Abstract
Background
It is unclear whether blood pressure control varies across the spectrum of atherosclerotic risk.
Methods
We used data from nonpregnant adults who had fasted laboratory samples drawn for the 2007-2009 cycle of the Canadian Health Measures Survey (CHMS) or the 2005-2008 US National Health and Nutrition Examination Survey (NHANES).
Results
The 1692 CHMS subjects and 3541 NHANES participants were demographically similar (aged a mean of 45 years), although NHANES participants exhibited higher obesity rates (33.8% vs 22.2%, P < 0.001). Over 80% of CHMS and NHANES subjects with hypertension had at least 1 other cardiovascular risk factor. As the number of atherosclerotic risk factors increased, hypertension prevalence increased, but blood pressure control rates improved (from 48% among hypertensives with no other risk factors in CHMS to 77% among those with 3 or more risk factors, and from 35% to 53% in NHANES). However, the converse was not true: The distribution of Framingham risk scores for those subjects with “controlled hypertension” was nearly identical to the distribution among those adults with uncontrolled hypertension in both CHMS and NHANES and substantially higher than scores in normotensive subjects.
Conclusions
Although control of blood pressure was better in patients with multiple atherosclerotic risk factors, hypertensives with controlled blood pressures exhibited risk-factor profiles similar to those of participants with uncontrolled blood pressures. This suggests the need, in educational messaging and therapy decision making, for an increased focus on total atherosclerotic risk rather than just blood pressure control.
Cardiovascular (CV) guidelines and continuing medical education activities have traditionally emphasized the treatment and attainment of “target levels” for individual risk factors such as hypertension, dyslipidemia, or dysglycemia.1 Although it has long been recognized that an individual’s absolute CV risk depends on the level of each of their CV risk factors,2-7 the interplay between multiple CV risk factors and their control rates is unclear in Canadian and US adults. While some studies have demonstrated that hypertensive patients with poorly controlled blood pressure but multiple comorbidities were less likely to have their antihypertensive therapy intensified,8 others have reported better blood pressure control and treatment intensification rates in those with multiple comorbidities.9 However, prior studies have focused on patients attending physician clinics and having discrete comorbidities (such as angina, chronic pulmonary disease, arthritis, depression, or diabetes), and it is unknown whether adults with other CV risk factors are more or less likely to have their blood pressure (BP) treated and controlled. We designed this study to explore whether BP control rates differed by CV risk profiles in nationally representative samples of individuals from Canada and the United States and to examine the extent to which other CV risk factors are optimized in Canadian and US adults with and without hypertension.
Methods
We used 2 North American population-based surveys that randomly sampled (using complex, multistage probability sampling) community-dwelling individuals, employed similar face-to-face questionnaires to ascertain medical history and medication use, measured physical attributes such as body mass index and BP levels, and collected fasting laboratory samples in a random sample of participants. The methodologies of cycle 1 of the Canadian Health Measures Survey (CHMS) 2007-2009 and the US National Health And Nutrition Examination Survey (NHANES) 2005-2008 have been described in full elsewhere.10-12
The NHANES collected up to 3 BP measurements with manual mercury sphygmomanometers, and for this analysis we averaged the second and third BP measurement in each subject. In the CHMS, BP was measured with an electronic oscillometric monitor (the BpTRU device, BpTRU Medical Devices Ltd, Coquitlam, British Columbia), and 6 readings were taken, with the last 5 averaged to determine the BP reading for each respondent. Although for this report we used the BpTRU measures for CHMS participants, in a prior publication12 we reported control rates after converting BpTRU measurements to manual sphygmomanometer estimates using a previously validated linear regression equation.13
We limited our analyses to participants aged 20 to 79 years with at least 2 BP measurements and excluded any subjects who were pregnant. We defined hypertension as being present if a subject had mean systolic BP (SBP) ≥ 140 mm Hg and/or mean diastolic BP (DBP) ≥ 90 mm Hg (or mean SBP ≥ 130 mm Hg and/or DBP ≥ 80 mm Hg in those with diabetes mellitus in a sensitivity analysis) and/or self-reported current use of BP-lowering medication or health care provider–assigned diagnosis of hypertension. We defined study participants with hypertension as having “controlled” BP if their SBP was < 140 mm Hg and their DBP was < 90 mm Hg (< 130/80 mm Hg for those with diabetes in a sensitivity analysis).
