with genotype and session as factors was performed on the object exploration data in the temporal ordering task. For dendritic branch length and node number, individual unpaired, two-tail Student's t-tests were performed. A repeated-measures ANOVA was performed for Sholl analysis and branch order, using genotype and either distance from soma, or branch order, as factors for Sholl and branch-order analyses, respectively. The repeated-measures ANOVA was followed by planned comparisons of least square means between genotypes. Differences were considered to be statistically significant when P < 0.05.
Results
Reduced NMDA:AMPA Receptor Ratio in Dentate Granule Cells of GluN2A−/− Mice
Western blot analysis of NMDA receptor subunits expression in the GluN2A−/− mouse demonstrated the complete deletion of GluN2A in all regions of the hippocampus, including the DG, without influencing GluN1 or GluN2B expression (Supplementary Fig. 1). To examine if GluN2A deletion modifies the function of postsynaptic ionotropic glutamate receptors in the DG, whole-cell recordings from mature dentate granule cells were performed. Mature dentate granule cells were defined by their well-characterized hyperpolarized resting membrane potential, low input resistance, and soma location in the outer half of the dentate granular cell layer, as visualized using oblique illumination (Liu et al. 1996; Schmidt-Hieber et al. 2004; Redila and Christie 2006; Mongiat et al. 2009; Fig. 1A). There were no significant differences between AMPA-EPSCs recorded from WT and GluN2A−/− mice (mean AMPA-EPSCWT: −1096.8 ± 137.4 pA; n = 7 cells, 6 mice; mean AMPA-EPSCGluN2A−/−: −1313.4 ± 197.2 pA; n = 8 cells, 5 mice; t(13) = 0.94, P > 0.1; Fig. 1B,C). However, NMDA-EPSCs isolated from these same cells were significantly attenuated in the GluN2A−/− mice when compared with WT (mean NMDA-EPSCWT: −1123.3 ± 173.8 pA; mean NMDA-EPSCGluN2A−/−: −253.8 ± 42.4 pA; t(13) = −5.93, P < 0.0001). This effect was also evidenced as an ∼80% reduction in the NMDAR:AMPAR ratio (WT: 1.03 ± 0.08, GluN2A−/−: 0.20 ± 0.03, t(13) = 10.53, P < 0.000001; Fig. 1D)—a substantially larger reduction than the ratio observed in CA1 pyramidal cells (∼50%, WT: 0.89 ± 0.19, GluN2A−/−: 0.44 ± 0.06, data not shown).
We next examined how loss of the GluN2A subunit could alter NMDA receptor properties in dentate granule cells. NMDA-EPSC charge transfer was significantly reduced in GluN2A−/− mice (WT: 95.06 ± 12.39, GluN2A−/−: 42.96 ± 8.57, t(13) = 3.80, P < 0.005; Fig. 1E). GluN2A−/− cells also demonstrated a significantly longer NMDA-EPSC decay time constant than that observed in WT cells (WT: 56.6 ± 8.40 ms, GluN2A−/−: 128.5 ± 8.28 ms, t(13) = −6.53, P < 0.00005; Fig. 1F,G), indicative of a functional loss of GluN2A subunits (Flint et al. 1997). Together, these results demonstrate that GluN2A deletion significantly influences NMDA-EPSC kinetics in the DG.
Normal Presynaptic Properties in the DG of GluN2A−/− Mice
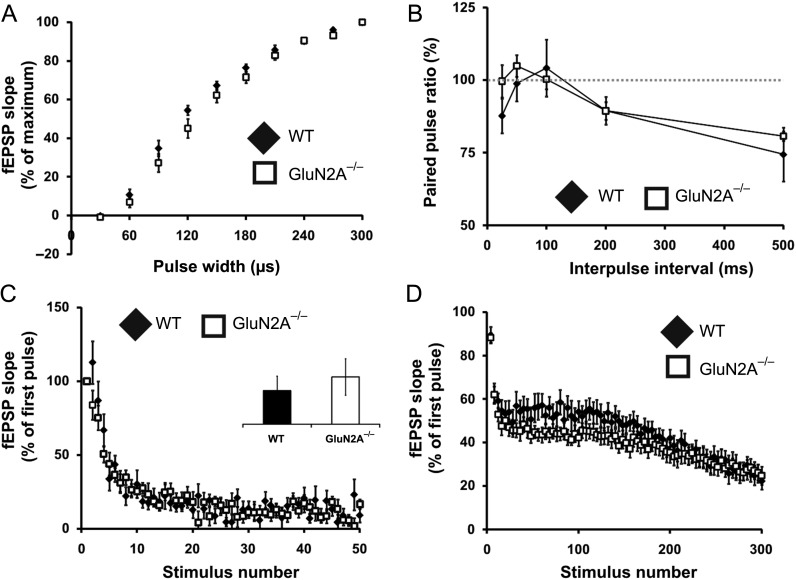
We next sought to determine if the deletion of the GluN2A subunit alters basal synaptic properties in the DG. Input–output curves for WT and GluN2A−/− mice were not significantly different, suggesting intact excitatory synaptic transmission (Fig. 2A). In addition, there was no effect of genotype observed in assays that investigated the probability of vesicular release (Fig. 2B), the number of vesicles in the readily releasable pool (Fig. 2C), or the rate of reserve vesicle pool replenishment (Fig. 2D), conducted as previously described (Cabin et al. 2002; Woo et al. 2005). Overall, these experiments indicate that presynaptic release properties are not significantly altered in the DG of GluN2A−/− mice.
Figure 2.
