Abstract
Cortical pyramidal neuron activity is regulated in part through inhibitory inputs mediated by GABAA receptors. The subunit composition of these receptors confers distinct functional properties. Thus, developmental shifts in subunit expression will likely influence the characteristics of pyramidal cell firing and the functional maturation of processes that depend on these neurons. We used laser microdissection and PCR to quantify postnatal developmental changes in the expression of GABAA receptor subunits (α1, α2, α5, β2, γ2, and δ) in layer 3 pyramidal cells of monkey prefrontal cortex, which are critical for working memory. To determine the specificity of these changes, we examined glutamate receptor subunits (AMPA Glur1 and NMDA Grin1) and conducted the same analyses in layer 5 pyramidal cells. Expression of GABAA receptor subunit mRNAs changed substantially, whereas glutamate receptor subunit changes were modest over postnatal development. Some transcripts (e.g., GABAA α1) progressively increased from birth until adulthood, whereas others (e.g., GABAA α2) declined with age. Changes in some transcripts were present in only one layer (e.g., GABAA δ). The development of GABAA receptor subunit expression in primate prefrontal pyramidal neurons is protracted and subunit- and layer-specific. These trajectories might contribute to the molecular basis for the maturation of working memory.
Keywords: GABA, glutamate, neurodevelopment, prefrontal cortex, pyramidal cells
Introduction
The primate dorsolateral prefrontal cortex (DLPFC) plays a critical role in cognition, particularly in tasks involving spatial working memory, the ability to transiently maintain and manipulate a limited amount of spatial information in order to guide thought or behavior. In both monkeys and humans, performance on spatial working memory tasks progressively improves from early childhood through adolescence, with mature levels of performance not achieved until late adolescence or early adulthood (Goldman 1971; Diamond 2002; Luna et al. 2010). This age-related improvement in performance appears to reflect the maturation of the functional architecture of the DLPFC, and consequently, its increased participation in the neural circuits that mediate spatial working memory (Alexander and Goldman 1978; Alexander 1982).
Within DLPFC circuitry, pyramidal cells in layer 3 appear to be preferentially involved in mediating working memory. For example neural activity during the delay period of spatial working memory tasks is most pronounced in the supragranular layers of the monkey DLPFC, with the greatest activity in layer 3 pyramidal cells (Sawaguchi et al. 1991; Friedman and Goldman-Rakic 1994). In addition, reciprocal connections among populations of spatially segregated clusters of layer 3 pyramidal cells are thought to provide the anatomical substrate for the recurrent excitation that sustains the firing of these neurons during the delay period of working memory tasks, serving as the cellular basis for keeping information “on-line” (Goldman-Rakic 1995; Arnsten et al. 2012).
The time course of spatial working memory maturation is correlated with a developmental increase in the proportion of DLPFC neurons that exhibit delay period firing (Alexander 1982). This age-related change in neural activity patterns is associated with developmental refinements in structural markers of excitatory inputs to layer 3 pyramidal cells. For example in layer 3 of both monkey and human DLPFC, the density of pyramidal neuron dendritic spines, the site of most excitatory synapses, and the density of axospinous synapses increase markedly during early postnatal life, remain at a plateau during childhood, and then decrease by 40–50% during adolescence (Huttenlocher 1979; Bourgeois et al. 1994; Anderson et al. 1995; Petanjek et al. 2011).
Importantly, the activity of layer 3 pyramidal neurons during working memory tasks is also spatially tuned by inhibitory inputs. For example interneurons in the monkey DLPFC demonstrate delay period activity that is isodirectionally tuned to nearby pyramidal neurons (Rao et al. 1999). Furthermore, pharmacological blockade with GABA antagonists in the DLPFC disrupts both the spatial tuning of pyramidal neurons and behavioral performance during working memory tasks (Rao et al. 2000; Constantinidis et al. 2002). Consistent with this role of inhibition in spatial working memory, the axon terminals of the subset of GABA neurons (basket and chandelier cells) that express the calcium-binding protein parvalbumin also undergo substantial developmental refinements in layer 3 of monkey DLPFC. For example parvalbumin protein levels in the axon terminals of basket neurons progressively increase during postnatal development, whereas the density of chandelier neuron axon terminals decreases (Erickson and Lewis 2002; Cruz et al. 2003; Fish et al. 2013). Basket and chandelier neurons, and other classes of GABA neurons, selectively target different domains on pyramidal cells and regulate their activity through GABAA receptors with different subunit compositions (Farrant and Nusser 2005; Farrant and Kaila 2007; Jacob et al. 2008). Moreover, the subunit composition of GABAA receptors contributes to the specificity of their electrophysiological properties. For example α1-containing GABAA receptors generate currents with a much faster decay time relative to α2- or α5-containing GABAA receptors (Farrant and Nusser 2005). Thus, different GABAA receptor subunits appear to play specialized roles in regulating the activity of layer 3 pyramidal cells, and changes in the expression of these subunits with age may contribute to the maturation of spatial working memory performance.
Consequently, we sought to determine whether the expression of different GABAA receptor subunits in layer 3 pyramidal neurons have distinctive developmental trajectories, whether these trajectories differ from those of major markers (AMPA Glur1 and NMDA Grin1 subunits) of excitatory neurotransmission, and whether these developmental changes are specific to layer 3 pyramidal cells. In order to address these questions, we used laser microdissection techniques to capture individual layer 3 pyramidal cells from the DLPFC of monkeys ranging in age from postnatal 1 week to 11.5 years and quantified the expression levels of the transcripts of interest by qPCR. Identical studies were conducted in layer 5 pyramidal cells which differ from layer 3 pyramidal cells based on their birth date, source of afferents, principal projection targets (Jones 1984), and role in working memory circuitry (Arnsten et al. 2012). Moreover, available evidence indicates that recurrent excitatory connections between layer 3 and 5 pyramidal cells (Kritzer and Goldman-Rakic 1995) might be critical for the persistent firing of these cells that is required to sustain mental representation in the absence of ongoing sensory input (Arnsten et al. 2012).
Materials and Methods
Animals
We used 26 rhesus (Macacca mulatta) monkeys ranging in age from 1 week to 11.5 years (Supplementary Table 1). All monkeys were female except for one adult male. Monkeys younger than 6 months of age were housed with their mothers; juveniles 6–24 months were housed in groups; and those older than 24 months were housed either in pairs, or in single cages, in the same social setting as previously described (Erickson and Lewis 2002). All housing and experimental procedures were conducted in accordance with guidelines set by the US Department of Agriculture and the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with approval of the University of Pittsburgh's Institutional Animal Care and Use Committee.