While prior studies of risk factors have used patient self-report to classify patients, we a priori decided to include fasting laboratory samples and medication reviews to derive “gold standard” case definitions for each of the CV risk factors we considered. For example, we classified a patient as having dyslipidemia if the patient had fasting low-density lipoprotein cholesterol ≥ 3.5 mmol/L plus elevated ratio of total to high-density lipoprotein cholesterol (≥ 5.5 among men, ≥ 4.5 among women) or if the patient was a current user of lipid-lowering medications. Other case definitions are given in the footnote to Table 1.14,15 We estimated each subject’s risk of incident CV disease (coronary death, nonfatal myocardial infarction, angina, stroke or transient ischemic attack, peripheral arterial disease, or heart failure) using the Framingham 10-year general CV risk prediction equation for those subjects aged between 30 and 74 years.16 We calculated the crude score for each subject and compared between patients with hypertension (controlled vs uncontrolled) and without hypertension.
Table 1.
Baseline characteristics of the 2007-2009 CHMS fasted sample
| Overall |
Nonhypertensives |
Hypertensives, controlled* |
Hypertensives, uncontrolled |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean or % |
95% CI | N | Mean or % |
95% CI | N | Mean or % |
95% CI | N | Mean or % |
95% CI | |
| Mean age | 1692 | 45.3 | 44.9-45.8 | 1233 | 41.6 | 41.1-42.0 | 269 | 60.4 | 58.6-62.2 | 190 | 57.5 | 54.9-60.1 |
| Men (%) | 807 | 49.7 | 49.1-50.3 | 569 | 49.6 | 48.3-51.0 | 139 | 48.8 | 42.0-55.6 | 99 | 51.7 | 41.6-61.8 |
| Mean systolic BP (mm Hg) | 1692 | 112.0 | 110.4-113.6 | 1233 | 107.7 | 106.6-108.8 | 269 | 119.9 | 117.7-122.2 | 190 | 139.0 | 131.9-146.2 |
| In men | 807 | 114.1 | 112.6-115.5 | 569 | 110.9 | 109.8-112.1 | 139 | 120.7 | 117.6-123.7 | 99 | 132.4 | 126.5-138.2 |
| In women | 885 | 109.9 | 107.7-112.1 | 664 | 104.6 | 102.8-106.4 | 130 | 119.2 | 115.9-122.5 | 91 | 146.2 | 136.9-155.4 |
| Current smoker | 324 | 23.0 | 20.5-25.5 | 253 | 24.0 | 20.6-27.3 | 27 | 11.1** | 4.8-17.3** | 44 | 31.7 | 23.1-40.3 |
| Diabetes† | 129 | 6.9 | 5.2-8.5 | 41 | 3.6** | 1.7-5.5** | 68 | 26.1** | 15.3-37.0** | †† | †† | †† |
| Dyslipidemia‡ | 443 | 22.5 | 18.7-26.3 | 224 | 16.5 | 12.6-20.5 | 152 | 55.5 | 48.6-62.3 | 67 | 29.1** | 15.9-42.3** |
| Obesity§ | 423 | 22.2 | 18.3-26.1 | 227 | 17.2 | 13.4-21.0 | 123 | 45.7 | 34.7-56.6 | 73 | 33.3 | 21.5-45.2 |
| Mean LDL-C (mmol/L) | 1692 | 3.07 | 2.97-3.16 | 1233 | 3.1 | 3.0-3.2 | 269 | 3.0 | 2.8-3.2 | 190 | 3.2 | 3.0-3.4 |
| Mean HDL-C (mmol/L) | 1692 | 1.34 | 1.28-1.39 | 1233 | 1.3 | 1.3-1.4 | 269 | 1.3 | 1.2-1.3 | 190 | 1.4 | 1.3-1.5 |
| Mean triglycerides (mmol/L) | 1692 | 1.47 | 1.09-1.84 | 1233 | 1.4 | 1.0-1.9 | 269 | 1.6 | 1.5-1.7 | 190 | 1.5 | 1.3-1.7 |
| Mean HbA1c (%) | 1648 | 5.4 | 5.3-5.6 | 1206 | 5.5 | 5.4-5.7 | 259 | 6.1 | 5.7-6.5 | 183 | 5.7 | 5.5-5.9 |
