Abstract
Viewing a person perform an action activates the observer's motor system. Whether this phenomenon reflects the action's kinematics or its final goal remains a matter of debate. One alternative to this apparent controversy is that the relative influence of goal and kinematics depends on the information available to the observer. Here, we addressed this possibility. For this purpose, we measured corticospinal excitability (CSE) while subjects viewed 3 different grasping actions with 2 goals: a large and a small object. Actions were directed to the large object, the small object, or corrected online in which case the goal switched during the movement. We first determined the kinematics and dynamics of the 3 actions during execution. This information was used in 2 other experiments to measure CSE while observers viewed videos of the same actions. CSE was recorded prior to movement onset and at 3 time points during the observed action. To discern between goal and kinematics, information about the goal was manipulated across experiments. We found that the goal influenced CSE only when its identity was known before movement onset. In contrast, a kinematic modulation of CSE was observed whether or not information regarding the goal was provided.
Keywords: action observation, corticospinal excitability, goal, kinematics, motor facilitation, transcranial magnetic stimulation
Introduction
Observing someone else perform an action recruits a set of sensorimotor brain regions that are active during movement execution (Rizzolatti and Craighero 2004; Grafton 2009). Whether motor facilitation reflects the action's goal or kinematics has been a matter of debate ever since the discovery of mirror neurons (Di Pellegrino et al. 1992; Gallese et al. 1996). For example, electrophysiological experiments have shown that neuronal activity in F5 increases during observation of grasping movements when the monkey knows there is an object to be grasped (visible or occluded) but not when the object is absent (Umiltà et al. 2001), suggesting a modulatory effect of the goal. In humans, activity of the motor system can be inferred from the level of corticospinal excitability (CSE) of the primary motor cortex (M1). Using this method, 2 studies were conducted to address this issue during observation of grasping movements with regular or reverse pliers. Yet, whereas one of them (Cattaneo et al. 2009) claimed an influence of the goal, the other (Cavallo et al. 2012) supported the kinematics view. Finally, a recent study of object grasping suggests that CSE reflects the kinematics of the observed movement (Sartori et al. 2012).
One alternative to this apparent discrepancy is that both goal and kinematics modulate the observer's motor system, but their relative influence may go unnoticed depending on the chosen experimental design. Factors such as the amount of information provided in the visual scene and/or the time at which motor facilitation is measured may bias the results toward one or the other component. Most studies carried out to date have used long videos in which transcranial magnetic stimulation (TMS) is applied at 1 or 2 time points during the action (maximal aperture or object contact), and where the observer has unequivocal knowledge of the action's goal. The latter is often provided indirectly by showing the object to be grasped long before movement onset. Experimental evidence, however, indicates that viewing a static picture, a 3D object, or a tool may activate motor representations involved in its manipulation (Grafton et al. 1997; Craighero et al. 2008; Tucker and Ellis 1998; Urgesi et al. 2006). Thus, observing the action's goal in advance is likely to influence the state of the motor system. One way to address this confound would involve having more than one potential target for the same action and not specifying the final goal until later in the movement. Such an approach would be more in line with natural prehension, where the identity of the final goal often remains unknown to the observer until near the time of object contact. Assessing motor facilitation before movement onset would also be essential to identify the influence of the goal in the absence of movement kinematics.
In this study, we examined the possibility that both goal and kinematics influence motor facilitation in the observer. For this purpose, we recorded the time course of CSE during static and dynamic phases of 3 grasping actions with 2 potential goals. Here, we use goal to refer to the object to be grasped. One action was directed to a large object and another one to a small object. Moreover, to dissociate goal from kinematics, an action in which the goal unexpectedly switched during the movement from the large to the small object (e.g., Tunik et al. 2005), was included. Unlike most goal-directed actions, which unfold according to the original motor plan, these actions require a modification of the initial motor plan during the movement to achieve the final goal (e.g., Pélisson et al. 1986; Castiello and Jeannerod 1991; Prablanc and Martin 1992). We refer to these actions as being corrected online.
In a first experiment, we established the correspondence between action kinematics and muscle activity by measuring grasp aperture and electromyographic (EMG) signal during execution of the 3 actions (movement duration: ∼1 s). We used this information to select 4 relevant time points, which guided the measurement of CSE in 2 other experiments in which subjects viewed videos of the same 3 actions. CSE was recorded prior to movement onset and at 3 time points during the observed action. To determine the influence of the goal, knowledge of the object to be grasped was provided (Experiment 2) or not (Experiment 3) before movement onset.
We reasoned that if motor facilitation during action observation reflects solely the kinematics of the action, the time course of CSE during Experiments 2 and 3 should be very similar and will follow closely that of muscle activity (Experiment 1). If instead it responds solely to the final goal, we expect the time course of CSE in Experiment 2 to be similar for the action directed to the small object and the action corrected online, and no temporal modulation of CSE in Experiment 3. Finally, if motor facilitation is influenced by goal and kinematics we expect knowledge of the goal to influence CSE in Experiment 2 but not in Experiment 3. In addition, we expect a similar, muscle-based, pattern of CSE during the dynamic phase of Experiments 2 and 3.
Materials and Methods
Participants
Thirty-five right-handed subjects (14 males and 21 females; age = 23.6 ± 4.5 years) participated in this study after giving written, informed consent. They did not have any neurological or psychiatric disorders and had no family history of epilepsy. The experimental procedure was approved by the local Ethics Committee and carried out according to the Declaration of Helsinki.
Experiment 1: Action Execution
Ten subjects (mean ± SE = 24.9 ± 1.16 years old; 4 males) took part in this Experiment.
Experimental Paradigm
Subjects sat on a comfortable chair and placed their right hand over a table, with their index finger lightly pressing a key on a keypad. Their elbow lay on the chair's armrest. A large and a small object (1.8 × 0.8 cm and 1.8 × 8.8 cm) were fixed on the same structure on a platform located at a height of 17 cm from the tabletop (see Fig. 2 for clarification). This configuration served to keep the arm kinematics constant while reaching to either object. The platform was aligned to the subject's right shoulder. The keypad was placed 22 cm before the platform, and was also aligned to the objects and the subject's shoulder. The distance between the chest and the platform was adjusted for each subject to allow natural reaching to grasp movements and to prevent them from slanting forward.
Figure 2.