Eleven monkeys were perfused transcardially with ice-cold artificial cerebrospinal fluid under deep anesthesia with ketamine and pentobarbital (Gonzalez-Burgos et al. 2008); in 2 of these monkeys, a small tissue block was surgically excised from the rostral third of the principal sulcus in the left hemisphere for electrophysiology studies 2–4 weeks prior to perfusion. The remaining 15 monkeys, all experimentally naïve, were deeply anesthetized with ketamine and pentobarbital. After the monkey was deeply anesthetized, a scalpel and heavy-duty scissors were used to quickly remove the head. Rongeurs were then used to remove the top of the skull and fine scissors used to resect the dura. The brain was then removed intact. Standard coronal blocks (∼5 mm thick) were cut from the right hemisphere, placed on 2″ × 3″ glass slides and immersed in isopentane chilled on dry ice. Each frozen block was then placed in an appropriately labeled bag and stored in a −80°C freezer.
Monkeys were divided into the following 4 age groups based on the previously identified inflection points in the developmental trajectories of excitatory inputs to layer 3 pyramidal cells in monkey DLPFC (Bourgeois et al. 1994; Anderson et al. 1995): 1) perinatal (n = 6), monkeys from 0.1 to 1.5 months of age, within the period of a rapid increase in density of excitatory synapses and spines; 2) prepubertal (n = 7), monkeys from 3 to 9 months of age, within the period when the density of excitatory synapses and spines is at a plateau; 3) peripubertal (n = 7), monkeys from 16 to 32 months of age, within the period of excitatory synapse and spine pruning; 4) adult (n = 6), monkeys from 45 to 138 months of age, during the period when excitatory synapse and spine density are at stable adult levels.
Laser Microdissection Analyses
Cryostat sections (12 μm) were cut from coronal blocks containing DLPFC areas 9 and 46 (Fig. 1A–C), thaw-mounted onto glass polyethylene naphthalate membrane slides (Leica Microsystems, Bannockburn, IL, USA) that had been previously UV-treated at 254 nm for 30 min, dried briefly, and stored at −80°C. On the day of the microdissection, slides were immersed in an ethanol–acetic acid fixation solution, stained with thionin, dehydrated through 100% ethanol, and air-dried. Using a Leica microdissection system (LMD 6500; ×40 objective; power, 15; aperture, 9; speed, 12; balance, 14; and offset, 180), individual pyramidal neurons in deep layer 3 or layer 5 were captured as previously described (Arion and Lewis 2011). Approximately 150 Nissl-stained pyramidal cells from each of layer 3 and 5 were collected from each of 3 different slides per animal. Pyramidal cells were identified based on their characteristic somal morphology and the presence of a prominent apical dendrite directed radially toward the pia mater (Fig. 1D–E). The pyramidal cells from a given layer for each monkey were pooled together in 0.5-mL microtube caps (Ambion/Applied Biosystems, Foster City, CA, USA), resulting in a sample of ∼450 pyramidal cells per layer per subject, and lysed by vortexing for 30 s in 200 μL of RLT Buffer Plus (QIAGEN, Valencia, CA, USA). The RNA was purified using the RNeasy Plus Micro kit (QIAGEN).
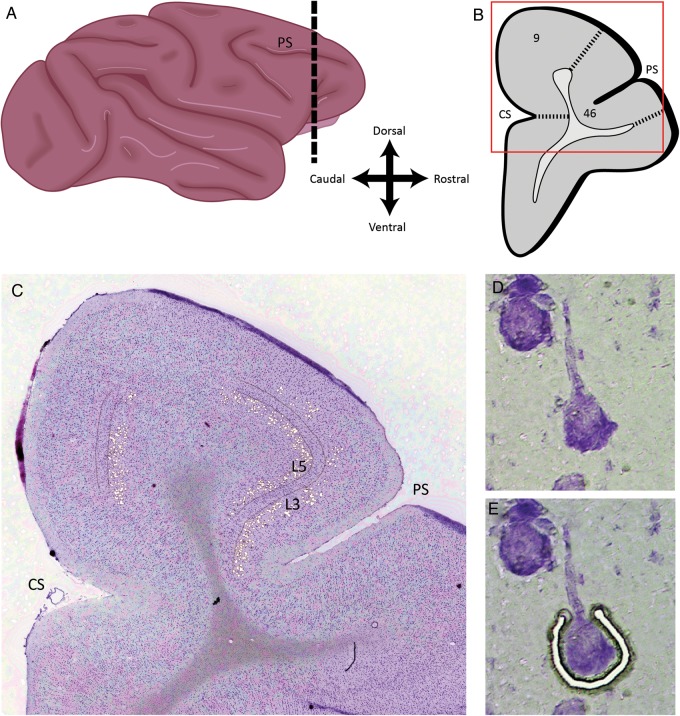
Figure 1.
Dissection of individual pyramidal cells in different laminar locations from monkey DLPFC. (A) Schematic drawing of the lateral view of the macaque monkey brain showing the approximate location (dotted black lines) of DLPFC sections used in the study. PS indicates principal sulcus and CS indicates cingulate sulcus. (B) Schematic drawing of a representative coronal section of monkey DLPFC, at the rostral–caudal level shown in A, showing the location of areas 9 and 46. Box indicates the portion of the section shown in C. (C) Thionin-stained coronal section showing the locations where pyramidal cells were captured in layer 3 and layer 5. (D) Digitally optimized representative image of a pyramidal cell in layer 3. (E) The same pyramidal cell in the process of being captured by laser microdissection.
In order to validate the cell type specificity of the dissected neurons, and the absence of glial or GABA cell contamination, we obtained LMD samples of Nissl-stained 1) pyramidal neurons identified using the criteria described, 2) nonpyramidal neurons (presumably interneurons), and 3) glial cells from the same tissue sections. The cDNA from each sample was used to quantify levels of specific markers of interneurons (glutamic acid decarboxylase 67, GAD67) and glial cells (myelin basic protein, MBP) by qPCR. In pyramidal cells, expression levels of GAD67 were only 3.3% of those in interneurons and MBP only 0.6% of those in glial cells. In concert, these findings demonstrate that our dissection method results in the selective collection of pyramidal neurons without contamination by other cortical cell types.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was converted to complementary DNA using the qScript™ cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD, USA). Priming was performed with a mix of poly dT and random hexamers, according to the manufacturer's recommendations. The efficiency for each primer set was between 90% and 100%, and the amplified product resulted in a specific single amplicon in dissociation curve analysis (Supplementary Table 2). Given the limitations on the number of cells that could be captured, and the resulting limits on the amount of available cDNA from each sample, we were able to profile a total of 8 transcripts of interest (and 2 reference genes) from each sample. We determined transcript levels of the major postsynaptic GABAA receptor subunits (α1, α2, α5, β2, γ2, and δ) and of critical subunits for AMPA (Glur1) and NMDA (Grin1) glutamate receptors.