Synaptic transmission and presynaptic properties are normal in GluN2A−/− mice. (A) Normal input–output curve in GluN2A−/− mice. Curves were generated by plotting fEPSP slopes against stimulus pulse width. No differences were found between GluN2A−/− and their WT counterparts. (B) Normal paired-pulse activity in GluN2A−/− mice. Paired-pulse plasticity was calculated from the ratio of the second fEPSP slope to the first, at different interstimulus intervals. No differences were seen between GluN2A−/− and controls. (C) Normal responses to brief high-frequency stimulation in GluN2A−/− mice. Slices from GluN2A−/− and WT mice were supplied with 50 pulses, delivered at 100 Hz in the presence of APV (50 μM) and BMI (5 μM). No significant differences were observed between genotypes. Inset shows response at 40th stimuli. (D) Normal responses to repetitive stimulation in GluN2A−/− mice. Slices from GluN2A−/− and WT mice were supplied with 300 pulses, delivered at 14 Hz in the presence of APV (50 μM) and BMI (5 μM). No significant differences were observed between genotypes. In all graphs, wild-type (WT): black; GluN2A−/−: white. Data are represented as mean ± SEM.
Abolished LTP in the DG and Reduced LTP in the CA1 of GluN2A−/− Mice
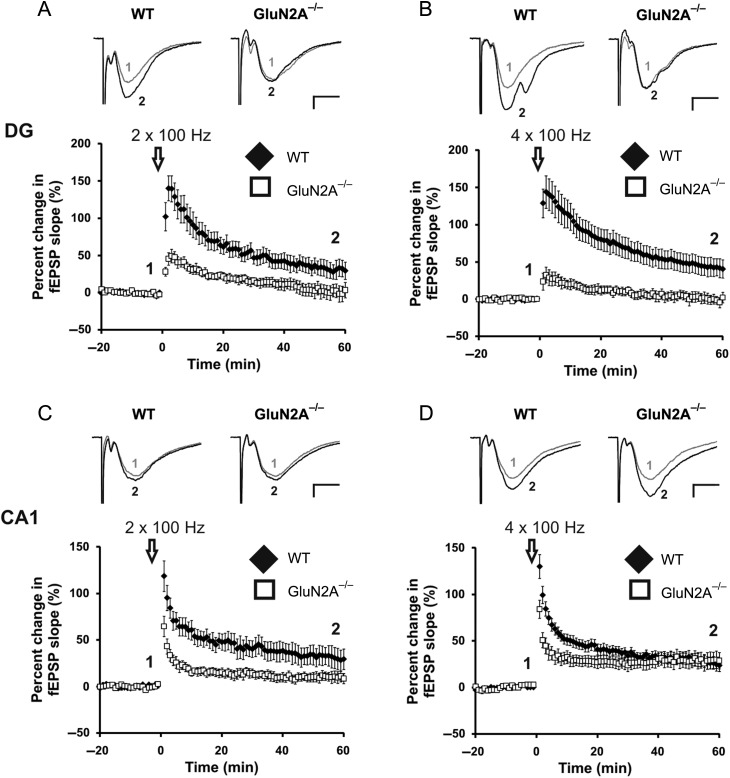
It had previously been reported that GluN2A−/− mice have reduced LTP in the CA1 region (Sakimura et al. 1995). This reduction appears to be the result of a higher threshold for induction, as LTP can be reinstated by increasing the intensity of the HFS (Kiyama et al. 1998). To test if there is a higher induction threshold for LTP in the DG, we tried inducing LTP using 2 HFS conditioning protocols: a weak (wHFS) protocol (2 × 100 Hz) and a strong (sHFS) protocol (4 × 100 Hz). The wHFS protocol produced LTP in WT mice (31.7 ± 10.9%; n = 8 slices, 7 mice), but was ineffective in GluN2A−/− mice (1.64 ± 9.47%, n = 7 slices, 7 mice, t(13) = 2.20, P < 0.05; Fig. 3A). The sHFS protocol produced robust LTP in WT mice (43.6 ± 12.2%; n = 13 slices, 7 mice), but failed to induce LTP in the GluN2A−/− DG (0.67 ± 6.65%, n = 7 slices, 5 mice, t(18) = 2.56, P < 0.05; Fig. 3B). LTP induced by the sHFS protocol was fully blocked by 50 µM APV, confirming that only NMDA receptor-dependent LTP was being induced (WT: −4.29 ± 5.38%, data not shown).
Figure 3.
GluN2A−/− mice have abolished LTP in the DG, altered LTP in the CA1. (A, B) Lack of LTP induced through a weak (2 × 100 Hz, A) and strong (4 × 100 Hz, B) HFS protocol at GluN2A−/− synapses in the DG. Representative traces from WT and GluN2A−/− DG slices, before (1) and after (2) the high-frequency conditioning protocol. (C) Partially reduced LTP induced through a weak high-frequency conditioning protocol (2 × 100 Hz) at GluN2A−/− synapses in the CA1. (D) Normal LTP induced through a strong high-frequency conditioning protocol (4 × 100 Hz) at GluN2A−/− synapses in the CA1. Representative traces from WT and GluN2A−/− CA1 slices, before (1) and after (2) the high-frequency conditioning protocols. In all graphs, wild-type (WT): black; GluN2A−/−: white. Data are represented as mean ± SEM. *P < 0.05 denotes statistical difference. Scale bar = 0.2 mV, 5 ms.
In the CA1, wHFS induced robust LTP in WT animals (31.3 ± 10.6%; n = 8 slices, 6 mice). The GluN2A−/− mice showed modest LTP (10.30 ± 5.40%, n = 9 slices, 6 mice) that was significantly less than that observed in WT animals (t(14) = 2.03 (one-tail), P < 0.05; Fig. 3C). When the sHFS protocol was employed, WT and GluN2A−/− mice showed equivalent LTP in the CA1 (WT: 27.3 ± 6.54%, n = 11 slices, 8 mice, GluN2A−/−: 29.0 ± 8.39%, n = 9 slices, 6 mice, t(18) = −0.17, P > 0.1; Fig. 3D). Together, these results demonstrate that LTP is significant impairment in the DG, but not the CA1, of adult GluN2A−/− mice.