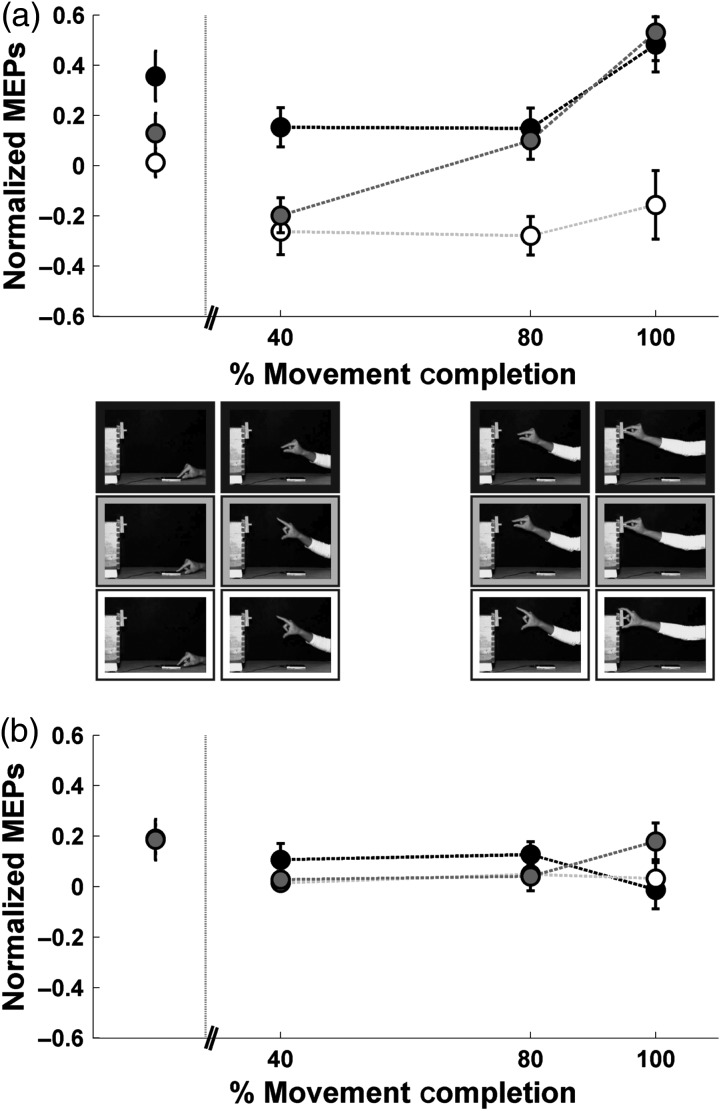
Experiment 2: time course of CSE during observation of grasping actions with previous knowledge of the goal. Symbols represent the mean ± SE of the MEPs evoked from (a) the FDI muscle and (b) the ADM muscle. Pictures depict the frames of the videos where stimulation occurred. White symbols: action directed to the large target; black symbols: action directed to the small target; gray symbols: action corrected online. Vertical dotted line indicates movement onset. For practicality, the MEPs evoked at the first time point (during observation of the static hand) have been moved to the left to avoid superimposition over the movement onset line.
To record muscle activity during grasping, superficial cup electrodes were placed using a belly-tendon mount over the “first dorsal interosseous” (FDI) and the “abductor digiti minimi” (ADM) muscles. Ground electrodes for each muscle were placed over the wrist. The skin was thoroughly cleaned with alcohol before placing the electrodes. EMG activity was amplified using 2 AC amplifiers (P5 series, Grass Instruments Co., MA, USA) with a bandwidth between 20 and 500 Hz and digitized at 2800 Hz (National Instruments, Inc., Austin, TX, USA). Data were collected in a PC using a program written in LabView (LabView 8.1, National Instruments).
To record movement kinematics a 3D motion tracker was used (Vicon MX Ultranet HD, Vicon Motion Systems, UK). Markers were placed at the tip of the thumb and index fingers and also at the index and little finger metacarpophalangeal joints. Data were collected at 400 Hz.
Procedure
Before starting the experiment, subjects were trained to perform the actions in form and time. A small computer screen was placed next to the objects. Each trial begun with the appearance on the screen of either a large or a small vertical rectangle indicating the size of the object to be grasped. After the appearance of the visual stimulus, a beep generated at a variable interval of 1–2.5 s signaled the start of the movement (go signal). Subjects were instructed to lift their index finger from the keypad as soon as they heard the beep and grasp the corresponding object. The visual stimuli remained on the screen for 1 s. To attain movement duration of approximately 1 s, subjects were instructed to match the end of the movement to the offset of the visual stimulus. They were told to slightly press on the grasped object and immediately return to the start position. No feedback was provided. In trials that required an online correction, a second beep of a different frequency was generated 100 ms after movement onset. Subjects were instructed to switch to the other target immediately after detecting the beep (only the initial goal was displayed on the computer screen). The training block consisted of 75 trials, of which 70% were uncorrected movements directed to the large or small object and 30% were movements that involved an online correction from the large to the small target or vice versa.
By the second half of the training block, all subjects showed good performance in accuracy and movement duration (data not shown). Next, they performed 2 experimental blocks during which movement kinematics and muscle activity were recorded. Each block consisted of 75 trials, of which 30% were corrected movements. The task was programmed in Matlab's psychtoolbox (The Mathworks, Inc., MA, USA) (Brainard 1997).
Data Processing
Kinematics and EMG time series were segmented into individual trials based on movement onset, defined as the moment when subjects lifted their index finger off the keypad, and movement offset determined as the time when the hand reached 5% of peak velocity (Della-Maggiore et al. 2004). Next, both measures were time normalized to achieve the same number of samples per trial (0–100% of the movement). The EMG data were rectified, and the root mean square computed to estimate muscle activity in 20 ms bins. EMG amplitude was normalized between 0 and 1 within subject and within condition across muscles, thereby preserving existing differences in muscle activity. Kinematics were also normalized for differences in amplitude between 0 and 1. This normalization was performed within subject and within condition.
Experiments 2 and 3: Action Observation
Twenty-five subjects took part in these experiments. Thirteen subjects (mean ± SE = 25.7 ± 1.66 years old; 4 males) participated in Experiment 2, whereas 12 subjects (mean ± SE = 24 ± 1 years old; 6 males) took part in Experiment 3. None of these subjects participated in Experiment 1.
Experimental Set-up
Participants sat on a comfortable armchair. They were instructed to keep their hands still and relaxed throughout the experiment. Their right hand was placed over their lap in a prone position underneath the table to prevent its vision. Their elbow rested comfortably over the armrest. Two superficial cup electrodes were placed using a belly-tendon mount over the FDI and the ADM muscles of the right hand. Ground electrodes for each muscle were placed over the wrist and elbow. The skin was thoroughly cleaned with alcohol before placing the electrodes. EMG activity was amplified using 2 AC amplifiers (P5 series, Grass Instruments Co.) with a bandwidth between 10 and 1000 Hz. The signal was amplified 1000 times, digitized at 5000 Hz (National Instruments, Inc), and collected in a PC using a program written in LabView (LabView 8.1, National Instruments).
Experimental Paradigm
The experimental stimuli consisted of 3 video clips showing a lateral view of an actor's arm performing the actions that were executed in Experiment 1, that is, reaching to grasp the small object, reaching to grasp the large object and the action corrected online from the large to the small object. Given that both objects were mounted on the same physical structure, no knowledge of the final goal could be extracted before movement onset unless this information was explicitly provided. The videos were presented on a 17″ LCD monitor (Samsung, 732N PLUS) placed 80 cm in front of the subjects and consisted of 50 frames each lasting 40 ms. Each video had a total duration of 2100 ms. The first 6 frames (240 ms) depicted a static hand pressing the button on the keypad, the next 29 frames covered the hand in motion from movement onset to movement offset which coincided with the time of object contact (1160 ms), and the last 15 frames covered the remaining of the grasp. Thus, movement duration was 1160 ms.