Samples from both layers of a given subject were always assayed on the same plate. For each sample, amplified product differences for each transcript were measured with 4 replicates using SYBR Green chemistry-based detection (Mimmack et al. 2004). β-Actin and cyclophilin A were used as endogenous reference genes to normalize the expression levels of transcripts, as these 2 transcripts have been previously shown to have stable levels of expression across development in monkey DLPFC (Volk et al. 2011; Hoftman et al. 2013). The qPCR reactions were carried out in StepOnePlus thermal cycler (Applied Biosystems) using the StepOnePlus software with the automatic baseline and threshold detection options selected. These data were exported to Microsoft Excel (Microsoft, Redmond, Washington) and delta cycle thresholds (dCTs) were calculated for each sample by using the geometric mean of the 2 endogenous reference genes as the normalization factor [i.e., cycle threshold (CT) for each transcript in a sample minus the geometric mean of β-actin and cyclophilin A CTs for the same sample]. Since the dCT represents the log2-transformed expression ratio of each transcript of interest to the geometric mean of the 2 reference genes, we calculated the more intuitive expression ratio (i.e., expression ratio = 2−dCTs) of each transcript and reported the results as expression ratios (Hashimoto et al. 2008; Volk et al. 2010).
Statistical Analysis
To assess the effect of age on transcript levels, we conducted both Pearson regression analyses for individual animal data and analyses of variance (ANOVA) for age group data for each transcript in layer 3 and layer 5 pyramidal cells. We also determined the mean and standard deviation of the expression ratios for each age group. Tukey's post hoc test was used for comparisons between age groups with α = 0.05. The reported P values for both the regression analyses and the ANOVAs were corrected for multiple comparisons (8 transcripts per layer times 2 layers equals 16 comparisons) using the Holm's simultaneous inference procedure (Volk et al. 2000).
Results
Postnatal Expression of GABAA Receptor Subunit mRNAs in Pyramidal Cells
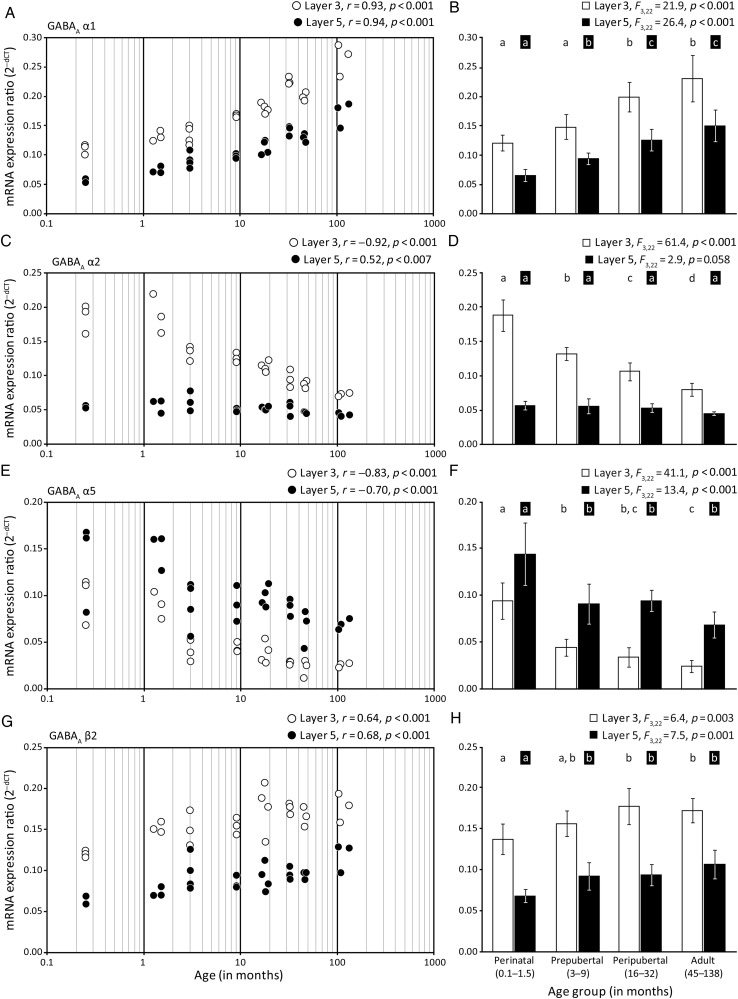
In monkey DLPFC, the levels of GABAA receptor α1 subunit mRNA significantly increased with age in both layer 3 (r = 0.93, P < 0.001) and layer 5 (r = 0.94, P < 0.001) pyramidal neurons (Fig. 2A). From the youngest (3 animals 1 week of age) to oldest (3 animals over 8 years of age) monkeys (Supplementary Table 1), mean levels of the α1 subunit mRNA increased by 137% in layer 3 and by 199% in layer 5 (Table 1). Analysis by age group also revealed significant differences in mRNA levels in both layer 3 (F3,22 = 21.9, P < 0.001) and layer 5 (F3,22 = 26.5, P < 0.001) pyramidal neurons, with post hoc analyses revealing significant (P < 0.05) increases in expression of α1 subunit mRNA between the perinatal to peripubertal age groups in layer 3 and between the perinatal to prepubertal and the prepubertal to peripubertal age groups in layer 5 (Fig. 2B). In addition, GABAA α1 subunit mRNA levels were higher in layer 3 than in layer 5 pyramidal cells in every animal, with expression levels ∼2-fold higher in layer 3 in each age group.
Figure 2.
Developmental trajectories of GABAA receptor α1, α2, α5, and β2 subunit mRNAs in layer 3 and 5 pyramidal cells in monkey DLPFC. The left panels show the expression ratios for each transcript in individual subjects for layer 3 (white circles) or layer 5 (black circles) pyramidal cells. The Pearson's correlation coefficient (r) and corresponding P-value are indicated for each panel on the left. The right panels show the mean (SD) expression ratios for each age group; age groups not sharing the same letter are significantly different (P < 0.05). (A and B) GABAA α1 subunit mRNA expression increased throughout postnatal development. (C and D) GABAA α2 subunit mRNA expression decreased selectively in layer 3 pyramidal cells from perinatal to adult. (E and F) GABAA α5 subunit mRNA expression decreased in both layers throughout development. (G and H) GABAA β2 subunit mRNA expression increased in both layers during development, paralleling the trajectory of GABAA α1 subunit mRNA.
Table 1.
