Abstract
Intestinal microbial communities regulate a range of host physiological functions, from energy harvest and glucose homeostasis to immune development and regulation. Suez and colleagues (2014) recently demonstrated that artificial sweeteners alter gut microbial communities, leading to glucose intolerance in both mice and humans.
Microbial communities populate the mammalian gastrointestinal tract, closely associating with the host throughout its lifespan. The gut is an important site for metabolic and immune regulation, and microbial cells here substantially outnumber human cells in the entire body, making it a prime location for interaction (Human Microbiome Project Consortium, 2012). Microbial communities are involved in regulation of numerous host physiological processes, including metabolism, immunity, and growth (Cox and Blaser, 2013). Diet is a major driver of microbial composition and function within the gut, and distinct microbial populations have been associated with both host adiposity and metabolic diseases (Cox and Blaser, 2013). Because host diet, metabolic and immune regulation, and microbiota are deeply interconnected, disturbance of this homeostasis can have long-lasting implications for host development and health (Cox and Blaser, 2013). Suez and colleagues (2014) now provide more evidence of how diet-induced microbial disturbances alter host health, demonstrating that dietary sugar alternatives increase glucose intolerance in mice and human patients.
The last century has seen profound changes in the way industrialized humans live, eat, work, and receive medical treatment, impacting the microbial consortia that live in and on us (Blaser and Falkow, 2009). Modern humans consume diets that are increasingly high-fat, processed, and lower in plant matter, differing substantially from the foods on which our ancestors subsisted prior to the industrial revolution. Such changes have affected both human physiology and our microbial inhabitants. In parallel with modernization, rates of non-communicable, “post-modern” diseases — such as diabetes, obesity, allergies, and asthma — have increased alarmingly (Blaser and Falkow, 2009). To combat this trend without compromising our penchant for sweet foods, dietary alternatives are frequently marketed for reducing caloric intake.
The new study by Suez and colleagues (2014) described the effects of one such dietary change — increasing use of non-caloric artificial sweeteners (NAS) — on host glucose tolerance. The authors found that glucose intolerance, a marker of metabolic diseases such as diabetes mellitus, was increased in mice by regular consumption of the sweeteners saccharin, sucralose, or aspartame (Figure 1A). These changes accompanied altered intestinal bacterial communities, including several organisms that are associated with obesity, diabetes, and metabolic disease, and were suppressed by antibiotic treatment, suggesting a direct microbial role (Figure 1B).
Figure 1. Non-caloric artificial sweeteners (NAS) induce glucose intolerance via microbial dysbiosis.
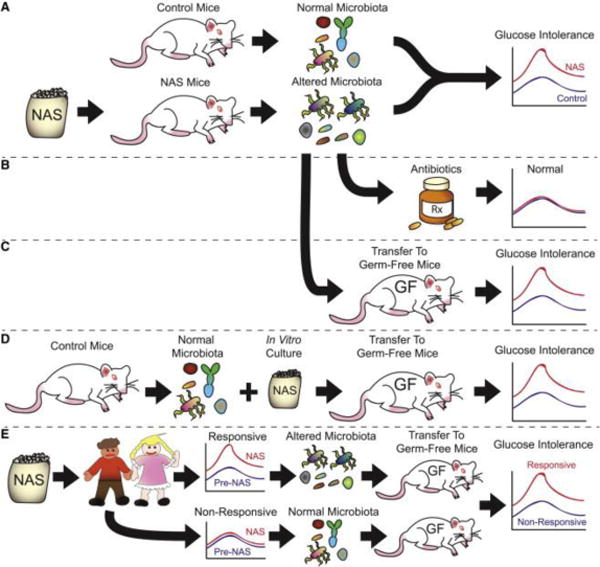
Schematic of the experimental design (Suez et al., 2014). A, NAS induction: Mice fed NAS developed altered intestinal microbial communities and glucose intolerance. B, Antibiotic suppression: treating these mice with antibiotics countered this effect, indicating microbial involvement. C, Microbial transfer from NAS-fed mice to germ-free (GF) mice fed normal chow induced glucose intolerance, compared to GF mice receiving control microbiota. D, NAS directly affects microbiota. Microbiota from control mice were grown in the presence of NAS in vitro and transferred to GF mice, inducing glucose intolerance compared to microbiota cultured without NAS. E, Personalized human response depends on microbiota. The responsiveness of adult human patients to NAS-induced glucose intolerance depended on prior microbial composition. When transferred to GF mice, microbiota from NAS-responsive patients induced glucose intolerance, while microbiota from NAS-non-responsive patients did not.