| Mean fasting glucose (mmol/L) | 1689 | 5.1 | 5.1-5.2 | 1233 | 5.0 | 4.9-5.1 | 268 | 5.8 | 5.5-6.1 | 188 | 5.4 | 5.2-5.6 |
| Mean BMI | 1690 | 27.0 | 26.4-27.6 | 1231 | 26.3 | 25.7-26.9 | 269 | 30.2 | 29.1-31.3 | 190 | 29.4 | 27.6-31.2 |
| Target organ damage | ||||||||||||
| Heart disease | 98 | 4.3** | 2.6-5.9** | 32 | 1.6** | 0.5-2.6** | 50 | 19.3 | 10.9-27.6 | 16 | 7.4 | 2.0-12.7 |
| Stroke or TIA | 20 | 1.1** | 0.6-1.6** | †† | †† | †† | 11 | 3.6** | 1.3-5.9** | †† | †† | †† |
| Cardiovascular disease¶ | 124 | 5.5 | 3.9-7.2 | †† | †† | †† | 66 | 23.6 | 16.1-31.1 | 20 | 10.1** | 4.8-15.3** |
| Chronic kidney disease∥ | 212 | 10.8 | 8.8-12.8 | 94 | 8.1 | 5.8-10.4 | 68 | 21.5 | 14.4-28.5 | 50 | 20.1** | 9.0-31.3** |
| Mean creatinine (μmol/L) | 1679 | 74.2 | 71.9-76.5 | 1225 | 72.6 | 70.6-74.7 | 267 | 81.8 | 71.9-91.7 | 187 | 77.4 | 68.0-86.8 |
| Number of total prescribed medications |
962 | 1.5 | 1.4-1.6 | 564 | 1.0 | 0.8-1.1 | 266 | 4.5 | 4.1-4.9 | 132 | 2.1 | 1.5-2.7 |
BMI, body mass index; BP, blood pressure; CI, confidence limit; HbA1c, hemoglobin A1c;, HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TIA, transient ischemic attack.
Hypertension control defined as an average systolic pressure < 140 mm Hg AND an average diastolic pressure < 90 mm Hg.
Diabetes defined as self-report of health professional-assigned diagnosis OR HbA1c ≥ 6.5 OR fasting plasma glucose > 7 mmol/L OR current use of glucose-lowering medications.
Dyslipidemia defined as fasting LDL > 3.5 mmol/L AND ratio of total cholesterol to HDL-C > 5.5 in men or > 4.5 in women, OR use of statins or other lipid-lowering medications.
Obesity defined as a body mass index [weight (kg)/height(m)2] ≥ 30.0.
Cardiovascular disease defined as history of either heart disease (coronary artery disease or heart failure) OR stroke or TIA.
Chronic kidney disease defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 (using isotope dilution mass spectrometry calibrated serum creatinine) [eGFR (mL/min/1.73 m2) = 175 × (serum creatinine)−1.154 × (age)−0.203 × (0.742 if female) × (1.210 if African American) (conventional units)] OR urinary albumin-to-creatinine ratio ≥ 30 mg/g.
Interpret with caution (coefficient of variation 16.6% to 33.3%).
Cannot be reported (coefficient of variation > 33.3% or N < 10).
Survey weights were applied to the CHMS results according to Statistics Canada CHMS Data User Guide: Cycle 1 (http://www.statcan.gc.ca/imdb-bmdi/document/5071_D2_T1_V1-eng.pdf). The weighted CHMS data were analyzed with SAS software (Enterprise Guide Version 4.1, SAS Institute Inc, Cary, NC). All NHANES analyses were conducted with the SUDAAN software package, which incorporates the survey’s complex sample design and the fasting sample weights, according to the NHANES Analytic and Reporting Guidelines (http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/analytical_guidelines.htm).