Summary of differences, by layer, magnitude, and time of maturation, of the developmental trajectories for GABA and glutamate receptor subunits in layers 3 and 5 pyramidal cells of the monkey DLPFC
| Transcript | Layer 3 |
Layer 5 |
Magnitude of expression | ||
|---|---|---|---|---|---|
| Maximal % change from youngest to oldest | Period when adult level of expression achieved | Maximal % change from youngest to oldest | Period when adult level of expression achieved | ||
| GABAA α1 | 137 | Adult | 199 | Peripubertal | L3>L5 |
| GABAA α2 | −61 | Adult | – | Perinatal | L3>L5 |
| GABAA α5 | −74 | Peripubertal | −50 | Prepubertal | L5>L3 |
| GABAA β2 | 47 | Peripubertal | 89 | Prepubertal | L3>L5 |
| GABAA γ2 | 92 | Adult | 163 | Adult | L3>L5 |
| GABAA δ | – | Perinatal | 193 | Adult | L3>L5 |
| AMPA Glur1 | −37 | Peripubertal | – | Perinatal | L5>L3 |
| NMDA Grin1 | – | Perinatal | – | Perinatal | L3>L5 |
The youngest age group comprises of 3 animals of 1 week of age and the oldest animals comprises of 3 animals that were over 8 years of age.
In contrast, expression levels of the GABAA α2 subunit mRNA selectively decreased across postnatal development in layer 3 pyramidal cells (r = −0.92, P < 0.001), and did not change with age in layer 5 pyramidal neurons (Fig. 2C). From the youngest (3 animals 1 week of age) to oldest (3 animals over 8 years of age) monkeys, mean levels of the α2 subunit mRNA decreased by 61% in layer 3 (Table 1). Analysis by age group also revealed significant differences in mRNA levels in layer 3 (F3,22 = 61.5, P < 0.001), with post hoc analyses revealing significant (P < 0.05) decreases in α2 subunit expression between each pair of adjacent age groups (Fig. 2D). Although the level of GABAA α2 subunit mRNA was higher in layer 3 than layer 5 in every age group, this difference declined from ∼4-fold higher in layer 3 in the perinatal age group to <2-fold in the adult age group.
In contrast to the developmental trajectories exhibited by α1 and α2 subunits, expression of α5 subunit mRNA significantly decreased in both layer 3 (r = −0.83, P < 0.001) and layer 5 (r = −0.70, P < 0.001) pyramidal neurons (Fig. 2E). From the youngest (3 animals 1 week of age) to oldest (3 animals over 8 years of age) monkeys, mean levels of the α5 subunit mRNA decreased by 74% in layer 3 and by 50% in layer 5 (Table 1). Analysis by age group also revealed significant differences in mRNA levels in both layer 3 (F3,22 = 41.1, P < 0.001) and layer 5 (F3,22 = 13.5, P < 0.001) pyramidal neurons, with post hoc analyses showing significant (P < 0.05) decreases in α5 subunit expression between the perinatal to prepubertal and prepubertal to adult age groups in layer 3 pyramidal cells and between the perinatal to prepubertal age group in layer 5 pyramidal cells (Fig. 2F). The laminar pattern of GABAA α5 subunit mRNA expression also differed from α1 and α2 subunits as α5 levels were ∼2-fold higher in layer 5 than layer 3 pyramidal cells across all age groups.
The GABAA β2 subunit, which preferentially assembles with the α1 subunit (Farrant and Nusser 2005), showed a developmental pattern of expression similar to that of the α1 subunit. Transcript levels of β2 significantly increased in both layer 3 (r = 0.64, P < 0.001) and layer 5 (r = 0.68, P < 0.001) pyramidal cells (Fig. 2G). From the youngest (3 animals 1 week of age) to oldest (3 animals over 8 years of age) monkeys, mean levels of the β2 subunit mRNA increased by 47% in layer 3 and by 89% in layer 5 (Table 1). Analysis by age group also revealed significant differences in mRNA levels in both layer 3 (F3,22 = 6.4, P = 0.003) and layer 5 (F3,22 = 7.5, P = 0.001) (Fig. 2H) pyramidal neurons, with post hoc analyses revealing significant (P < 0.05) increases in β2 subunit expression between the perinatal to peripubertal age groups in layer 3 and between the perinatal to prepubertal age groups in layer 5 pyramidal neurons (Fig. 2H). Similar to the α1 subunit, laminar analyses showed that the expression of GABAA β2 subunit was ∼2-fold higher in layer 3 than layer 5 pyramidal cells in every age group.
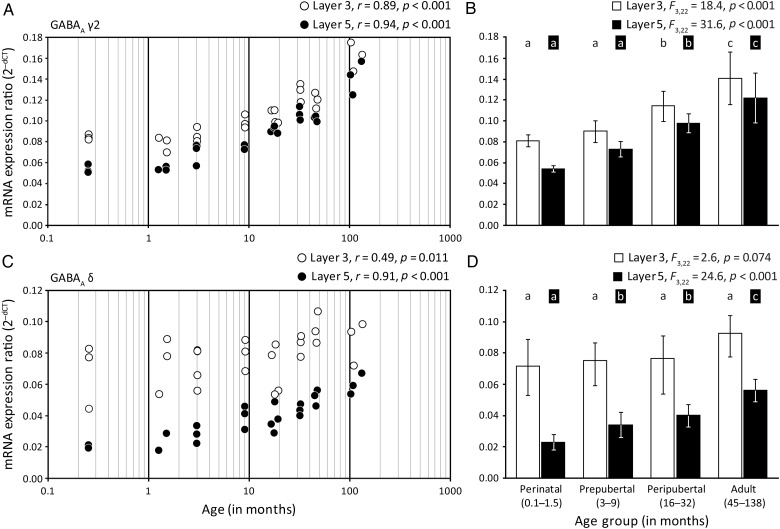
In postsynaptic locations, most α1-, α2-, and α5-containing GABAA receptors also contain a γ2 subunit (Farrant and Nusser 2005; Jacob et al. 2008). Expression of GABAA receptor γ2 subunit mRNA significantly increased in both layer 3 (r = 0.89, P < 0.001) and layer 5 (r = 0.94, P < 0.001) pyramidal neurons (Fig. 3A). From the youngest (3 animals 1 week of age) to oldest (3 animals over 8 years of age) monkeys, mean levels of the γ2 subunit mRNA increased by 92% in layer 3 and by 163% in layer 5 (Table 1). Analysis by age group also revealed significant differences in mRNA levels in both layer 3 (F3,22 = 18.5, P < 0.001) and layer 5 (F3,22 = 31.7, P < 0.001) pyramidal neurons, with post hoc analyses revealing significant (P < 0.05) increases in γ2 subunit expression between the perinatal to peripubertal and peripubertal to adult age groups in both layer 3 and layer 5 pyramidal cells (Fig. 3B). The magnitude of expression of the γ2 subunit was similar between layers with a slightly higher expression in layer 3 pyramidal cells at all developmental age groups.