The opposite correction (from the small to the large object) was not used in the action observation experiments, because a pilot study revealed that 6 subjects could not distinguish this action from the unperturbed action directed to the large object (data not shown). This was probably due to the use of videos with real or close to real-time movement duration, and the subtlety in detecting kinematic features between these 2 actions at this rapid rate of movement. While artificially slow movements might enhance the observer's discrimination performance, the use of videos in real time is crucial to maintain the time course of CSE within the physiological range of natural actions. Therefore, we decided not to include the small-to-large correction in Experiments 2 and 3 as it would not be directly comparable to the large-to-small correction.
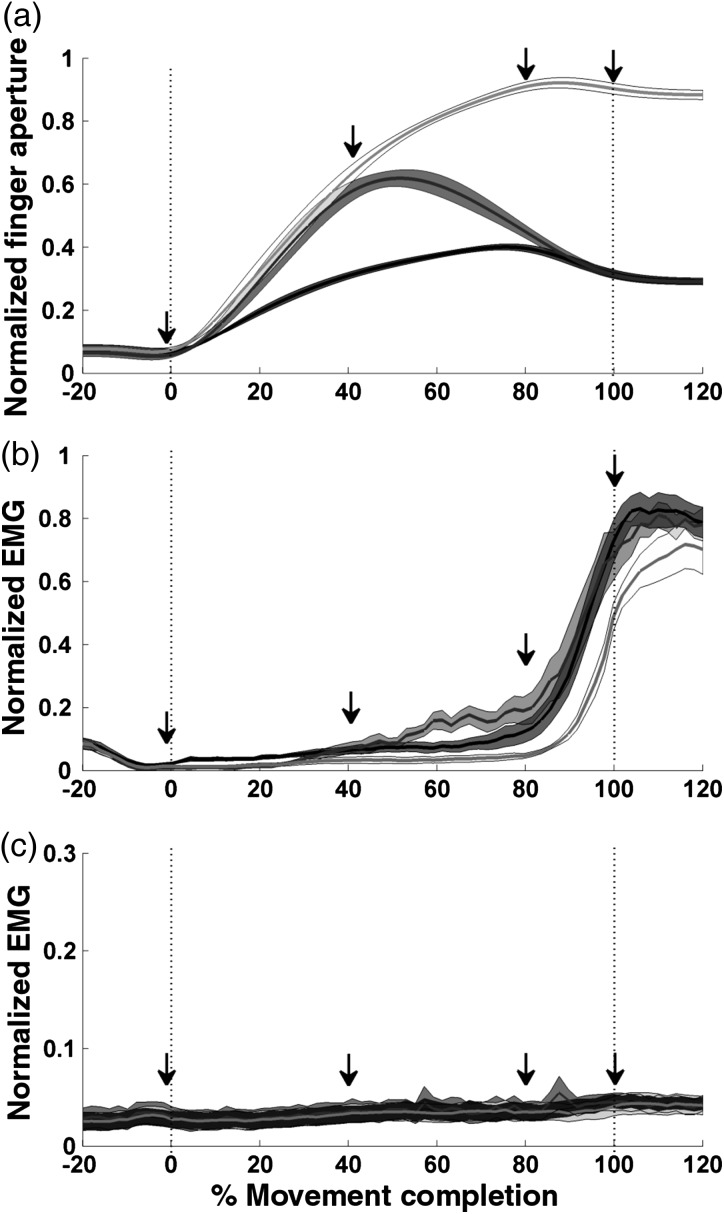
Based on the data acquired in Experiment 1, we chose 4 TMS stimulation points to measure CSE during action observation (see arrows in Fig. 1). The first time point corresponded to the frame prior to movement onset (0% of movement completion), when the 3 videos depicted a static hand touching the keypad. The second time point was chosen right before the online correction started (40% of movement completion). The third time point was chosen right after the correction took place (80% movement completion). The final time point was chosen when the hand contacted the object (100% movement completion). These time points best captured the time course of movement kinematics for all 3 actions, allowing the identification of modulatory effects through static and dynamic phases of the grasp (see Results for details). The task was programmed using Matlab's psychtoolbox (The Mathworks, Inc.) (Brainard 1997).
Figure 1.
Kinematics and muscle activity during execution of grasping actions. Shown are the mean values of these variables (solid line) ± 1 SE (shadow). (a) Kinematic description of executed actions measured as hand aperture. (b) EMG activity of the FDI muscle during execution. (c) EMG activity of the ADM muscle during execution. Light gray traces represent the movement directed toward the large object; dark traces the movement directed toward the small object and medium gray represents the online corrected action. The beep signaling the online correction took place 100 ms after movement onset, which would roughly correspond to 10% of movement completion. The arrows indicate the time points chosen for TMS stimulation during the action observation experiments.
Procedure
Three blocks of 20 video clips (total 60 video clips) for each action were presented in a pseudorandomazied order to avoid habituation. This consisted of shuffling the order of presentation of the 3 videos every 3 trials, so that a maximum of one repetition could take place every 6 trials with a probability of 33%. Each block lasted approximately 10 min. The interval between videos was of at least 7 s to avoid cumulative effects of TMS (Chen et al. 1997). Single TMS pulses were delivered every single trial at 1 of the 4 chosen time points. A total of five TMS pulses were delivered per time point during each block, yielding to 15 trials per stimulation time for each action.
Subjects were told to observe the videos carefully. To ensure they followed the hand's trajectory, in 30% of the trials they were asked to report either the type (corrected or uncorrected) or the goal (large or small) of the action last seen. Requests appeared in written form after the end of the trial. Participants responded by pressing a pedal with their right foot when the option presented on the screen matched the observed video.
To assess the influence of the kinematics and the goal of the observed action, we conducted 2 different experiments. In Experiment 2 (n = 13), information regarding the action's goal was provided before movement onset. This was accomplished by painting the object corresponding to the goal on the frames depicting a static hand for 2 s. For the action corrected online, the large object (initial goal) and the small object (final goal) were painted sequentially indicating the goal switch. In Experiment 3 (n = 12), information regarding the action's goal was not provided.
Transcranial Magnetic Stimulation
CSE was assessed based on the amplitude of motor evoked potentials (MEPs) elicited by single pulses of transcranial magnetic stimulation (Magstim 200; Magstim Co., Whitland, UK). For this purpose, a 70-mm figure-of-eight coil was positioned over the optimum scalp location corresponding to the left motor cortex, with the handle pointing backward at 45° from the midline. Earplugs were provided to protect participants' hearing. Before starting each block, the optimal scalp position to evoke MEPs from the FDI was identified. This location was marked on a rubber cap with a soft-tipped pen. Subsequently, the coil was moved around slightly until the spot evoking the largest MEPs from the ADM while still producing MEPs of at least similar amplitude from the FDI was found. A second mark was made for this location on the cap, which was then used as a reference to determine the resting motor threshold and to stimulate throughout the experiment. The head coil was fixed in position using an articulated arm (Manfrotto, Venice, Italy). Subjects were instructed to maintain their head still throughout the block. Motor threshold was determined as the intensity to evoke 5 out of 10 MEPs of at least 50 µV. The intensity used to measure CSE was 120% of the resting motor threshold.