Abolished LTD in the DG and Intact LTD in the CA1 of GluN2A−/− Mice
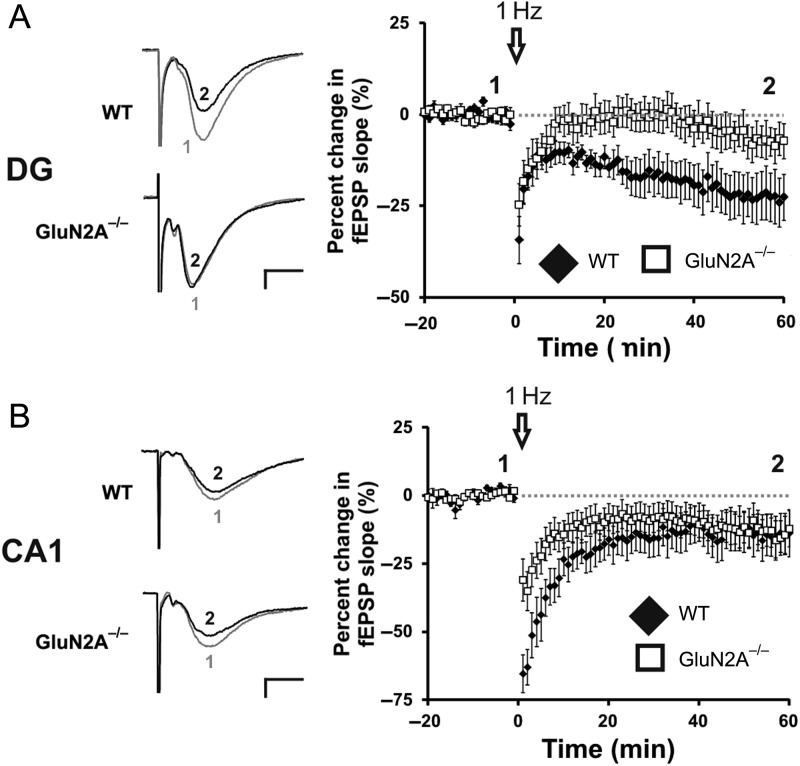
A function for the GluN2B subunit in CA1 LTD has been postulated by some (Kutsuwada et al. 1996; Liu et al. 2004; Izumi et al. 2006), but this hypothesis remains controversial (Bartlett et al. 2007; Morishita et al. 2007). We have previously shown that the GluN2A antagonist NVP-AAM077, but not the GluN2B-specfic antagonist ifenprodil, blocks LTD in the DG of animals that exercise, but not in control animals (Vasuta et al. 2007), suggesting a complex role for GluN2A subunits in LTD in this region. To further examine this issue, we induced LTD in GluN2A−/− mice using a LFS conditioning protocol that we have previously shown to be NMDA receptor-dependent (Eadie et al. 2012). This protocol produced strong LTD in WT animals (−22.6 ± 6.54%; n = 9 slices, 8 mice) but failed to induce LTD in GluN2A−/− mice (−5.03 ± 5.40%, n = 13 slices, 7 mice, t(18) = 2.21, P < 0.05; Fig. 4A). In contrast to the DG, LFS applied to the CA1 region produced equivalent LTD in both GluN2A−/− and WT mice (WT: −14.3 ± 7.43%, n = 8 slices, 5 mice; GluN2A−/−: −13.1 ± 6.23%, n = 13 slices, 6 mice, t(19) = −0.12, P > 0.1; Fig. 4B). These findings indicate that LTD is significantly impaired in the DG, but not CA1, of the GluN2A−/− mouse.
Figure 4.
GluN2A−/− mice have abolished LTD in the DG, intact LTD in the CA1. (A) Lack of LTD induced through a LFS conditioning protocol (1 Hz, 900 pulses) at GluN2A−/− synapses in the DG. Representative traces from WT and GluN2A−/− DG slices, before (1) and after (2) the low-frequency conditioning protocol. (B) Normal LTD induced through a low-frequency conditioning protocol (1 Hz, 900 pulses) at GluN2A−/− synapses in the CA1. Representative traces from WT and GluN2A−/− DG slices, before (1) and after (2) the low-frequency conditioning protocol. In all graphs, wild-type (WT): black; GluN2A−/−: white. Data are represented as means ± SEM. *P < 0.05 denotes statistical difference. Scale bar = 0.2 mV, 5 ms.
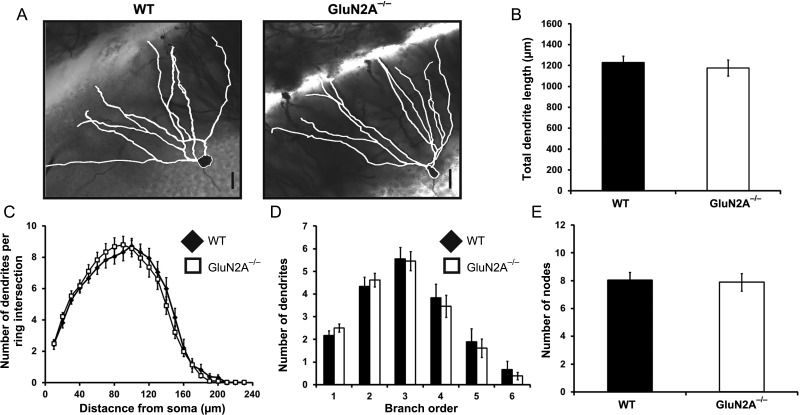
Intact Dendritic Structure of Dentate Granule Cells in GluN2A−/− Mice
Individual synaptic changes are integrated in dendrites, and are therefore greatly influenced by the morphology of the granule cell dendritic tree (Schmidt-Hieber et al. 2007). Our results show clear bidirectional synaptic plasticity impairments in the DG in the GluN2A−/− mice, and we next examined whether these impairments were due to reductions in dendritic morphology. Golgi impregnation was used to label the soma and dendritic tree of individual mature dentate granule cells in WT and GluN2A−/− mice.