Figure 3.
Developmental trajectories of GABAA receptor γ2 and δ subunit mRNAs in layer 3 and layer 5 pyramidal cells in monkey DLPFC. The left panels show the expression ratios for each transcript in individual subjects for layer 3 (white circles) or layer 5 (black circles) pyramidal cells. The Pearson's correlation coefficient (r) and corresponding P-value are indicated for each panel on the left. The right panels show the mean (SD) expression ratios for each age group; age groups not sharing the same letter are significantly different (P < 0.05). (A and B) GABAA γ2 subunit mRNA expression increased throughout postnatal development in both layers. (C and D) GABAA δ receptor subunit mRNA expression increased selectively in layer 5 pyramidal cells although the magnitude of expression was higher in layer 3 pyramidal cells.
Cortical GABAA receptors containing δ subunits are localized extrasynaptically, have a high affinity for GABA, and mediate tonic inhibition, defined as the constant activation of extrasynaptic receptors that, by increasing input conductance, reduces the probability of generating an action potential (Nusser et al. 1998; Wei et al. 2003; Farrant and Nusser 2005). Our results showed that the expression of the GABAA receptor δ subunit mRNA significantly increased in layer 5 (r = 0.91, P < 0.001) pyramidal cells, but did not change in layer 3 pyramidal cells across postnatal development (Fig. 3C). From the youngest (3 animals 1 week of age) to oldest (3 animals over 8 years of age) monkeys, mean levels of the δ subunit mRNA increased by 193% in layer 5 (Table 1). Analysis by age group revealed significant differences in mRNA levels in layer 5 (F3,22 = 24.7, P < 0.001) pyramidal neurons, with post hoc analyses revealing significant (P < 0.05) increases between the perinatal to prepubertal and prepubertal to adult age groups (Fig. 3D). Expression level of the δ subunit was ∼4-fold higher during the perinatal period in layer 3 compared with layer 5 pyramidal cells, with the levels of expression becoming similar in both layers in the adult age group.
Postnatal Expression of Glutamate AMPA and NMDA Receptor Subunit mRNAs in Pyramidal Cells
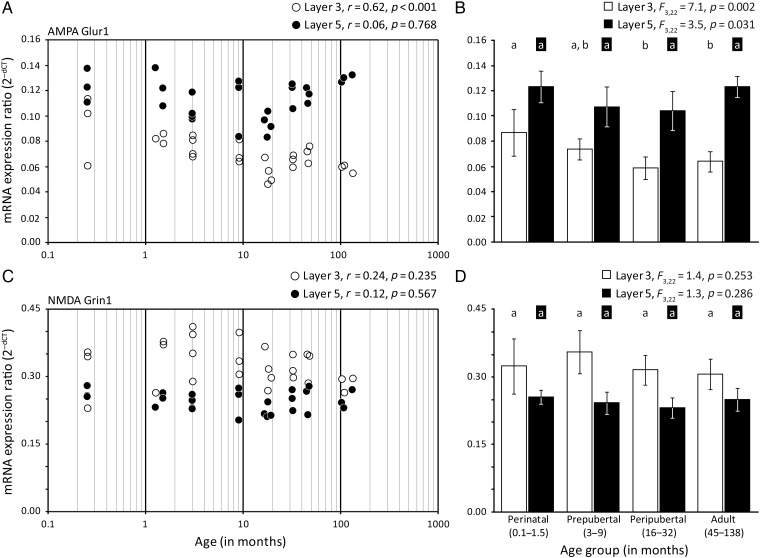
In order to determine the specificity of these developmental changes in expression of GABAA receptor subunits, we also evaluated the expression patterns for 2 glutamate receptor subunits that are critical mediators of excitatory neurotransmission (Cull-Candy et al. 2001; Traynelis et al. 2010). Expression of AMPA receptor Glur1 subunit mRNA significantly decreased in layer 3 (r = −0.62, P < 0.001) pyramidal cells, and did not change in layer 5 pyramidal cells (Fig. 4A). From the youngest (3 animals 1 week of age) to oldest (3 animals over 8 years of age) monkeys, mean levels of the AMPA Glur1 subunit mRNA decreased by 37% in layer 3 (Table 1). Analysis by age group revealed significant differences in mRNA levels in layer 3 (F3,22 = 7.2, P = 0.002) pyramidal cells, with post hoc analyses revealing significant (P < 0.05), albeit modest, decreases in AMPA Glur1 subunit expression between the perinatal to peripubertal age group in layer 3 pyramidal cells (Fig. 4B). Expression levels of the AMPA Glur1 subunit in layer 5 pyramidal cells were almost 2-fold higher in layer 5 compared with layer 3 pyramidal cells in every animal across all ages.
Figure 4.
Developmental trajectories of glutamate receptor subunits AMPA Glur1 and NMDA Grin1 mRNAs in layers 3 and 5 pyramidal cells of monkey. The left panels show the expression ratios for each transcript in individual subjects for layer 3 (white circles) or layer 5 (black circles) pyramidal cells. The Pearson's correlation coefficient (r) and corresponding P-value are indicated for each panel on the left. The right panels show the mean (SD) expression ratios for each age group; age groups not sharing the same letter are significantly different (P < 0.05). (A and B) AMPA Glur1 subunit mRNA expression decreased between the perinatal and peripubertal age groups during development in layer 3 pyramidal cells. (C and D) NMDA Grin1 subunit mRNA expression was largely unchanged throughout development.
The developmental trajectory of the obligatory subunit for NMDA receptors, Grin1 (Monyer et al. 1992; Schorge and Colquhoun 2003; Ulbrich and Isacoff 2008), did not show any significant changes in either layer 3 or layer 5 pyramidal cells during postnatal development (Fig. 4C,D). The expression of the NMDA Grin1 subunit was higher in layer 3 than layer 5 pyramidal cells across all age groups (perinatal = 26.6%; prepubertal = 46.7%; peripubertal = 36.2%; adult = 22.9%).
Discussion
In this study, we used a cell type-specific approach to determine if the expression of GABAA receptor subunits specifically in layer 3 pyramidal neurons in monkey DLPFC exhibit distinctive developmental trajectories, whether these trajectories differ from those of excitatory receptor subunits in these neurons, and whether these developmental patterns of receptor transcript expression differ from those in layer 5 pyramidal cells.