Results
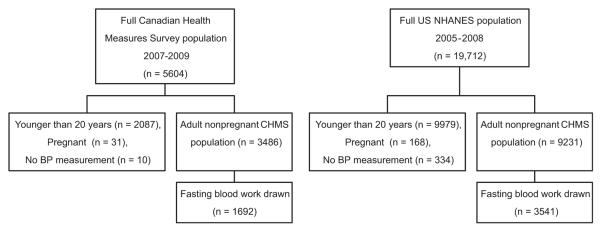
We analyzed data from 1692 CHMS participants and 3541 NHANES participants aged 20 to 79 years, not pregnant, had at least 2 BP readings, and had fasting blood work done (Fig. 1). The household response rates for the 2 surveys were comparable (70% in CHMS and 76% in NHANES).
Figure 1.
Derivation of both samples. BP, blood pressure; NHANES, National Health and Nutrition Examination Survey.
The CHMS (Table 1) and NHANES (Table 2) subjects were demographically similar and exhibited similar serum creatinine and fasting lipid profiles; however, NHANES participants were more likely to be obese (33.8% vs 22.2%, P < 0.001) or diabetic (11.1% vs 6.9%, P < 0.001) and had higher mean SBP (120 mm Hg vs 112 mm Hg, P < 0.001), body mass index (28.5 vs 27.0), and fasting serum glucose (5.8 mmol/L vs 5.1 mmol/L). Even after adjusting the CHMS BpTRU measurements to estimate manual sphygmomanometer readings (115.5 mm Hg with 95% confidence interval (CI) of 114.1-117.0 mm Hg), there was still a statistically significant difference between mean SBP in the Canadian and US samples (P < 0.001). There was no evidence of systematic bias in the 2 surveys between those who did and did not have fasting blood work done (data not shown). Of the 3541 fasted subjects in NHANES, 735 (21%) were at low CV risk (men younger than 55 years or women younger than 60 years without hypertension, target organ damage, or other atherosclerotic risk factors); 515 (30%) of the 1692 fasted CHMS subjects met the same definition of low CV risk.
Table 2.
Baseline characteristics of the 2005-2008 NHANES fasted sample
| Overall |
Nonhypertensives |
Hypertensives, controlled |
Hypertensives, uncontrolled |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean or % |
95% CI | N | Mean or % |
95% CI | N | Mean or % | 95% CI | N | Mean or % |
95% CI | |
| Mean age (years) | 3541 | 45.4 | 44.3-46.5 | 2119 | 40.8 | 39.7-41.9 | 603 | 57.0 | 55.2-58.7 | 819 | 51.9 | 50.4-53.3 |
| Men (%) | 1832 | 50.4 | 48.5-52.3 | 1096 | 49.7 | 46.9-52.5 | 298 | 49.9 | 45.3-54.6 | 438 | 53.3 | 48.7-57.8 |
| Mean systolic BP (mm Hg) | 3541 | 120.0 | 119-120.9 | 2119 | 114.0 | 113.2-114.8 | 603 | 120.3 | 119.3-121.4 | 819 | 140.0 | 138.3-141.6 |
| In men | 1832 | 121.7 | 120.9-122.6 | 1096 | 116.7 | 116.0-117.5 | 298 | 120.4 | 119.0-121.8 | 438 | 138.5 | 136.2-140.8 |
| In women | 1709 | 118.2 | 116.9-119.5 | 1023 | 111.3 | 110.4-112.2 | 305 | 120.2 | 118.5-122.0 | 381 | 141.7 | 139.1-144.3 |
| Current smoker | 825 | 24.8 | 22.0-27.7 | 514 | 25.0 | 22.0-28.2 | 115 | 20.1 | 15.1-26.2 | 196 | 27.7 | 24.2-31.6 |
| Diabetes (measured) | 590 | 11.1 | 9.7-12.7 | 153 | 4.8 | 3.8-6.0 | 220 | 29.1 | 24.5-34.1 | 217 | 18.4 | 15.6-21.6 |