Dendritic length did not differ between genotypes (WT: 1229 ± 63.25 µm, n = 18, GluN2A−/−: 1176 ± 76.92 µm, n = 26, t(42) = 0.51, P > 0.5; Fig. 5B). Several analyses were then used to examine dendritic complexity. No main effects of genotype were observed when dendritic complexity was analyzed using a Sholl analysis (F1,41 = 0.018, P > 0.5; Fig. 5C). Similarly, no effect of genotype was observed in branch order (F1,42 = 0.075, P > 0.5; Fig. 5D) or number of dendritic nodes (WT: 8.06 ± 0.542, GluN2A−/−: 7.88 ± 0.631, t(42) = 0.20, P > 0.5; Fig. 5E). Thus, the loss of the GluN2A does not impact dendritic morphology in the DG, indicating that morphological changes do not contribute to the bidirectional synaptic plasticity impairments observed in the DG of GluN2A−/− mice.
Figure 5.
Normal dendritic morphology of dentate granule cells in GluN2A−/− mice. (A) Representative light micrographs, with neurolucida-generated tracing overlay (white), of golgi-impregnated dentate granule cells, acquired at ×40 magnification. (B) GluN2A−/− cells did not show alterations in total dendritic length. (C) A Sholl analysis revealed no main effect of genotype between WT and GluN2A−/− dentate granule cells. (D) Branch-order analysis revealed no main effect of genotype between WT and GluN2A−/− dentate granule cells. (E) GluN2A−/− cells contained similar amounts of dendritic node points. In all graphs, wild-type (WT): black; GluN2A−/−: white. Data are represented as mean ± SEM. *P < 0.05 denotes statistical difference. Scale bar = 20 µm.
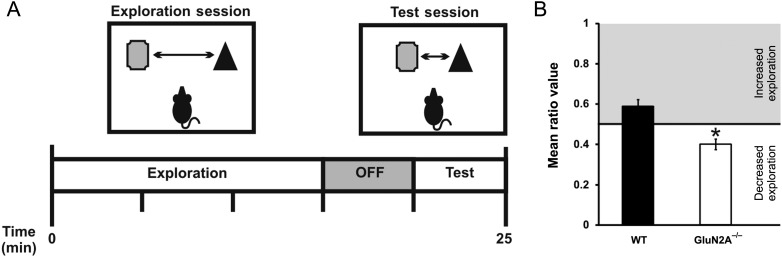
Compromised Spatial Pattern Processing in GluN2A−/− Mice
GluN2A−/− mice have previously been shown to demonstrate impairments at the Morris water maze task (Sakimura et al. 1995). This well-established task, however, is dependent on the entire hippocampus (Morris et al. 1982), and does not provide the sensitivity needed to examine hippocampal region-specific deficits. Having established reduced bidirectional synaptic plasticity only in the DG of GluN2A−/− mice, we sought to investigate if these deficits were accompanied by impairments in behaviors dependent on the DG. We tested mice on a metric spatial change task previously shown to be DG-specific (Goodrich-Hunsaker et al. 2005, 2008; Hunsaker et al. 2008). The metric spatial change task, used to examine spatial pattern processing, allows mice to explore a pair of different objects spaced 40 cm apart (Fig. 6A). Mice are then re-exposed to the environment, with the same objects moved closer together (10 cm apart). Normal rodents are able to recognize the pair of objects as being metrically displaced and re-explore the pair of objects for a significant amount of time, while rodents with impaired DG functioning fail to detect such differences and spend less time re-exploring the pair of objects (Gilbert et al. 2001; Goodrich-Hunsaker et al. 2008).
Figure 6.
Compromised spatial pattern separation in GluN2A−/− mice. (A) Behavioral paradigm for the metric spatial change task. (B) Decreased performance in metric spatial change task in GluN2A−/− mice. Mean ratio score, reflecting the proportion of time exploring the metrically displaced objects during the test session, was significantly lower in GluN2A−/− mice in comparison with controls. In graphs, wild-type (WT): black; GluN2A−/−: white. Data are represented as mean ± SEM. *P < 0.05 denotes statistical difference.
As expected, object exploration time during the 15-min exploration period decreased significantly in both WT (one-way ANOVA; F2,21 = 9.13, P < 0.01) and GluN2A−/− (one-way ANOVA; F2,21 = 12.16, P < 0.001) mice, reflecting habituation during this period. Furthermore, a two-way repeated-measures ANOVA (with genotype as the between-group factor and exploration during the three 5-min time blocks as the within-group factor) also revealed that there was no main effect of genotype (P > 0.05). This indicates that GluN2A−/− and WT mice showed similar habituation during the initial 15-min exploration period. However, the GluN2A−/− group showed significantly lower mean ratio scores, indicating that compared with controls, GluN2A−/− mice re-explored the pair of objects to a lesser extent after the metric change (mean ratioWT: 0.59 ± 0.03, n = 8, mean ratioGluN2A−/−: 0.40 ± 0.02, n = 8, t(14) = 4.04, P < 0.001, Fig. 6B). Thus, GluN2A−/− deletion was sufficient to compromise the DG-specific behavior of spatial pattern processing.