Expression levels of all 6 GABAA receptor subunits studied changed over a protracted period of postnatal development. Some transcripts (e.g., GABAA receptor α1 subunit) in layer 3 pyramidal neurons progressively increased from birth until the adult age group, whereas others (e.g., GABAA receptor α2 subunit) declined across the same developmental period. In contrast, postnatal developmental changes in both glutamate receptor subunits were modest, with adult levels reached early in life. Some transcripts exhibited a similar direction of developmental change in both layer 3 and 5 pyramidal cells (e.g., GABAA α5 decreased in both layers and GABAA β2 increased in both layers), whereas others (e.g., GABAA receptor α2 and δ subunits and AMPA Glur1) changed with age only in one layer. Transcripts also differed in terms of whether expression levels were consistently higher in layer 3 (e.g., GABAA receptor γ2 subunit) or layer 5 (e.g., glutamate AMPA receptor Glur1 subunit) pyramidal cells.
Our focus on gene expression patterns in a specific population of neurons may be particularly informative since measures of transcript levels in total gray matter may be diluted by other cell types. For example previous studies of total gray matter suggested a less robust increase in GABAA α1 subunit levels during development (Hoftman et al. 2013) than those observed in layer 3 and 5 pyramidal cells in the present study. Moreover, the collection of the same number of neurons per subject also excludes the potential confound of gray matter measures that age-related differences in transcript levels reflect changes in neuron number or density rather than changes in gene expression.
Relationships among Developmental Trajectories of GABA Receptor Subunits
The postsynaptic influence of GABA released into the synaptic cleft is determined, in part, by the subunit composition of the GABAA receptor to which it binds. The majority of GABAA receptors assemble according to a 2α:2β:γ stoichiometry (Farrant and Kaila 2007; Olsen and Sieghart 2009; Luscher et al. 2011). The resulting receptor heterogeneity is associated with differences in 2 key parameters of synaptic transmission: the duration of inhibitory postsynaptic currents (IPSCs) and the nature of inhibition (i.e., phasic vs. tonic). Because GABAA receptor α subunits contribute to differences in the decay rate of IPSCs, the developmental trajectories reported here may underlie important changes in the functional properties of DLPFC circuitry. For example the decay time of GABA-activated currents is 6-fold faster when mediated by α1- than by α2- or α5-containing receptors (Lavoie et al. 1997; McClellan and Twyman 1999; Farrant and Nusser 2005). Thus, the progressive increase in expression of GABAA α1 subunit and decrease in expression of α2 and α5 receptor subunits in layer 3 pyramidal cells across postnatal development is likely to substantially decrease the duration of IPSCs in these neurons with age. Consistent with this interpretation, the decay time constant of IPSCs in layer 3 pyramidal cells of monkey DLFPC was reported to decline significantly by ∼25% between 16 and 44 months of age (Hashimoto et al. 2009). A similar correlation between developmental changes in tissue levels of α GABAA receptor subunits and of IPSC decay time has been observed in rodent neocortex and hippocampus (Cohen et al. 2000; Bosman et al. 2002), although these changes are completed much earlier in rodents (Le Magueresse and Monyer 2013).
Whether these findings reflect a switch in the α subunit composition of GABAA receptors at particular synaptic sites or a change in the number of GABAA receptors containing a particular α subunit at different synaptic locations remains an important question for future studies. In either case, the apparent increase in the relative numbers of receptors containing fast versus slow decay kinetics may contribute to the substrate for developmental changes in cortical network oscillations. For example gamma band (30–80 Hz) oscillations require a fast decay of GABA currents, such as that associated with the GABAA α1 subunit, in order for ensembles of pyramidal cells to be quickly released from synchronous inhibition in order to fire at a fast frequency (Bartos et al. 2007). The developmental increase in the relative abundance of GABAA α1 subunits that confer fast IPSC decay properties shown here may increase the capacity of prefrontal networks to generate gamma oscillations. In addition, our finding that the increase in the relative ratio of α1 to α2 or α5 subunit expression is more prominent in layer 3 than in layer 5 pyramidal cells is particularly interesting given recent findings that gamma oscillations originate principally in layer 3 of adult monkey association cortices (Buffalo et al. 2011). Because prefrontal gamma oscillation power increases in proportion to working memory load (Tallon-Baudry et al. 1999; Howard et al. 2003; Roux et al. 2012), our findings may represent an important factor in the postnatal developmental increase in the power of gamma oscillations in humans (Uhlhaas et al. 2009) and in working memory performance from 3 months of age to adulthood in monkeys (Goldman 1971; Alexander and Goldman 1978).
It is important to note that the subcellular location of the receptors affected by the opposed developmental trajectories of GABAA α1 and α2 subunits in layer 3 pyramidal cells and the expression levels of the cognate proteins cannot be determined from the present study. However, in adult animals, α1-containing receptors predominate postsynaptic to PV basket cell inputs, whereas α2-containing receptors predominate postsynaptic to PV chandelier cell inputs (Nusser et al. 1996; Klausberger et al. 2002). Previous studies using electron microscopy revealed that the overall density of symmetric GABAergic synapses does not change during postnatal development in monkey DLPFC (Bourgeois et al. 1994). However, recent work using quantitative, multilabel, confocal microscopy suggests that the pruning of axon terminals may be a cell type- and membrane domain-specific phenomenon which is not detectable when all GABAergic terminals are assessed without regard to cell type (Fish et al. 2013). For example the mean number of chandelier cell boutons per pyramidal neuron axon initial segment was significantly lower in adult compared with 3-month-old monkeys, whereas the density of basket cell boutons did not differ (Fish et al. 2013). In contrast, the levels of parvalbumin protein in basket cell axon terminals were ∼2-fold higher in adult relative to 3-month-old animals, whereas no age-related differences were found for parvalbumin protein levels in chandelier cell axon terminals (Fish et al. 2013). Although speculative, these findings raise the possibility that the GABAA receptors postsynaptic to these 2 types of GABA axon terminals may also exhibit distinctive types of developmental changes in response to the changes in their presynaptic inputs. For example due to the developmental pruning of chandelier cell terminals, the number of GABAA α2-containing postsynaptic receptors may similarly decline, leading to a progressively lower demand for expression of α2 mRNA. Similarly, based on findings that parvalbumin levels are correlated with the probability of GABA release (Eggermann and Jonas 2012), the parallel increase in parvalbumin protein levels in basket cell terminals and the number of postsynaptic GABAA α1-containing receptors suggested by the current findings would provide a synergistic mechanism for increasing the strength of inhibitory inputs from parvalbumin basket cell inputs and thus a greater capacity to generate gamma oscillations with age.