Data Analysis
Peak-to-peak amplitudes of the MEPs for the ADM and the FDI muscles were measured for each condition. Trials in which MEP amplitudes were >2 SDs from the mean, as well as those in which muscle activity exceeded 100 μV, 100 ms prior to the TMS pulse, were excluded as outliers (on average, 2 trials were discarded out of 15).
Given that linear drifts were often observed within a block, we removed linear trends using the “detrend function” of Matlab. To avoid any nonspecific effects due to differences in coil position across blocks, we normalized MEP amplitudes by computing the z-scores across both muscles for each individual block. This procedure aimed to preserve any existing differences in amplitude across muscles.
For each Experiment, we performed repeated-measures analysis of variance (ANOVA) as needed. Sphericity of the data was verified before each test (Mauchly's P > 0.05). When a triple interaction was significant, it was split into individual two-way ANOVAs to facilitate the interpretation of the results according to Cohen (2007). Post hoc tests (Fisher's LSD) were conducted when needed to contrast CSE values for a given time point.
Results
Experiment 1
This experiment was designed to characterize the temporal correspondence between the kinematics and EMG activity associated with the 3 grasping actions of our experimental paradigm. This information served to guide the TMS stimulation times in the action observation experiments, where videos of the same actions were presented to naïve observers. Figure 1a shows the kinematics measured as the grasp aperture for the actions aimed to the large and small object. Given that only the action corrected online from the large to the small object was used in the action observation experiments, the results for the opposite correction are not shown. As seen in Figure 1a, during the first 40% of the movement, the grasp aperture of the action directed to the large object was similar to that of the action corrected online. The time to correction, determined based on the first time point at which hand aperture statistically differed across conditions (Saijo et al. 2005) was 330 ms (n = 10, P = 0.04; paired-t-test). This is in line with previous work by Castiello and Jeannerod (1991) for grip adjustments after an unexpected change in the section of a cylinder (330 ms) and with that reported by Grafton's group (Tunik et al. 2005) for corrections to perturbations in object length (∼300 ms). Therefore, we are confident that our videos reflect realistic kinematics changes in movement execution.
Comparison of the corresponding velocity profiles using the same method (Saijo et al. 2005) corroborated that both actions were kinematically identical until 320 ms into the movement (n = 10, P = 0.03), about the time when the correction began. This rules out the possibility that subjects relied on velocity to distinguish the large from the corrected action during the initial phase of the movement. Once the correction began, grip aperture decreased progressively until it matched that for the action directed to the small object. Consistent with previous descriptions (Borroni and Baldissera 2008), the time course of muscle activity showed low levels for the FDI during hand opening, but a strong increment during the second half of the movement as the hand closed to grasp the object (Fig. 1b). In contrast, the ADM showed no apparent modulation throughout the 3 actions (Fig. 1c).
Based on visual inspection of the kinematics and the EMG profiles, we chose 4 time points that served to characterize the time course of CSE in the action observation experiments. The first time point corresponded to the frame prior to movement onset (0% of movement completion), when the 3 videos depicted a static hand touching the keypad (no activity of the FDI or ADM was evident at this point, see Fig. 1b). The second time point was chosen before the online correction started (40% of movement completion). At this point, the hand aperture for the large and corrected movements was similar and clearly greater than that of the action directed to the small object. The third time point was chosen right after the correction took place (80% movement completion), when the small and corrected actions depicted a similar hand aperture that was clearly smaller than the aperture of the action directed to the large object. The final time point was chosen when the hand contacted the object (100% movement completion).
Experiment 2
This experiment was designed to assess the time course of CSE during observation of the same 3 actions when anticipated information about the goal was available. Note that knowledge of the goal is provided by default in most studies by presenting the object to be grasped in the visual scene long before movement onset.
The results of the attentional task showed that, on average, subjects responded correctly 90.9% of the time (standard error = 1.39), indicating that they were paying attention to the videos. Figure 2 illustrates the time course of CSE. A three-way repeated-measures ANOVA with “Muscle”, “Action”, and “Time Point” as factors yielded no main effect of Muscle (F1,12 = 0.94, P = 0.351), a significant main effect of Action (F2,24 = 18.59, P < 0.001), a significant main effect of Time Point (F3,36 = 8.24, P < 0.001) and a significant triple interaction (F6,72 = 2.26; P = 0.047). The effect size computed based on the partial eta-squared () was 0.61 for the main effect of Action, 0.41 for the main effect of Time Point, and 0.18 for the triple interaction, respectively.
To facilitate the interpretation of the results, we split the triple interaction into a separate two-way ANOVA for each muscle. The two-way ANOVA conducted for the FDI revealed a clear modulation of CSE (main effect of Action: F2,24 = 27.71, P < 0.001; main effect of Time Point: F3,36 = 10.17, P < 0.001; and a significant Action × Time Point interaction: F6,72 = 2.45, P = 0.03). Specifically, the MEPs for the action directed to the small object were larger than those for the action directed to the large object throughout the movement. This finding is consistent with the difference in hand aperture and muscle activity recorded during execution. Furthermore, the MEPs for the small target were even larger at the time of object contact, when muscle activity was stronger and hand aperture smaller than the action directed to the large target. Interestingly, the time course of the MEPs for the action corrected online resembled that of the action directed to the large object for the first 2 time points, when grasp aperture was large, but that of the action directed to the small object for the last 2 time points, when grasp aperture was small. In contrast, MEPs for the ADM showed no specific modulation as a function of time (main effect of Action: F2,24 = 0.50, P = 0.61; main effect of Time: F3,36 = 3.09, P = 0.39; Action × Time Point interaction: F6,72 = 1.54, P = 0.18), which is in line with the lack of modulation observed for the EMG during execution (Experiment 1).
A closer inspection of Figure 2 revealed that unlike time points 2 through 4, the CSE corresponding to time point 1 was not congruent with the EMG and kinematics during the static phase of the movement. In fact, CSE values corresponding to the first time point were far above the grand mean at a point when the EMG and kinematics were zero or near zero. To assess the specificity of this effect, we contrasted the MEP values across the 3 conditions for the first time point of the FDI. Post hoc tests of the two-way ANOVA referred to above (with Action and Time Point as factors) yielded larger MEP values for the movement directed to the small target than for the other two, despite the fact that all 3 videos depicted the same static hand (movement directed to small vs. large object: P < 0.001; movement directed to small object vs. movement corrected online: P < 0.05). In other words, when the upcoming video was of an action directed toward the small target, the MEPs appeared to be larger than when the upcoming video was an action directed toward the large target or corrected online from the large to the small object. No differences were found between the action directed to the large object and the action corrected online (movement corrected online vs. movement directed to large object: P = 0.68). The fact that the goal of the action corrected online switched from the large to the small target, and that subjects knew about this switch beforehand, suggests that the premovement MEP pattern reflected the initial rather than the final action's goal. This finding is in agreement with a mechanism coding upcoming action phases. Note that a CSE pattern consistent with the final goal would have been expected if action understanding emerge from this level of processing.