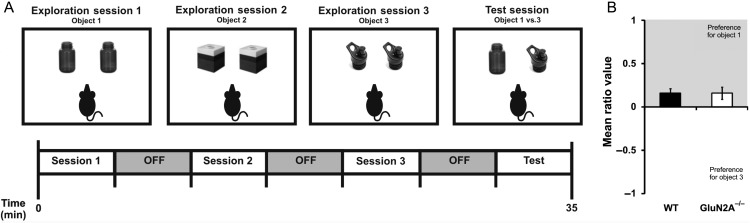
Intact Temporal Pattern Processing in GluN2A−/− Mice
To test whether there were also changes in CA1-dependent behavior in GluN2A−/− mice, we used a temporal ordering task (Fig. 7A). The temporal ordering task allows mice to explore three pairs of objects in sequence, followed by a test period, where they are exposed to the first and third object of the sequence. This task takes advantage of a rodent's tendency to explore the earlier object in a sequence of objects, if given the choice between two, and performance of this task has been attributed to CA1 functioning (Gilbert et al. 2001; Hoge and Kesner 2007). All mice similarly explored objects across sessions 1–3 and no significant differences in total object exploration during the test session were detected. Indeed, a two-way repeated-measures ANOVA with genotype (WT, GluN2A−/−) and session (session 1, session 2, session 3, test session) as factors revealed no significant main effects of genotype (F1,10 = 1.16, P > 0.1) and session (F3,10 = 1.40, P > 0.1), and no significant interaction between genotype and session (F3,30 = 0.57, P > 0.5). To determine the performance of GluN2A−/− mice in temporal ordering, a mean ratio score was calculated for both groups, with values constrained between −1 and 1. The mean ratio score for the temporal ordering task, which reflects the proportion of time spent exploring the first sequential object (object 1) during the test session, was similar between both genotypes (mean ratioWT: 0.22 ± 0.07, n = 6, mean ratioGluN2A−/−: 0.16 ± 0.05, n = 6, t(10) = 0.007, P > 0.5; Fig. 7B). Thus, loss of GluN2A had no impact on the CA1-specific behavior of temporal pattern processing.
Figure 7.
Normal temporal pattern separation in GluN2A−/− mice. (A) Behavioral paradigm for the temporal ordering task. (B) Normal performance in temporal ordering task in GluN2A−/− mice. Mean ratio score, reflecting the proportion of time spent exploring object 1 or object 3 during the test session, did not differ significantly between genotypes. In graphs, wild-type (WT): black; GluN2A−/−: white. Data are represented as mean ± SEM.
Discussion
The present study demonstrates the compromised physiological and behavioral functions of the DG in GluN2A−/− mice, and that these alterations may be different from those observed in the CA1. Specifically, we found 1) impaired bidirectional synaptic plasticity in the DG of GluN2A−/− mice, whereas minor alterations in plasticity were observed in the neighboring CA1 region; 2) diminished performance on the DG-specific metric spatial change task, but not the CA1-specific temporal ordering task in the GluN2A−/− mouse. Together, these results present a unique link between DG synaptic plasticity and behavioral function that may be dependent on NMDA receptor function.
Dentate granule cells from GluN2A−/− mice had normal AMPA-EPSCs, but reduced NMDA-EPSCs, corresponding to an ∼80% reduction in NMDA:AMPA receptor ratio. This is even greater than the change in NMDA:AMPA receptor ratio previously shown in the CA1 (Sakimura et al. 1995). Interestingly, the NMDA:AMPA receptor ratio observed in WT cells was much higher in this study in comparison with those observed by Sakimura et al. (1995) (∼43%). This discrepancy most likely reflects methodological differences in how NMDA receptor currents are isolated: factors such as holding potential, stimulation method, and extracellular Mg2+ concentration can alter the measured NMDA:AMPA receptor ratio (Myme et al. 2003). Despite these methodological differences, reduced NMDA-EPSCs and decreased NMDA:AMPA receptor ratios in GluN2A−/− mice were observed in both studies, and importantly, the observed reduction in CA1 GluN2A−/− cells was comparable.
The kinetics of the NMDA-EPSCs were drastically different in mutant mice, with decay rates in GluN2A−/− mice being more than twice that of controls. This is consistent with the reported fast decay rates of GluN2A-containing receptors (Flint et al. 1997; Bellone and Nicoll 2007; Rauner and Kohr 2011). In combination with the decreased charge transfer, the increase in the decay time constant in mutant mice drastically alters the amount and time-line of calcium influx. In mutant mice, calcium entry via NMDA receptor activation would produce an initially small, but prolonged influx of ions. This may modify the activity of several calcium-dependent enzymes including Calcium–calmodulin-dependent kinase II (CaMKII), and protein phosphatases, whose balance have been suggested to regulate synaptic plasticity (Lisman 1994; Winder and Sweatt 2001).
Using pharmacological agents, several studies have associated the GluN2A subunit with LTP in the CA1 (Liu et al. 2004; Zhang et al. 2009), DG (Vasuta et al. 2007), and other nonhippocampal regions (Massey et al. 2004; Dalton et al. 2012). However, the poor subunit specificity for the antagonists used in these studies (NVP-AAM077 and zinc) leaves these results open to question (Berberich et al. 2005; Kohr 2006; Neyton and Paoletti 2006; Paoletti and Neyton 2007; Lorca et al. 2011). Here, we use GluN2A−/− mice to show that NMDA receptor-dependent LTP is completely abolished in the DG, while confirming that LTP is only partially reduced in the CA1 (Sakimura et al. 1995; Kiyama et al. 1998). These results indicate that unlike the CA1, the DG requires the presence of the GluN2A subunit for LTP. Notably, recent evidence suggests that GluN2B-containing receptors are also required for LTP in the DG. Ifenprodil inhibits LTP induction in the DG (Vasuta et al. 2007), and GluN2B-containing receptors play a critical role in LTP in adult-born dentate granule cells (Snyder et al. 2001; Ge et al. 2007; Kheirbek et al. 2012). Together, these results indicate that both GluN2A and GluN2B are required for LTP induction in the in the DG.