Comparison of Perisomatic and Dendritic Inhibition in Pyramidal Cells During Postnatal Development
In contrast to the perisomatic location of GABAA α1 and α2 subunits, GABAA receptors containing α5 subunits are localized on pyramidal cell apical dendrites (Ali and Thomson 2008) postsynaptic to GABA inputs from the Martinotti class of GABA neurons that express the neuropeptide somatostatin (SST) (Kawaguchi and Kubota 1996; Melchitzky and Lewis 2008). The SST-containing interneurons are low threshold-spiking cells that form electrically coupled networks which oscillate at theta frequency (4–8 Hz) (Beierlein et al. 2000; Gibson et al. 2005). Moreover, these inputs are critical mediators of dendritic inhibition which controls the local integration of inputs and mediates strong disynaptic inhibition in cortical circuits (Silberberg and Markram 2007; Kapfer et al. 2007; Murayama et al. 2009). Our findings of a developmental decline in expression of the GABAA α5 subunit in both layer 3 and layer 5 pyramidal cells suggests that dendritic inhibition decreases progressively during postnatal maturation in the primate DLPFC (Caraiscos et al. 2004). Expression of the mRNA for SST, which inhibits pyramidal neurons (Boehm and Betz 1997), also decreases significantly during postnatal development in the human PFC (Fung et al. 2010). Although the developmental trajectories of molecules regulating GABA release from SST terminals and of SST receptors in pyramidal neurons remain unknown, the available data suggest that both presynaptic (Fung et al. 2010) and postsynaptic components of dendritic inhibition onto layer 3 and 5 pyramidal cells decrease during maturation of the DLPFC. Thus, our results may provide the substrate for a progressive developmental shift from dendritic inhibition to perisomatic inhibition as the principal means to modulate pyramidal cell output. Alternatively, the apparent decrease in dendritic inhibition could parallel the decrease in density of dendritic spines and excitatory axospinous synapses during postnatal development (Bourgeois et al. 1994; Anderson et al. 1995; Petanjek et al. 2011). This notion is supported by recent findings suggesting that a significant proportion of dendrite-targeting GABA synapses are made onto individual spines to focally regulate Ca2+ signals and that GABA inputs onto spines have high rates of turnover in vivo (Kubota et al. 2007; Chen et al. 2012; van Versendaal et al. 2012; Chiu et al. 2013). Hence, the progressive decline in dendritic inhibition during postnatal development could be a response to the pruning of dendritic spines in apical dendrites.
Contrasting Development of Markers of Phasic Versus Tonic Inhibition of Pyramidal Cells
The γ2 subunit co-assembles with α1 and β2 subunits to form ∼60% of GABAA receptors in the adult cortex (Mohler 2006). Our data indicate that these 3 subunits all increase in a similar fashion across postnatal development, suggesting a progressive increase in phasic inhibition with age in both layer 3 and 5 pyramidal neurons. In contrast, expression of the GABAA δ receptor subunit increased exclusively in layer 5 pyramidal cells during postnatal development, consistent with the findings of a prior in situ hybridization study showing a selective increase in GABAA δ receptor subunit expression in layer 5 relative to layer 3 (Maldonado-Aviles et al. 2009). Interestingly, the γ2 to δ subunit ratio increased by 49% from the youngest (3 animals 1 week of age) to the oldest animals (3 animals over 8 years of age) in layer 3 pyramidal cells and decreased by 10% in layer 5 pyramidal cells. These comparisons suggest that the ratio of phasic to tonic inhibition becomes increasingly different between layer 3 and 5 pyramidal cells across postnatal development. The laminar-specific pattern of changes in the nature of GABA inhibition suggest that phasic inhibition predominates over tonic inhibition with age in layer 3 pyramidal cells during circuit maturation while the ratio of phasic to tonic inhibition remains unchanged in layer 5 pyramidal cells.
Development Trajectories of Molecular Markers of Inhibition Versus Excitation of DLPFC Pyramidal Neurons
Our experiments revealed marked differences in the magnitude of developmental changes in GABAA and glutamate receptor subunits in monkey DLPFC. In contrast to the robust developmental changes in GABAA receptor subunits, the developmental trajectories for the AMPA Glur1 and NMDA Grin1 subunits were relatively flat in both layers 3 and 5 pyramidal cells. Among other possibilities, the difference in trajectories may contribute to the molecular substrate for electrophysiological findings that excitatory postsynaptic currents in layer 3 pyramidal cells of monkey DLPFC achieve mature properties much earlier in postnatal development than do IPSCs (Gonzalez-Burgos et al. 2008; Hashimoto et al. 2009).
Conclusion
In aggregate, the developmental trajectories of transcripts critical in mediating GABA neurotransmission in pyramidal cells in the DLPFC support a dynamic and complex process of circuit maturation. Our findings suggest that the molecular determinants of GABA neurotransmission have distinctive developmental trajectories that differ in magnitude and direction of change, whereas the molecular determinants of glutamate neurotransmission are relatively stable and achieve adult expression levels early in development. As a result, the components of GABA neurotransmission in DLPFC circuitry may have distinctive developmentally sensitive periods during which environmental events may have a particular strength in either enriching or impairing circuit development.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by NIH grant MH051234 (D.A.L.) from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Supplementary Material
Notes
We thank Mary Brady for her technical expertise. Conflict of Interest: D.A.L. currently receives investigator-initiated research support from Bristol-Myers Squibb and Pfizer and in 2012–2014 served as a consultant in the areas of target identification and validation and new compound development to Autifony, Bristol-Myers Squibb, Concert Pharmaceuticals, and Sunovion.