Differences in CSE at time point 1 were also examined for the ADM. Post hoc tests of the two-way ANOVA conducted on the ADM (with Action and Time Point as factors) yielded the following results: action directed to the small versus large object: P = 0.98; action directed to the small versus corrected action: P = 0.98; action directed to the large versus corrected action: P = 0.96. Therefore, CSE did not differ across conditions for the ADM during observation of the static hand.
Experiment 3
This experiment was designed to dissociate the contribution of the action's goal from that of action kinematics. For this purpose, a different set of subjects performed the same protocol used in Experiment 2 but no information regarding the goal/s was provided.
The results from the attentional task showed that, on average, subjects responded to the questions correctly 93.8% of the times (standard error = 0.73), demonstrating that they were paying attention to the videos. Figure 3 shows the normalized MEPs recorded for each video at the 4 time points chosen. A three-way repeated-measures ANOVA (Muscle × Action × Time Point) revealed a significant main effect of Action (F2,22 = 12.52, P < 0.001), a significant main effect of Time Point (F3,33 = 8.19, P < 0.001), a nonsignificant main effect of Muscle (F1,11 = 2,32, P = 0.16), and a significant triple interaction (F6,66 = 3.31; P < 0.01). The effect size computed based on the partial eta-squared () was 0.53 for the main effect of Action, 0.43 for the main effect of Time Point and 0.24 for the triple interaction, respectively.
Figure 3.
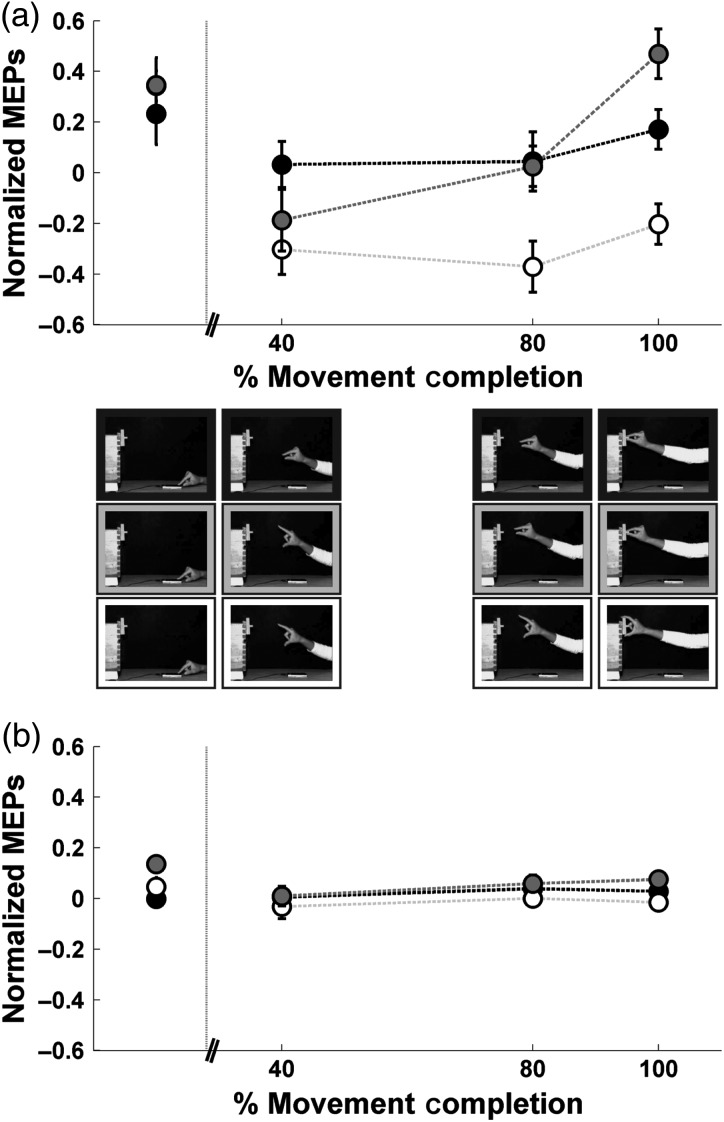
Experiment 3: time course of CSE during observation of grasping actions without previous knowledge of the goal. Symbols represent the mean ± SE of the MEPs evoked from (a) the FDI muscle and (b) the ADM muscle. Pictures depict the frames of the videos where stimulation occurred. White symbols: action directed to the large target; black symbols: action directed to the small target; gray symbols: action corrected online. The symbol corresponding to the action directed to the large target overlaps with that of the action corrected online. Vertical dotted line indicates movement onset. For practicality, the MEPs evoked at the first time point (during observation of the static hand) have been moved to the left to avoid superimposition over the movement onset line.
To facilitate the interpretation of the triple interaction, we broke it down into a two-way repeated-measures ANOVA per muscle. As in Experiment 2, we detected a clear modulation of the MEPs for the FDI that was in line with the time course of the EMG and kinematics of Experiment 1 (main effect of Action: F2,22 = 12.58, P < 0.001; main effect of Time Point: F3,33 = 9.21, P < 0.001; Action × Time Point interaction: F6,66 = 3.79; P < 0.01). In contrast, the two-way ANOVA for the ADM showed no evidence of a specific modulation (main effect of Action: F2,22 = 5.32, P = 0.013; main effect of Time Point: F3,33 = 2.48, P = 0.08; Action × Time interaction: F6,66 = 0.89; P = 0.50). This is in line with the lack of modulation observed for the EMG during execution (Experiment 1).
In line with Experiment 2, we found an increment in CSE for the FDI during the static phase prior to action onset (see first time point of Fig. 3a). Yet, in contrast with Experiment 2, no significant differences were found across conditions (movement directed to small vs. large object: P = 0.46; movement directed to small object vs. movement corrected online: P = 0.48; movement directed to large object vs. movement corrected online: P = 0.98). These results are consistent with a lack of modulation by the goal and are instead probably due to a nonspecific enhancement of CSE driven by attention or arousal in anticipation of any one of the upcoming actions.
Differences in CSE at time point 1 were also examined for the ADM. Post hoc tests of the two-way ANOVA conducted on the ADM (with Action and Time Point as factors) yielded the following results: Action directed to the small versus large object: P = 0.60; action directed to the small versus corrected action: P = 0.01; action directed to the large versus corrected action: P = 0.04. Given that the Action × Time Point interaction was not significant, alpha was adjusted to 0.017 (0.05/3), following a Bonferroni correction. The results indicate that the action corrected online was significantly different from the action directed to the small object. No other comparisons reached significance. Given that knowledge of the goal was not provided in Experiment 3, it is likely that this result is not physiologically meaningful. The fact that a direct comparison to the FDI (two-way ANOVA on T1 with Muscle and Action as factors) yielded no significant Muscle × Action interaction but only a main effect of muscle (main effect of muscle: F1,11 = 21.42, P = 0.001; main effect of Action: F2,22 = 1.2, P = 0.32; Muscle × Action interaction: F2,22 = 0.27, P = 0.762), suggests that the pattern detected for the ADM at T1 when analyzed alone is not robust and might reflect, like the FDI, a nonspecific increment in CSE due to attention or arousal, which was not strong enough in the other 2 conditions.