In the DG, GluN2A−/− mice also demonstrated disrupted NMDA receptor-dependent LTD. As GluN2B antagonists also do not alter LTD induction in the DG (Vasuta et al. 2007), we suggest that GluN2A, and not GluN2B is required for LTD in the DG. This is in contrast to the CA1 region, where loss of the GluN2A subunit does not affect LTD, a finding consistent with previous studies in the GluN2A−/− mouse (Longordo et al. 2009). As several GluN2B antagonists also do not appear to alter LTD induction (Morishita et al. 2007), it is currently unclear whether any GluN2 subunit plays a predominant role in LTD in the CA1. Interestingly, a conditional knockout of the GluN2B subunit in the CA1 has shown to cause impairments in LTD; however, this form of plasticity was elicited with LFS in conjunction with tPDC, a glutamate transporter antagonist that was in itself sufficient to elicit LTD (Brigman et al. 2010).
Glutamate receptors, specifically AMPA and NMDA receptors, have been suggested to regulate the integration and modification of synaptic inputs (Myme et al. 2003), which might manifest through the alteration of dendritic morphology. As changes in dendritic morphology could alter synaptic plasticity and behavior, it was prudent to examine the dendritic structure of dentate granule cells in the GluN2A−/− mice. The dendritic structure was found to be largely intact, as no differences were observed in dendritic length and dendritic arbourization in GluN2A−/− mice. Our results are in contrast to hippocampal culture work that showed increases in both dendritic length and arbourization after GluN2A RNA interference (Sepulveda et al. 2010). This difference could be attributed to many factors, including cell type examined, the differences between the cell culture system and the in vivo brain experiments presented here. Work in Xenopus tadpoles also suggests a complex role for the GluN2A subunit in morphology, as both overexpression and knockdown of GluN2A expression decreases branch clustering (Ewald et al. 2008). Overall, this study and others allude to a multifaceted role of GluN2 subunits in neuronal structure, and further experiments will need to be completed before these mechanisms are fully understood.
Although it has long been known that the hippocampus is important for the acquisition of memories (Morris et al. 1982), the contributions of the different hippocampal subfields have only been investigated more recently. Targeted lesion studies have associated the CA1 region with memory encoding and temporal pattern separation, the CA3 with spatial pattern completion, and the DG with spatial pattern separation (reviewed in Kesner et al. 2004). In this study, the deficits in spatial information associated with the DG were seen in GluN2A−/− mice, while processing associated with the CA1 was intact. This result is consistent with reports of compromised rapid spatial pattern separation in mutant mice lacking the obligatory GluN1 subunit in the DG (McHugh et al. 2007). Alterations in the production of newborn neurons in the adult DG, via a process referred to as adult neurogenesis, have also been associated with spatial pattern separation (Clelland et al. 2009). Notably, adult neurogenesis is unaltered in GluN2A−/− mice (Kitamura et al. 2003). Our findings therefore provide a novel link between GluN2A-containing receptors at DG synapses and the ability to discriminate similar spatial distance.
While our observations strongly support a role for the GluN2A subunit in the spatial pattern separation, 2 important caveats should be considered. First, while the metric spatial change task has been used by others to demonstrate the capacity for spatial pattern separation in rodents (Goodrich-Hunsaker et al. 2008; Hunsaker et al. 2008), this behavioral task does not completely allow for us to truly discern if disruptions in pattern separation at the level of the hippocampal neuronal network are altered. Second, it should be noted that due to the global deletion of the GluN2A subunit, it is possible that other areas of the brain may contribute to the observed behavioral alterations in GluN2A−/− mice during the metric spatial change task. For example, a recent fMRI study showed parahippocampal activity in human participants completing a dissociating spatial retrieval task (Ekstrom et al. 2011). It is therefore possible that dysfunction in other brain areas of the GluN2A−/− mice may influence performance in the behavior tasks.
The observed results demonstrate that the loss of the GluN2A subunit considerably disrupts synaptic plasticity and spatial pattern separation in the DG, suggesting a primary role for the GluN2A subunit in DG function. However, given the major reduction in NMDA receptor response and charge transfer, it is also possible that the lack of bidirectional plasticity and behavioral deficits observed in the GluN2A−/− DG is not due to a lack of GluN2A-containing receptors, but rather a lack of synaptic NMDA receptor in the DG. Interestingly, this would suggest that synaptic NMDA receptors are predominantly GluN2A-containing in the DG. While GluN2A-containing receptors appear to be located centrally in the synapse of superior colliculus neurons (Zhao and Constantine-Paton 2007), previous work in the DG has shown the presence of both synaptic GluN2A- and GluN2B-containing NMDA receptors (Harney et al. 2008). Disruptions in plasticity in the GluN2A−/− DG may therefore be due to an alteration in the ratio of synaptic GluN2A to GluN2B subunits. Combining our observations of an ∼80% reduction in NMDA-EPSC amplitude in GluN2A−/− dentate granule cells with previous reports of an ∼28% reduction in NMDA-EPSC amplitude in dentate granule cells in response to ifenprodil (Harney et al. 2008) results in an approximate 3:1 ratio of synaptic GluN2A-containing to synaptic GluN2B-containing receptors. However, due to maximum efficiency of ifenprodil inhibition being ∼ 80% (Neyton and Paoletti 2006) and the unknown contribution of triheteromeric receptors in the dentate granule cells (Rauner and Kohr 2011; Tovar et al. 2013), the true ratio of synaptic GluN2 subunits in the DG is unclear.