| Dyslipidemia (measured) | 1032 | 25.9 | 23.6-28.4 | 368 | 16.2 | 14.4-18.2 | 356 | 55.3 | 48.2-62.1 | 308 | 36.1 | 30.3-42.3 |
| Obesity | 1303 | 33.8 | 31.4-36.3 | 603 | 26.4 | 23.8-29.1 | 332 | 53.5 | 48.1-58.9 | 368 | 43.6 | 40.6-46.8 |
| Mean LDL-C (mmol/L) | 3424 | 3.0 | 3.0-3.0 | 2065 | 3.0 | 3.0-3.1 | 575 | 2.8 | 2.7-2.9 | 784 | 3.1 | 3.1-3.2 |
| Mean HDL-C (mmol/L) | 3466 | 1.4 | 1.4-1.4 | 2074 | 1.4 | 1.4-1.5 | 594 | 1.3 | 1.3-1.4 | 798 | 1.4 | 1.4-1.4 |
| Mean triglycerides (mmol/L) | 3466 | 1.5 | 1.4-1.5 | 2074 | 1.4 | 1.3-1.4 | 594 | 1.7 | 1.6-1.8 | 798 | 1.8 | 1.6-1.9 |
| Mean HbA1c (%) | 3539 | 5.5 | 5.5-5.6 | 2119 | 5.4 | 5.3-5.4 | 602 | 5.9 | 5.8-6.0 | 818 | 5.7 | 5.6-5.8 |
| Mean fasting glucose (mmol/L) | 3541 | 5.8 | 5.8-5.9 | 2119 | 5.6 | 5.5-5.6 | 603 | 6.5 | 6.3-6.7 | 819 | 6.2 | 6.0-6.4 |
| Mean BMI | 3510 | 28.5 | 28.2-28.8 | 2106 | 27.4 | 27.0-27.7 | 597 | 31.3 | 30.6-32 | 807 | 30.2 | 29.7-30.8 |
| Target organ damage | ||||||||||||
| Heart disease | 281 | 6.3 | 5.3-7.4 | 68 | 2.4 | 1.8-3.3 | 115 | 16.3 | 12.5-20.9 | 98 | 11.5 | 9.2-14.2 |
| Stroke or TIA | 119 | 2.3 | 1.8-3.0 | 18 | 0.7 | 0.4-1.4 | 53 | 6.7 | 4.5-9.7 | 48 | 4.2 | 3.0-5.9 |
| Cardiovascular disease | 354 | 7.6 | 6.4-9.0 | 79 | 2.9 | 2.2-3.8 | 146 | 19.8 | 15.4-25.0 | 129 | 14.2 | 11.6-17.3 |
| Chronic kidney disease | 518 | 11.0 | 9.5-12.8 | 159 | 6.3 | 5.0-8.0 | 154 | 20.7 | 16.5-25.7 | 205 | 19.4 | 16.2-23.2 |
| Mean creatinine (umol/Ll) | 3512 | 76.9 | 75.7-78.0 | 2103 | 74.5 | 73.5-75.5 | 600 | 82.0 | 80.0-84.1 | 809 | 80.8 | 77.2-84.4 |
| Number of total prescribed medications |
3541 | 1.8 | 1.7-2 | 2119 | 1.1 | 1.0-1.2 | 603 | 4.4 | 4.2-4.7 | 819 | 2.4 | 2.1-2.7 |
BMI, body mass index; BP, blood pressure; CI, confidence limit; HbA1c, hemoglobin A1c;, HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TIA, transient ischemic attack.
Comparing subject self-report with the gold-standard case definitions we used in this analysis (based on laboratory measurements plus review of prescribed medications plus self-report), we found that although self-report by itself was reasonably accurate for diabetes mellitus (k, 0.73 [95% CI, 0.67-0.77]; sensitivity, 64% [95% CI, 59%-67%]; specificity, 99.5% [95% CI, 99.1%-99.7%]), self-report was considerably less accurate for dyslipidemia (k, 0.52 [95% CI, 0.47-0.57]; sensitivity, 69% [95% CI, 65%-72%]; specificity, 83% [95% CI, 82%-85%]) and chronic kidney disease (k, 0.08 [95% CI, 0.47-0.57]; sensitivity, 7% [95% CI, 5%-8%]; specificity, 99% [95% CI, 98%-99%]). This finding supported our decision to use case definitions that relied on more than just self-report in the analyses outlined below.