Experiment 2 versus Experiment 3
Attentional Task
A two-way ANOVA conducted on the percent of correct responses with Action and “Knowledge of the Goal” as factors, yielded a significant main effect of Action (F2,46 = 116, P < 0.001), a nonsignificant main effect of Knowledge of the Goal (F1,23 = 3.24; P = 0.09), and a nonsignificant interaction (F2,46 = 0.27, P = 0.77). The fact that neither the main effect of group nor the interaction were significant suggests that differences in the time course of CSE across groups were not driven by differences in attention. Post hoc comparisons detected relatively lower levels of performance for the action corrected online, both for Experiments 2 and 3 (Experiment 2: large vs. small target, P = 0.45; large vs. online corrected, P < 0.001; small vs. online corrected, P < 0.001; Experiment 3: large vs. small target, P = 0.98; large vs. online corrected, P < 0.001; small vs. online corrected, P < 0.001). Although performance was significantly lower for this condition, it was above 80% (80.43 ± 2.24% for Experiment 2 vs. 84.17 ± 1.48% for Experiment 3).
Corticospinal Excitability
To assess statistically the effect of the manipulation (Knowledge of the Goal), we directly compared Experiments 2 and 3. Given the distinct modulation recorded during observation of the static hand, we conducted a two-way ANOVA for the CSE corresponding to the first time point of the FDI (with Knowledge of the Goal and Action as factors) and a three-way ANOVA including time points 2 through 4 (with Knowledge of the Goal, Action, and Time Point as factors). The results yielded a significant Knowledge of the Goal × Action interaction during observation of the static hand (F2,46 = 4.26; P = 0.05), but not during time points 2 through 4 (triple interaction: F4,92 = 0.27 ; P = 0.89). In contrast, the same analysis conducted on the ADM yielded no significant effects either during the static phase of the action (Knowledge of the Goal × Action interaction: F2,46 = 0.87, P = 0.43) or during its dynamic phase (Knowledge of the Goal × Action × Time Point interaction: F4,92 = 1.22, P = 0.31). Our findings suggest that a modulatory effect of the goal can be detected specifically for the FDI when its identity is known in advance. In addition, they indicate that movement kinematics modulate CSE in the observer whether or not information about the action's goal is provided.
Discussion
The main goal of our study was to assess the possibility that both goal and kinematics influence activity of the motor system in the observer. We began by characterizing the kinematics and EMG during the execution of 3 grasping actions. We found that the time course of the kinematics closely followed the underlying muscle activity of the FDI. Based on this information, we chose 4 relevant time points that best characterized the movements and served to guide the recording of CSE during observation of the same actions. To dissociate the effect of goal and kinematics, knowledge of the goal was manipulated in Experiments 2 and 3. We found an influence of the goal only when the identity of the object to be grasped was known before movement onset. On the other hand, we found that movement kinematics modulated CSE whether or not information about the goal was provided. Our results suggest that, depending on the information available, both goal and kinematics may drive motor facilitation during action observation.
Even though several studies have used TMS to measure motor facilitation during action observation, only a handful have examined the time course of CSE (Gangitano et al. 2001, 2004; Borroni et al. 2005; Borroni and Baldissera 2008; Cavallo et al. 2012). Most studies only assessed CSE at 1 or 2 time points, often at the time of maximal aperture/muscle activity and/or object contact (e.g., Fadiga et al. 1995; Catmur et al. 2007; Petroni et al. 2010). Although this may not be a problem when actions involve simple one-jointed movements such as finger abduction, it may lead to inaccuracies when multijoint, longer-lasting movements such as reaching to grasp are involved. This is because the discrepancy between the time of TMS stimulation and the peak of activity in the observers' M1 is likely to increase as processing times become longer and the number of potential distractors grows. On the other hand, using a small time window to sample CSE may limit the understanding of modulatory effects that vary as a function of movement dynamics, possibly leading to some of the inconsistencies reported in the literature. To prevent these shortcomings here we measured CSE before movement onset and at 3 time points that best characterized the action kinematics during movement execution. On the other hand, the choice of videos with natural movement durations (∼1.2 s from movement onset to movement offset) together with the inclusion of an attentional task that forced visually tracking the hand on the screen, helped reduce the impact of potential distractors on CSE.
Two of the studies that have effectively measured the time course of CSE during reaching to grasp (Gangitano et al. 2001, 2004) claimed that motor facilitation in the observer closely matched the action kinematics. Even though at first sight these findings are partly in line with ours, there is a major discrepancy: in these studies, the maximum CSE for the FDI was found at the time of maximal hand aperture, whereas in ours it took place toward the end of the movement as the hand closed to grasp the object. The results from Experiment 1, however, are in agreement with 2 studies (Montagna et al. 2005; Borroni and Baldissera 2008) where the time course of EMG and CSE was assessed during grasping of a single object. This work shows that CSE appeared to follow the time course of muscle activity, with larger MEPs during the closing phase of the movement.
In recent years, Urgesi and collaborators proposed an alternative view in which motor facilitation during action observation does not reflect movement features of the ongoing action but its future kinematic states. This idea arose from a study where CSE was measured during the random presentation of static pictures with implied motion depicting key phases of ongoing actions (Urgesi et al. 2010). In this work, motor facilitation was largest for ongoing but incomplete actions, whereas lowest values of CSE were detected during observation of pictures depicting the final action phase. Some of our findings, particularly the pattern of CSE obtained while viewing the hand at rest (Experiment 2), may be explained by a such a predictive mechanism involving coding of upcoming action phases in the observers' motor system (Kilner et al. 2004). Nevertheless, the time course of CSE from time points 2 through 4 does not appear to reflect future but actual action phases. This is supported by the tight congruency with the EMG pattern obtained during execution. In contrast with Urgesis's study, visual inspection of Figure 2a suggests that the strongest CSE modulation was evident at the time of object contact (time point 4 of Experiment 2), when the FDI was most contracted. Therefore, although viewing static pictures with implied action can increase CSE (Urgesi et al. 2006), observing a picture as an isolated snapshot or in the context of a movement may not recruit the same neural processes. Our results open the possibility that motor facilitation may either be predictive or time locked to the observed action depending on the task design.
A number of TMS studies carried out in the last few years point to a preponderant role of kinematics in driving motor facilitation during observation of grasping actions. In one of them, Cattaneo et al. (2009) recorded MEPs from the “opponens pollicis” (OP), while subjects observed the experimenter grasp one object with regular or reversed pliers during the extension and flexion of the hand, respectively. The results showed a pattern of CSE consistent with the distal part of the pliers rather than the underlying muscle activity. Although, at first sight, these results appear to be in line with a modulation based on the action's goal, a more recent study where CSE was also measured from the FDI during grasping with regular, reverse and magnetic pliers, suggests otherwise (Cavallo et al. 2012). Although MEPs recorded from the OP were similar when grasping was performed with regular or reversed pliers, those recorded from the FDI were higher during observation of grasping movements performed with classic pliers than with reversed pliers or magnetic pliers. The results suggest that the output of the motor system reflects the observed hand movements and not the distal goal. More recently, the same group (Sartori et al. 2012) measured CSE while participants observed videos of an actor grasping an object using normal or slightly altered hand kinematics. Although the difference in movement kinematics was not consciously detectable by the observer, it was sufficient to influence MEP amplitudes in a muscle-based manner.