Our findings implicate a common mechanism between synaptic plasticity and spatial pattern separation; however, how these two processes are related is currently unknown. It has been proposed that when an animal experiences a spatial setting or episode, combinations of afferent firing from entorhinal cortex cells arrive to the hippocampus and together act as a unique firing pattern. Dentate granule cells learn to respond to these patterns, which at a synaptic level could occur via LTP and LTD in a set of synapses. Spatial pattern separation requires the initial storage of patterns (Treves and Rolls 1992). Then loss of bidirectional plasticity would disallow proper encoding at the granule cell level, functionally disabling the “learning” processes of individual mature dentate granule cells, and producing a DG-specific behavioral deficit.
In conclusion, our results demonstrate that NMDA receptor-dependent bidirectional synaptic plasticity is abolished in the adult DG of GluN2A−/− mice. These deficits could not be attributed to alterations in presynaptic properties or synaptic transmission in the DG. Mature dentate granule cells exhibited significantly reduced NMDA receptor currents; however, their dendritic structure remained unaltered. The behavioral experiments provide evidence for spatial processing deficits in GluN2A−/− mice, specifically for processing metric relationships between objects, a process associated with DG function. We therefore conclude that both synaptic and behavioral plasticity in the adult DG are uniquely regulated by the GluN2A subunit.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada. T.S.K. and B.D.E. were supported by NSERC and CIHR awards, respectively. P.S.B. is supported by a postdoctoral fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; National Counsel of Technological and Scientific Development, Brazil). B.R.C. is supported by grants from CIHR and NSERC, and is a Michael Smith Senior Scholar.
Supplementary Material
Notes
The authors thank E. Wiebe, S. De Rham, and J. Graham for advice and technical assistance. The authors thank Dr R. P. Kesner for technical advice on the development of the behavioral tasks, and Dr J. Gil-Mohapel for helpful review of the manuscript. Conflict of Interest: None declared.
References
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. 2007. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 52:60–70. [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. 2007. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 55:779–785. [DOI] [PubMed] [Google Scholar]
- Berberich S, Jensen V, Hvalby O, Seeburg PH, Kohr G. 2007. The role of NMDAR subtypes and charge transfer during hippocampal LTP induction. Neuropharmacology. 52:77–86. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. 2005. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 25:6907–6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, et al. 2010. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 30:4590–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, et al. 2002. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 22:8797–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Luo T, Raymond LA. 1999. Subtype-dependence of NMDA receptor channel open probability. J Neurosci. 19:6844–6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. 2009. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 325:210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. 2004. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004:re16. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Wu DC, Wang YT, Floresco SB, Phillips AG. 2012. NMDA GluN2A and GluN2B receptors play separate roles in the induction of LTP and LTD in the amygdala and in the acquisition and extinction of conditioned fear. Neuropharmacology. 62:797–806. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. 2010. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 11:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie BD, Cushman J, Kannangara TS, Fanselow MS, Christie BR. 2012. NMDA receptor hypofunction in the dentate gyrus and impaired context discrimination in adult Fmr1 knockout mice. Hippocampus. 22:241–254. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Copara MS, Isham EA, Wang WC, Yonelinas AP. 2011. Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage. 56:1803–1813. [DOI] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. 2005. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 563:345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald RC, van Keuren-Jensen KR, Aizenman CD, Cline HT. 2008. Roles of NR2A and NR2B in the development of dendritic arbor morphology in vivo. J Neurosci. 28:850–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. 1997. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 17:2469–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CJ, Russell KI, Wang YT, Christie BR. 2006. Contribution of NR2A and NR2B NMDA subunits to bidirectional synaptic plasticity in the hippocampus in vivo. Hippocampus. 16:907–915. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. 2007. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 54:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb R, Kolb B. 1998. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods. 79:1–4. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. 2001. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 11:626–636. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. 2005. Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Behav Neurosci. 119:1307–1315. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. 2008. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behav Neurosci. 122:16–26. [DOI] [PubMed] [Google Scholar]
- Harney SC, Jane DE, Anwyl R. 2008. Extrasynaptic NR2D-containing NMDARs are recruited to the synapse during LTP of NMDAR-EPSCs. J Neurosci. 28:11685–11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge J, Kesner RP. 2007. Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiol Learn Mem. 88:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Goodrich-Hunsaker NJ, Willemsen R, Berman RF. 2010. Temporal ordering deficits in female CGG KI mice heterozygous for the fragile X premutation. Behav Brain Res. 213:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Rosenberg JS, Kesner RP. 2008. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 18:1064–1073. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Wenzel HJ, Willemsen R, Berman RF. 2009. Progressive spatial processing deficits in a mouse model of the fragile X premutation. Behav Neurosci. 123:1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Auberson YP, Zorumski CF. 2006. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J Neurosci. 26:7181–7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. 2007. A behavioral analysis of dentate gyrus function. Prog Brain Res. 163:567–576. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. 2004. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 15:333–351. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, Hen R. 2012. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci. 32:8696–8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Mishina M, Sugiyama H. 2003. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor epsilon 1 subunit. Neurosci Res. 47:55–63. [DOI] [PubMed] [Google Scholar]
- Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M. 1998. Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor epsilon1 subunit. J Neurosci. 18:6704–6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G. 2006. NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res. 326:439–446. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, et al. 1996. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 16:333–344. [DOI] [PubMed] [Google Scholar]
- Lisman J. 1994. The CaM kinase II hypothesis for the storage of synaptic memory. Trends Neurosci. 17:406–412. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. 2004. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 304:1021–1024. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, et al. 2007. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 27:2846–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YB, Lio PA, Pasternak JF, Trommer BL. 1996. Developmental changes in membrane properties and postsynaptic currents of granule cells in rat dentate gyrus. J Neurophysiol. 76:1074–1088. [DOI] [PubMed] [Google Scholar]
- Longordo F, Kopp C, Mishina M, Lujan R, Luthi A. 2009. NR2A at CA1 synapses is obligatory for the susceptibility of hippocampal plasticity to sleep loss. J Neurosci. 29:9026–9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca RA, Rozas C, Loyola S, Moreira-Ramos S, Zeise ML, Kirkwood A, Huidobro-Toro JP, Morales B. 2011. Zinc enhances long-term potentiation through P2X receptor modulation in the hippocampal CA1 region. Eur J Neurosci. 33:1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Christofi G, Miyakawa H, Christie B, Lasser-Ross N, Johnston D. 1995. Subthreshold synaptic activation of voltage-gated Ca2+ channels mediates a localized Ca2+ influx into the dendrites of hippocampal pyramidal neurons. J Neurophysiol. 74:1335–1342. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. 2004. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 24:7821–7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. 2007. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 317:94–99. [DOI] [PubMed] [Google Scholar]
- Mongiat LA, Esposito MS, Lombardi G, Schinder AF. 2009. Reliable activation of immature neurons in the adult hippocampus. PLoS One. 4:e5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. 1994. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 12:529–540. [DOI] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. 2007. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology. 52:71–76. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. 1986. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 319:774–776. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. 1982. Place navigation impaired in rats with hippocampal lesions. Nature. 297:681–683. [DOI] [PubMed] [Google Scholar]
- Myme CI, Sugino K, Turrigiano GG, Nelson SB. 2003. The NMDA-to-AMPA ratio at synapses onto layer 2/3 pyramidal neurons is conserved across prefrontal and visual cortices. J Neurophysiol. 90:771–779. [DOI] [PubMed] [Google Scholar]
- Nacher J, Varea E, Miguel Blasco-Ibanez J, Gomez-Climent MA, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, McEwen BS. 2007. N-methyl-d-aspartate receptor expression during adult neurogenesis in the rat dentate gyrus. Neuroscience. 144:855–864. [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. 2006. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 26:1331–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. 2007. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 7:39–47. [DOI] [PubMed] [Google Scholar]
- Rauner C, Kohr G. 2011. Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem. 286:7558–7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Christie BR. 2006. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 137:1299–1307. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. 1995. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 373:151–155. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. 2004. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 429:184–187. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. 2007. Subthreshold dendritic signal processing and coincidence detection in dentate gyrus granule cells. J Neurosci. 27:8430–8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda FJ, Bustos FJ, Inostroza E, Zuniga FA, Neve RL, Montecino MA, van Zundert B. 2010. Differential roles of NMDA receptor subtypes NR2A and NR2B in dendritic branch development and requirement of RasGRF1. J Neurophysiol. 103:1758–1770. [DOI] [PubMed] [Google Scholar]
- Sholl DA. 1956. The measurable parameters of the cerebral cortex and their significance in its organization. Prog Neurobiol. (2):324–333. [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. 2001. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 85:2423–2431. [DOI] [PubMed] [Google Scholar]
- Staubli U, Thibault O, DiLorenzo M, Lynch G. 1989. Antagonism of NMDA receptors impairs acquisition but not retention of olfactory memory. Behav Neurosci. 103:54–60. [DOI] [PubMed] [Google Scholar]
- Tovar KR, McGinley MJ, Westbrook GL. 2013. Triheteromeric NMDA receptors at hippocampal synapses. J Neurosci. 33:9150–9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M, Yoshii A, Mishina M, Constantine-Paton M. 2003. Developmental loss of miniature N-methyl-D-aspartate receptor currents in NR2A knockout mice. Proc Natl Acad Sci USA. 100:1340–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Burgess MF, Zheng F, Lyuboslavsky P, Powers JL. 1998. Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. J Neurosci. 18:6163–6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Rolls ET. 1992. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 2:189–199. [DOI] [PubMed] [Google Scholar]
- Trommer BL, Liu YB, Pasternak JF. 1996. Long-term depression at the medial perforant path-granule cell synapse in developing rat dentate gyrus. Brain Res Dev Brain Res. 96:97–108. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. 1996. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 87:1327–1338. [DOI] [PubMed] [Google Scholar]
- Vasuta C, Caunt C, James R, Samadi S, Schibuk E, Kannangara T, Titterness AK, Christie BR. 2007. Effects of exercise on NMDA receptor subunit contributions to bidirectional synaptic plasticity in the mouse dentate gyrus. Hippocampus. 17:1201–1208. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D. 1997. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 68:469–478. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. 1983. Large long-lasting potentiation in the dentate gyrus in vitro during blockade of inhibition. Brain Res. 275:153–158. [DOI] [PubMed] [Google Scholar]
- Winder DG, Sweatt JD. 2001. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat Rev Neurosci. 2:461–474. [DOI] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. 2005. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 8:1069–1077. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. 2008. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 55:1081–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SL, Bohenek DL, Fanselow MS. 1994. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behav Neurosci. 108:19–29. [DOI] [PubMed] [Google Scholar]
- Zhang L, Meng K, Li YH, Han TZ. 2009. NR2A-containing NMDA receptors are required for L-LTP induction and depotentiation in CA1 region of hippocampal slices. Eur J Neurosci. 29:2137–2144. [DOI] [PubMed] [Google Scholar]
- Zhao JP, Constantine-Paton M. 2007. NR2A−/− mice lack long-term potentiation but retain NMDA receptor and L-type Ca2+ channel-dependent long-term depression in the juvenile superior colliculus. J Neurosci. 27:13649–13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.