References
- Alexander GE. 1982. Functional development of frontal association cortex in monkeys: behavioural and electrophysiological studies. Neurosci Res Prog Bull. 20:471–479. [PubMed] [Google Scholar]
- Alexander GE, Goldman PS. 1978. Functional development of the dorsolateral prefrontal cortex: an analysis utilizing reversible cryogenic depression. Brain Res. 143:233–249. [DOI] [PubMed] [Google Scholar]
- Ali AB, Thomson AM. 2008. Synaptic alpha 5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex. 18:1260–1271. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Classey JD, Condé F, Lund JS, Lewis DA. 1995. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 67:7–22. [DOI] [PubMed] [Google Scholar]
- Arion D, Lewis DA. 2011. Altered expression of regulators of the cortical chloride transporters NKCC1 and KCC2 in schizophrenia. Arch Gen Psychiatry. 68:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Wang MJ, Paspalas CD. 2012. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 76:223–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. 2007. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 8:45–56. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. 2000. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 3:904–910. [DOI] [PubMed] [Google Scholar]
- Boehm S, Betz H. 1997. Somatostatin inhibits excitatory transmission at rat hippocampal synapses via presynaptic receptors. J Neurosci. 17:4066–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman LW, Rosahl TW, Brussaard AB. 2002. Neonatal development of the rat visual cortex: synaptic function of GABAA receptor alpha subunits. J Physiol. 545:169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois J-P, Goldman-Rakic PS, Rakic P. 1994. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 4:78–96. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. 2011. Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci USA. 108:11262–11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You T, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, et al. 2004. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 101:3662–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Villa KL, Cha JW, So PT, Kubota Y, Nedivi E. 2012. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron. 74:361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GC, Higley MJ. 2013. Compartmentalization of GABAergic inhibition by dendritic spines. Science. 340:759–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Coulter DA. 2000. Protracted postnatal development of inhibitory synaptic transmission in rat hippocampal area CA1 neurons. J Neurophysiol. 84:2465–2476. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Williams GV, Goldman-Rakic PS. 2002. A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nat Neurosci. 5:175–180. [DOI] [PubMed] [Google Scholar]
- Cruz DA, Eggan SM, Lewis DA. 2003. Postnatal development of pre- and post-synaptic GABA markers at chandelier cell inputs to pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 465:385–400. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. 2001. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 11:327–335. [DOI] [PubMed] [Google Scholar]
- Diamond A. 2002. Normal development of prefrontal cortex from birth to young adulthood: cognitive functions, anatomy and biochemistry. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. London: Oxford University Press; p. 466–503. [Google Scholar]
- Eggermann E, Jonas P. 2012. How the ‘slow’ Ca(2+) buffer parvalbumin affects transmitter release in nanodomain-coupling regimes. Nat Neurosci. 15:20–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson SL, Lewis DA. 2002. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 448:186–202. [DOI] [PubMed] [Google Scholar]
- Farrant M, Kaila K. 2007. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res. 160:59–87. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. 2005. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 6:215–229. [DOI] [PubMed] [Google Scholar]
- Fish KN, Hoftman GD, Sheikh W, Kitchens M, Lewis DA. 2013. Parvalbumin-containing chandelier and basket cell boutons have distinctive modes of maturation in monkey prefrontal cortex. J Neurosci. 33:8352–8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HR, Goldman-Rakic PS. 1994. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. J Neurosci. 14:2775–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. 2010. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 167:1479–1488. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. 2005. Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4. J Neurophysiol. 93:467–480. [DOI] [PubMed] [Google Scholar]
- Goldman PS. 1971. Functional development of the prefrontal cortex in early life and the problem of neuronal plasticity. Exp Neurol. 32:366–387. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. 1995. Cellular basis of working memory. Neuron. 14:477–485. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G, Lewis DA. 2008. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cereb Cortex. 18:626–637. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. 2008. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 165:479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, Gonzalez-Burgos G, Lewis DA. 2009. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 65:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA. Forthcoming 2013. Altered cortical expression of GABA-related genes in schizophrenia: illness progression versus developmental disturbance. Schizophr Bull. [DOI] [PMC free article] [PubMed]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. 2003. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 13:1369–1374. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. 1979. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 163:195–205. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. 2008. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 9:331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. 1984. Laminar distribution of cortical efferent cells. In: Peters A., Jones EG., eds. Cerebral cortex, Vol. 1. New York: Plenum Press; p521–p553. [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. 2007. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci. 10:743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. 1996. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci. 16:2701–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Roberts JD, Somogyi P. 2002. Cell type- and input-specific differences in the number and subtypes of synaptic GABAA receptors in the hippocampus. J Neurosci. 22:2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Goldman-Rakic PS. 1995. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J Comp Neurol. 359:131–143. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hatada S, Kondo S, Karube F, Kawaguchi Y. 2007. Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. J Neurosci. 27:1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. 1997. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on α-subunit isoform. Biophys J. 73:2518–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse C, Monyer H. 2013. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 77:388–405. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O'Hearn K. 2010. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 72:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. 2011. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 70:385–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Aviles JG, Curley AA, Hashimoto T, Morrow AL, Ramsey AJ, O'Donnell P, Volk DW, Lewis DA. 2009. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 166:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan AM, Twyman RE. 1999. Receptor system response kintetics reveal functional subtypes of native murine and recombinant human GABAA receptors. J Physiol. 515:711–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchitzky DS, Lewis DA. 2008. Dendritic-targeting GABA neurons in monkey prefrontal cortex: comparison of somatostatin- and calretinin-immunoreactive axon terminals. Synapse. 62:456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimmack ML, Brooking J, Bahn S. 2004. Quantitative polymerase chain reaction: validation of microarray results from postmortem brain studies. Biol Psychiatry. 55:337–345. [DOI] [PubMed] [Google Scholar]
- Mohler H. 2006. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 326:505–516. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. 1992. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 256:1217–1221. [DOI] [PubMed] [Google Scholar]
- Murayama M, Perez-Garci E, Nevian T, Bock T, Senn W, Larkum ME. 2009. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature. 457:1137–1141. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy J-M, Somogyi P. 1996. Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA. 93:11939–11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. 1998. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 18:1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. 2009. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 56:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. 2011. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. 2000. Destruction and creation of spatial tuning by disinhibition: GABAA blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 20:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. 1999. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 81:1903–1916. [DOI] [PubMed] [Google Scholar]
- Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ. 2012. Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J Neurosci. 32:12411–12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K. 1991. Catecholaminergic Effects on Neuronal Activity Related to a Delayed Response Task in Monkey Prefrontal Cortex. J Neurophysiol. 63(6):1385–1400. [DOI] [PubMed] [Google Scholar]
- Schorge S, Colquhoun D. 2003. Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J Neurosci. 23:1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G, Markram H. 2007. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 53:735–746. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Kreiter A, Bertrand O. 1999. Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans. Vis Neurosci. 16:449–459. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. 2010. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 62:405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. 2009. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci USA. 106:9866–9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich MH, Isacoff EY. 2008. Rules of engagement for NMDA receptor subunits. Proc Natl Acad Sci USA. 105:14163–14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Versendaal D, Rajendran R, Saiepour MH, Klooster J, Smit-Rigter L, Sommeijer JP, De Zeeuw CI, Hofer SB, Heimel JA, Levelt CN. 2012. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron. 74:374–383. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. 2000. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 57:237–245. [DOI] [PubMed] [Google Scholar]
- Volk DW, Eggan SM, Lewis DA. 2010. Alterations in metabotropic glutamate receptor 1alpha and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. Am J Psychiatry. 167:1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Radchenkova PV, Walker EM, Sengupta EJ, Lewis DA. 2011. Cortical opioid markers in schizophrenia and across postnatal development. Cereb Cortex. 22:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. 2003. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 23:10650–10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.