To test whether changes in microbial composition induced by NAS consumption led to glucose intolerance, the authors transferred intestinal microbiota from NAS-fed or control mice into germ-free mice, which are maintained under aseptic conditions, making their gastrointestinal tracts completely sterile. Thus, studies of microbial transfer into germ-free mice provide a unique opportunity to test the role of commensal microbiota on host physiology, since differences between control and treated animals can be attributed to the defined microbial inoculum given to the germ-free recipients, rather than to direct effects due to treatment. This approach has been employed with great success in defining how intestinal microbiota influence host metabolism under conditions of disturbance, such as comparing obese versus lean individuals (Turnbaugh et al., 2006) and during low-dose antibiotic exposure (Cox et al., 2014). Suez and colleagues found that germ-free mice inoculated with microbiota from NAS-fed mice became more glucose intolerant than mice conventionalized with control microbiota, demonstrating a causal role of the affected gut microbial communities (Figure 1C). Similar effects were also seen in germ-free mice receiving control microbiota that had been grown in vitro in the presence of NAS (Figure 1D). These results indicate that NAS consumption directly altered microbial composition and metabolism, leading to the important downstream metabolic effects.
In each of these experiments, similar impacts were seen on microbial gene composition, as assessed by shotgun metagenomic sequencing, indicating that NAS exerted an impact on microbial function. Glycan degradation pathways were strongly affected, leading to increased short-chain fatty acid (SCFA) abundance. Among other properties, SCFAs are consumed by intestinal epithelial cells, leading to enhanced energy harvest by the host (Turnbaugh et al., 2006), providing one possible mechanism for microbial alterations of glucose tolerance induced by NAS consumption. However, intestinal SCFA production also has been associated with increased secretion of the incretin hormone glucagon-like peptide (GLP)-1 and improved glucose tolerance (Tolhurst et al., 2012; Yadav et al., 2013), so the mechanisms of NAS-induced, microbial-mediated alterations in glucose tolerance are unclear and likely more complex than SCFAs alone.
The authors discovered a similar response to NAS consumption in non-diabetic humans, showing that these effects extend to human dietary choices. Healthy volunteers who did not normally consume NAS were fed saccharin daily for one week. The majority of these subjects developed poorer glycemic responses within one week, and had altered intestinal microbiota, distinguishing them from non-responders, who had neither altered glycemic responses nor microbial changes. Germ-free mice conventionalized with stool samples from NAS responders developed glucose intolerance compared to mice conventionalized with stools from the same patients pre-NAS or from non-responders (Figure 1E). These findings again provide evidence that NAS-induced dysbiosis had a causal role in inducing the glucose intolerance seen in these patients. These findings were consistent with the authors’ observations from a larger cohort of humans, in whom regular NAS consumption was positively correlated with intestinal microbial changes and multiple clinical parameters, including glucose intolerance and weight (Suez et al., 2014).
While specific microbial compositions clearly predispose human patients to NAS-induced metabolic effects, the factors that contribute to this susceptibility are unclear and warrant further investigation. Host genetics, diet, immune status, underlying diseases, and medical treatments all are features of patient history that influence human microbial composition and could determine individual responses to NAS consumption. We do not know whether NAS select against certain microbes by inhibiting their function, allowing their unaffected competitors to flourish, or whether they are direct stimulants of other organisms, or both. The mode of selection remains to be determined, but the problem is tractable. Also unclear is whether metabolic effects relate to differences in food and liquid intake between experimental groups. Future studies should carefully control intakes to minimize potential cofounder effects.
Biological variation similarly defines patient susceptibility to other microbe-mediated treatments, such as drug metabolism (Maurice et al., 2013) and dietary responses (Salonen et al., 2014). These studies all highlight the need to establish how microbial variation influences host responses to diet, therapies, and disease. The development and implementation of personalized treatments for complex diseases could likely involve manipulation of the microbiota.
In the interim, the findings of Suez and colleagues have more immediate consequences: that dietary sugar alternatives meant to stave off the risk of obesity and diabetes may actually increase disease risk due to microbial alterations. Other dietary additives may provoke similar microbial changes, and deserve further investigation. This is yet another indication that we are not alone and that microbial disturbances can lead to unexpected physiological effects.
Selected Reading
- Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nature reviews. Microbiology. 2009;7:887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Blaser MJ. Pathways in microbe-induced obesity. Cell metabolism. 2013;17:883–894. doi: 10.1016/j.cmet.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Human Microbiome Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39–50. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen A, Lahti L, Salojarvi J, Holtrop G, Korpela K, Duncan SH, Date P, Farquharson F, Johnstone AM, Lobley GE, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014 doi: 10.1038/ismej.2014.63. advance online publication 24 April 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014 doi: 10.1038/nature13793. advance online publication 17 September 2014. [DOI] [PubMed] [Google Scholar]
- Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein–coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. The Journal of Biological Chemistry. 2013;288:25088–25097. doi: 10.1074/jbc.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]