Hypertensive individuals were more likely to have other CV risk factors or target organ damage than were normotensive subjects in both CHMS and NHANES (Tables 1 and 2). Overall, 81% of hypertensive CHMS subjects (76% of those with controlled BPs and 85% of those with uncontrolled BPs) and 85% of hypertensive NHANES participants (88% of those with controlled BPs and 83% of those with uncontrolled BPs) had at least 1 other CV risk factor (compared with 49% and 60% of their normotensive peers, both P < 0.001). Target organ damage was more common in hypertensive participants in both surveys, particularly chronic kidney disease (21% vs 8% in CHMS and 20% vs 6% in NHANES, both P < 0.001); indeed, chronic kidney disease was more common than CV disease among these community-dwelling hypertensive participants. Of all 459 fasted hypertensive participants in CHMS, only 30 (7%) could be classified as “lower-risk hypertensives” (men younger than 55 years or women younger than 60 years without target organ damage or other risk factors, and with SBP < 160 mm Hg and DBP < 100 mm Hg). Of the 1422 fasted hypertensive subjects in NHANES, only 100 (7%) fit the same definition of “lower-risk hypertensives.”
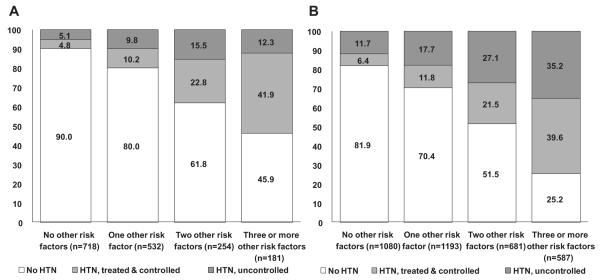
Hypertension prevalence, treatment, and control rates differed substantially across patient subgroups defined by comorbidity profiles (Figs. 2A and 2B). Although BP control rates were generally higher in Canada (59% if goal BP was defined as < 140/90 mm Hg in all patients and 55% if goal BP was defined as < 130/80 mm Hg in those with diabetes and < 140/90 mm Hg in all others) than in the United States (44% if goal BP was defined as < 140/90 mm Hg in all patients and 36% if goal BP was defined as < 130/80 mm Hg in those with diabetes and < 140/90 mm Hg in all others), the relative patterns were similar in Canada and the United States in that while those with overt CV disease, diabetes, or dyslipidemia exhibited the highest rates of BP treatment and control, those with hypertension and no other CV risk factors or who smoked or had chronic kidney disease exhibited the lowest rates of control. As the number of concomitant CV risk factors increased, hypertension prevalence increased in both Canada and the United States, as did the proportion of hypertensive individuals who were controlled (Figs. 3A and 3B).
Figure 2.
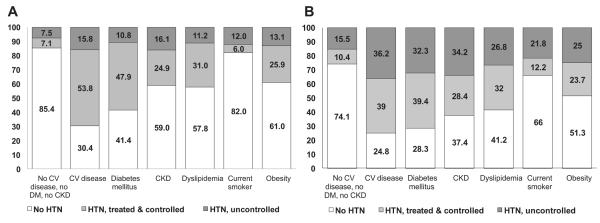
(A) Hypertension (HTN) prevalence, treatment, and control in Canadian Health Measures Survey participants, stratified by risk factors. Risk-factor groups are not mutually exclusive. Blood pressure (BP) control was defined as < 140/90 mm Hg in all groups. If definition of BP control is lowered to < 130/80 mm Hg in subjects with diabetes mellitus, the proportion with HTN treated and controlled is 54.6% and the proportion with HTN uncontrolled is 45.5%. (B) HTN prevalence, treatment, and control in National Health and Nutrition Examination Survey participants, stratified by risk factors. Risk-factor groups are not mutually exclusive. BP control was defined as < 140/90 mm Hg in all groups. If definition of BP control is lowered to < 130/80 mm Hg in subjects with diabetes mellitus, the proportion with HTN treated and controlled is 26% and the proportion with HTN uncontrolled is 52%. CKD, chronic kidney disease; CV, cardiovascular; DM, diabetes mellitus.
Figure 3.
(A) Hypertension (HTN) prevalence, treatment, and control in Canadian Health Measures Survey participants, stratified by number of other cardiovascular risk factors. Cardiovascular risk factors include smoking, obesity, diabetes mellitus, dyslipidemia, and chronic kidney disease (7 patients excluded from this figure because of missing data in at least 1 of these fields). (B) HTN prevalence, treatment, and control in National Health and Nutrition Examination Survey participants, stratified by number of other cardiovascular risk factors. Cardiovascular risk factors include smoking, obesity, diabetes mellitus, dyslipidemia, or chronic kidney disease.