A series of seminal studies by Fadiga's group have described a similar pattern of goal/kinematics dissociation during object lifting. Specifically, Alaerts and collaborators have shown that motor facilitation in the observer matches the force applied to lift an object when the intrinsic properties of the object and the observed kinematics are congruent (e.g., light object, low filling), but also when they are incongruent (e.g., light object, high filling) (Alaerts, Senot, et al. 2010; Alaerts, Swinnen, et al. 2010). In line with Sartori's findings (2012), Senot et al. (2011) have shown that CSE measured during lifting of 2 identical—opaque—objects filled with different weights reflects the distinct kinematics even though the observer is unaware of the weight difference. Interestingly, this differential modulation disappears when the conflicting information is provided explicitly, by labeling the light object as “heavy” and the heavy object as “light”. This would suggest that kinematics modulation appears to prevail when conflicting information regarding the goal and the kinematics is provided implicitly but not explicitly.
Our findings from the dynamic phase of Experiments 2 and 3 confirm a role of kinematics in modulating motor facilitation during action observation of goal-directed actions. It is important to emphasize, however, that participants of the studies mentioned above (Cattaneo et al. 2009; Cavallo et al. 2012; Sartori et al. 2012) unequivocally knew the final goal before movement onset, which may have influenced motor facilitation (Grafton et al. 1997; Craighero et al. 2008; Tucker and Ellis 1998). In contrast with these studies, by presenting 2 potential goals and manipulating the identity of the object to be grasped, we were able to evaluate the role of movement kinematics unambiguously. Finally, a major contribution of our experimental design over previous studies was the identification of a modulatory effect by the action's goal. This was achieved by recording MEPs from an additional time point before movement onset while the hand was at rest, that is, in the absence of the influence of movement kinematics.
A key manipulation of our work was the inclusion of an action corrected online. Unexpected perturbations in target size or position after movement onset trigger automatic corrections in hand/finger trajectories that reflect the amendment of the original motor plan to accommodate the switch in the action's goal (e.g., Pélisson et al. 1986; Castiello and Jeannerod 1991; Prablanc and Martin 1992). Previous studies have examined the time course of CSE during unexpected changes in movement kinematics (Gangitano et al. 2001, 2004). Yet, these actions involving unusual kinematics to grasp an object were not corrected online but planned before movement onset. The occurrence of unnatural kinematics has been reported to abolish motor facilitation during action perception (Gangitano et al. 2004). In contrast, here we found that the time course of CSE followed the natural change in kinematics triggered by a switch in the action's goal. This pattern is reminiscent of the amendment of the motor plan characteristic of movement execution. Our results are in line with those by Alaerts and collaborators (Alaerts, Swinnen et al. 2010) and Senot and collaborators (Senot et al. 2011), showing that kinematics prevail when incongruent information regarding the goal is delivered implicitly. Although explicit clues were delivered in Experiment 2, these were always congruent with the observed action, probably sparing kinematics matching. To the best of our knowledge, this is the first demonstration that motor facilitation follows movement kinematics even when the motor plan changes as the action unfolds. Additional work in which CSE is sampled at several time points throughout the correction would be necessary to draw further conclusions regarding the nature of the mechanism at the basis of this phenomenon.
Recently, Cavallo et al. (2013) tested the hypothesis posited elsewhere (Lago and Fernandez-del-Olmo 2011) that the influence of goal and kinematics on motor facilitation depends on the processing stage—static or dynamic—of the observed action. For this purpose, they recorded MEPs while subjects observed a hand grasping a bottle positioned in front or behind a physical barrier, using either a precision grip or a whole grasp. They found that the MEP ratio between the ADM and FDI was similar at movement onset and at movement contact both when a precision grip was used to grasp the bottle from behind the barrier, and when a power grasp was used to achieve the bottle located in front of the barrier. However, a switch in the MEP ratio was observed when the bottle located in front of the barrier was achieved using a precision grip. The authors claimed that the switch observed in the latter case was due to a mismatch between the initially facilitated motor program to achieve the bottle (power grasp) and the one activated as the hand approached it (precision grip). Based on these findings, they suggested that the final goal of an action modulates the excitability of the motor system at movement start, but the kinematics features take over as the action unfolds. Although this is an interesting study and certainly shares some commonalities with ours, a number of differences in the experimental design complicate the comparison with our results. On the one hand, the findings are based on the assumption that the motor program necessary to achieve a goal is activated by default at movement start. Yet, this is not directly addressed but inferred based on the MEP ratio. The fact that the first TMS pulse delivered at movement onset was applied once the hand was already in motion not static, further complicates this interpretation since one would expect muscle activity at that point. This introduces a potential kinematic confound that cannot be ruled out since the authors did not measure EMG during execution. Finally, whether the switch in the MEP pattern reflects a switch in movement kinematics cannot be established, because CSE was only sampled around movement onset and at contact time.
The fact that we were able to identify a modulation by the goal during the static phase of the observed action does not necessary support the hypothesis that goal modulation occurs exclusively during movement planning. We can be sure of an influence of the goal at this point because there is no kinematics confound, but this does not preclude a spread into the dynamic phase of the action. In fact, a close inspection of MEP values during the dynamic phase suggests that the influence of the goal appeared to persist at 40% of movement completion (compare time point 2 of Figs 2a and 3a). This may reflect a predictive influence of the goal on the accuracy of kinematics matching. Further work would be necessary to directly test this possibility. Another important contribution of our study related to the previous one is that activity of the observer's motor system was not modulated by the final goal but by the initial goal, before the switch took place. This is against the hypothesis postulated by Cavallo et al. (2013) that the motor plan necessary to achieve the action's goal is automatically loaded at movement onset, but rather suggests that the excitability of the motor system may be modulated based on the upcoming motor primitives in the action sequence. The latter hypothesis is in line with the framework of predictive coding mentioned above (Kilner et al. 2004).
In summary, we have provided solid evidence demonstrating a specific influence of both the action's goal and kinematics on the motor system of the observer. Whereas the goal appears to modulate CSE when its identity is known, movement kinematics modulate motor facilitation even when the motor plan of the action changes throughout the movement. The persistence of this switch when the identity of the goal is unknown to the observer suggests that a similar mechanism may drive motor facilitation during goal-directed and intransitive actions (e.g., Borroni et al. 2005; Borroni and Baldissera 2008; Borroni et al. 2008). Our results are compatible with the idea that goal and kinematics can influence different levels of action representation (Grafton and Hamilton 2007), which corresponding neural circuits may or not be invoked depending on the available information and task demands.
Funding
This work was supported by the Fogarty International Research Collaboration Award of the National Institutes of Health with Dr ST Grafton (R03 TW007815-01A1), the National Agency for the Promotion of Science and Technology of Argentina (PICT-0063), and the University of Buenos Aires (UBACyT-090100157).