Although hemoglobin A1c, fasting glucose, and body mass index were significantly lower in normotensive subjects than in hypertensive individuals in both surveys, there were no appreciable differences among those with hypertension who had controlled vs uncontrolled BPs in these risk factors or others such as cholesterol levels or serum creatinines in either CHMS (Table 1) or NHANES (Table 2). Even those subjects with hypertension who were defined as “controlled” since they were at (or below) target BPs had substantially higher Framingham scores than did those subjects without hypertension (Table 3). Indeed, the distribution of Framingham risk scores for the “controlled hypertension” subjects was nearly identical to the distribution among those adults with hypertension who were uncontrolled in both CHMS and NHANES (Table 3).
Table 3.
Framingham risk score distribution by hypertension status in individuals aged 30 to 74 years
| CHMS |
NHANES |
|||||
|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | |
| 10-year general cardiovascular disease risk (lipid based) | ||||||
| Including those with DM or CKD | 1263 | 7.6 | 7.1-8.0 | 2760 | 9.8 | 9.1-10.6 |
| Treated and controlled hypertension | 183 | 14.4 | 12.3-16.5 | 530 | 14.5 | 13.1-15.8 |
| Nonhypertensive | 942 | 5.5 | 5.1-5.8 | 1527 | 6.0 | 5.5-6.6 |
| Uncontrolled, untreated, or unaware hypertension | 138 | 15.9 | 11.6-20.2 | 703 | 16.7 | 15.4-18.0 |
| Excluding those with DM or CKD | 1065 | 6.7 | 6.3-7.0 | 2000 | 7.7 | 7.1-8.2 |
| Treated and controlled | 112 | 13.2 | 11.3-15.2 | 289 | 11.3 | 10.0-12.6 |
| Nonhypertensive | 852 | 5.1 | 4.7-5.4 | 1290 | 5.5 | 5.0-6.1 |
| Uncontrolled, untreated, or unaware | 101 | 14.9 | 10.2-19.7 | 421 | 12.5 | 11.5-13.5 |
| 10-year general cardiovascular disease risk (BMI based) | ||||||
| Including those with DM or CKD | 1261 | 9.0 | 8.5-9.5 | 2747 | 12.2 | 11.2-13.2 |
| Treated and controlled | 183 | 18.5 | 16.5-20.6 | 527 | 19.7 | 17.8-21.6 |
| Nonhypertensive | 940 | 6.1 | 5.7-6.5 | 1522 | 7.1 | 6.6-7.7 |
| Uncontrolled, untreated, or unaware | 138 | 20.2 | 14.8-25.5 | 698 | 20.3 | 18.7-21.9 |
| Excluding those with DM or CKD | 1064 | 8.0 | 7.5-8.5 | 1995 | 9.3 | 8.6-10.0 |
| Treated and controlled | 112 | 17.0 | 15.0-19.0 | 287 | 15.1 | 13.7-16.6 |
| Nonhypertensive | 851 | 5.8 | 5.4-6.2 | 1289 | 6.5 | 5.9-7.1 |
| Uncontrolled, untreated, or unaware | 101 | 19.2 | 13.2-25.2 | 419 | 14.8 | 13.6-16.0 |
For Framingham equation, diabetes is defined as self-reported diabetes medication use OR fasting plasma glucose > 7 mmol/L.
CHMS, Canadian Health Measures Survey; CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; NHANES, National Health and Nutrition Examination Survey.