Notes
We thank Claudia Vargas for her support. Conflict of Interest: None declared.
References
- Alaerts K, Senot P, Swinnen SP, Craighero L, Wenderoth N, Fadiga L. 2010. Force requirements of observed object lifting are encoded by the observer's motor system: a TMS study. Eur J Neurosci. 6(31):1144–1153. [DOI] [PubMed] [Google Scholar]
- Alaerts K, Swinnen SP, Wenderoth N. 2010. Observing how others lift light or heavy objects: which visual cues mediate the encoding of muscular force in the primary motor cortex? Neuropsychologia. 48:2082–2090. [DOI] [PubMed] [Google Scholar]
- Borroni P, Baldissera F. 2008. Activation of motor pathways during observation and execution of hand movements. Soc Neurosci. 3:276–288. [DOI] [PubMed] [Google Scholar]
- Borroni P, Montagna M, Cerri G, Baldissera F. 2008. Bilateral motor resonance evoked by observation of a one-hand movement: role of the primary motor cortex. Eur J Neurosci. 28(7):1427–1435. [DOI] [PubMed] [Google Scholar]
- Borroni P, Montagna M, Cerri G, Baldissera F. 2005. Cyclic time course of motor excitability modulation during the observation of a cyclic hand movement. Brain Res. 1065:115–124. [DOI] [PubMed] [Google Scholar]
- Brainard DH. 1997. The psychophysics toolbox. Spatial Vis. 10:433–436. [PubMed] [Google Scholar]
- Castiello U, Jeannerod M. 1991. Measuring time to awareness. NeuroReport. 2:797–800. [DOI] [PubMed] [Google Scholar]
- Catmur C, Walsh V, Heyes C. 2007. Sensorimotor learning configures the human mirror system. Curr Biol. 17:1527–1531. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Caruana F, Jezzini A, Rizzolatti G. 2009. Representation of goal and movements without overt motor behavior in the human motor cortex: a transcranial magnetic stimulation study. J Neurosci. 29:11134–11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo A, Becchio C, Sartori L, Bucchioni G, Castiello U. 2012. Grasping with tools: corticospinal excitability reflects observed hand movements. Cereb Cortex. 22:710–716. [DOI] [PubMed] [Google Scholar]
- Cavallo A, Bucchioni G, Castiello U, Becchio C. 2013. Goal or movement? Action representation within the primary motor cortex. Eur J Neurosci. 38:3507–3512. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. 1997. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 48:1398–1403. [DOI] [PubMed] [Google Scholar]
- Cohen BH. 2007. Explaining psychological statistics. 3rd ed. New York: John Wiley & Sons. [Google Scholar]
- Craighero L, Bonetti F, Massarenti L, Canto R, Fabbri Destro M, Fadiga L. 2008. Temporal prediction of touch instant during observation of human and robot grasping. Brain Res Bull. 75:770–774. [DOI] [PubMed] [Google Scholar]
- Della-Maggiore V, Malfait N, Ostry DJ, Paus T. 2004. Stimulation of the posterior parietal cortex interferes with arm trajectory adjustments during the learning of new dynamics. J Neurosci. 24:9971–9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. 1992. Understanding motor events: a neurophysiological study. Exp Brain Res. 91:176–180. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. 1995. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 73:2608–2611. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. 1996. Action recognition in the premotor cortex. Brain. 119:593–609. [DOI] [PubMed] [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual-Leone A. 2004. Modulation of premotor mirror neuron activity during observation of unpredictable grasping movements. Eur J Neurosci. 20:2193–2202. [DOI] [PubMed] [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual-Leone A. 2001. Phase-specific modulation of cortical motor output during movement observation. NeuroReport. 12:1489–1492. [DOI] [PubMed] [Google Scholar]
- Grafton ST. 2009. Embodied cognition and the simulation of action to understand others. Ann N Y Acad Sci. 1156:97–117. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fadiga L, Arbib MA, Rizzolatti G. 1997. Premotor cortex activation during observation and naming of familiar tools. Neuroimage. 6:231–236. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hamilton A. 2007. Evidence for a distributed hierarchy of action representation in the brain. Hum Mov Sci. 26:590–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Vargas C, Duval S, Blakemore SJ, Sirigu A. 2004. Motor activation prior to observation of a predicted movement. Nat Neurosci. 12:1299–1301. [DOI] [PubMed] [Google Scholar]
- Lago A, Fernandez-del-Olmo M. 2011. Movement observation specifies motor programs activated by the action observed objective. Neurosci Lett. 493:102–106. [DOI] [PubMed] [Google Scholar]
- Montagna M, Cerri G, Borroni P, Baldissera F. 2005. Excitability changes in human corticospinal projections to muscles moving hand and fingers while viewing a reaching and grasping action. Eur J Neurosci. 22(6):1513–1520. [DOI] [PubMed] [Google Scholar]
- Pélisson D, Prablanc C, Goodale MA, Jeannerod M. 1986. Visual control of reaching movements without vision of the limb. II. Evidence of fast unconscious processes correcting the trajectory of the hand to the final position of a double step stimulus. Exp Brain Res. 62:303–311. [DOI] [PubMed] [Google Scholar]
- Petroni A, Baguear F, Della-Maggiore V. 2010. Motor resonance may originate from sensorimotor experience. J Neurophysiol. 104:1867–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prablanc C, Martin O. 1992. Automatic control during hand reaching at undetected two-dimensional target displacements. J Neurophysiol. 67(2):455–469. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. 2004. The mirror neuron system. Annu Rev Neurosci. 27:169–192. [DOI] [PubMed] [Google Scholar]
- Saijo N, Murakami I, Nishida S, Gomi H. 2005. Large-field visual motion directly induces an involuntary rapid manual following response. J Neurosci. 25(20):4941–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori L, Bucchioni G, Castiello U. 2012. Motor cortex excitability is tightly coupled to observed movements. Neuropsychologia. 50:2341–2347. [DOI] [PubMed] [Google Scholar]
- Senot P, D'Ausilio A, Franca M, Caselli L, Craighero L, Fadiga L. 2011. Effect of weight-related labels on corticospinal excitability during observation of grasping: a TMS study. Exp Brain Res. 211:161–167. [DOI] [PubMed] [Google Scholar]
- Tucker M, Ellis R. 1998. On the relations between seen objects and components of potential actions. J Exp Psychol Hum Percept Perform. 24:830–846. [DOI] [PubMed] [Google Scholar]
- Tunik E, Frey SH, Grafton ST. 2005. Virtual lesions of the anterior intraparietal area disrupt goal-dependent on-line adjustments of grasp. Nat Neurosci. 8:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umiltà MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G . 2001. I know what you are doing. A neurophysiological study. Neuron. 31:155–165. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Maieron M, Avenanti A, Tidoni E, Fabbro F, Aglioti SM. 2010. Simulating the future of actions in the human corticospinal system. Cereb Cortex. 20:2511–2521. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Moro V, Candidi M, Aglioti SM. 2006. Mapping implied body actions in the human motor system. J Neurosci. 26:7942–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]