Discussion
Our study confirms that even at the end of the first decade of the 21st century, more than 80% of Canadian and US adults with hypertension have at least 1 other CV risk factor and that hypertension prevalence increased as the number of atherosclerotic risk factors increased. This is consistent with data from the Reduction of Atherothrombosis for Continued Health (REACH) registry17 and mirrors findings from North American population-based surveys and primary care medical record audits from earlier decades.18-20 However, our more important finding was that despite concerns that patients with multiple comorbidities may have their BP less well treated,8 we found that BP control rates actually improved as the number of other risk factors increased in both Canada and the United States. This pattern was also reported in medical record audits from a group of primary care practices in Southwestern Ontario in 200421 but has not been seen in other jurisdictions.22,23
Thus, while control rates for hypertension have improved markedly in both Canada12 and the United States24 during the past 2 decades, the persistently high prevalence of additional CV risk factors in hypertensive individuals will blunt the potential magnitude of the reductions in CV morbidity and mortality that would have been expected otherwise. Indeed, although other atherosclerotic risk factors were more common in hypertensive than in normotensive individuals, we found that subjects with controlled hypertension did not exhibit any better control of their other risk factors, with the exception of current smoking, than did those with uncontrolled hypertension. As a result, even those hypertensive individuals with BPs controlled to guideline-recommended target levels still exhibited substantially higher Framingham risk scores for subsequent CV disease than did normotensive individuals in both surveys. Previous studies have also reported that treated hypertensives have poorer CV prognoses than do untreated normotensives with the same BPs.25-27 Analyses of the NHANES III Linked Mortality File28 and the Investigations Preventives et Cliniques cohort29 suggested that this was a result of undertreatment of their other atherosclerotic risk factors.
Although our study comparing data from the most recent cycles of the CHMS and NHANES has many strengths, it is not without limitations. A particular strength of our study was the use of fasting laboratory measurements and review of medication profiles to supplement self-report in identifying CV risk factors. Thus, although we found higher rates for all CV risk factors than reported in recent papers that relied on self-report alone,30 we likely still underestimated the prevalence of multiple atherosclerotic risk factors in hypertensive individuals since both the CHMS and NHANES excluded institutionalized adults and older persons living in nursing homes who do have higher comorbidity burdens than community-dwelling adults.31 Restriction of our analysis to those survey participants who provided fasting blood work resulted in slightly different estimates of hypertension prevalence, treatment, and control rates than those in prior CHMS publications.10,12 While the Framingham equations have been validated in North American populations and can discriminate which patients are at elevated risk, their accuracy in estimating absolute CV event rates is still debated.32 As such, in this paper we reported only the crude scores for those subjects aged 30 to 74 years in both CHMS and NHANES, rather than trying to convert those scores to estimated event rates.
In conclusion, mortality rates (both all-cause and CV) are directly related to the number of poorly controlled CV risk factors in Canadian and US adults.5 While others have reported that less than 1% of American adults exhibit ideal CV health,33,34 our study expands on these earlier reports by focusing on the issue of CV health in community-dwelling hypertensive individuals. Our important finding was that although hypertensive individuals with multiple risk factors exhibited better BP control rates than did individuals with uncomplicated hypertension, the converse was not true: hypertensive individuals with BPs controlled below target levels recommended in guidelines still had Framingham absolute risk scores that were not appreciably different than those with uncontrolled BP levels. Although educational programs appear to have been successful in improving BP control rates, future efforts need to expand beyond the focus on BP levels to address all atherosclerotic risk factors in hypertensive individuals.35
Acknowledgements
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Public Health Agency of Canada. C.R. and D.R. had full access to all of the CHMS data in this study and take responsibility for the integrity of the CHMS data and the accuracy of the data analysis; C.G. and K.Y. had full access to all of the NHANES data used in this study and take responsibility for the integrity of the NHANES data and the accuracy of the data analysis.
Funding Sources
F.A.M. is supported by an Alberta Innovates-Health Solutions Senior Health Scholar Award. N.C. holds the Heart and Stroke Foundation of Canada Canadian Institutes of Health Research Chair in Hypertension Prevention and Control.
Footnotes
Disclosures
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Public Health Agency of Canada. The authors have no conflicts of interest.
References
- 1.McAlister FA, Campbell NRC, Zarnke K, Levine M, Graham I. The management of hypertension in Canada: a review of current guidelines, their shortcomings, and implications for the future. CMAJ. 2001;164:517–22. [PMC free article] [PubMed] [Google Scholar]
- 2.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell MJ, Zavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–23. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 4.MacMahon S, Neal B, Rodgers A. Hypertensiondtime to move on. Lancet. 2005;365:1108–9. doi: 10.1016/S0140-6736(05)71148-X. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Cogswell ME, Flanders WD, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–83. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn R, Robertson RM, Smith R, Eddy D. The impact of prevention on reducing the burden of cardiovascular disease. Circulation. 2008;118:576–85. doi: 10.1161/CIRCULATIONAHA.108.190186. [DOI] [PubMed] [Google Scholar]
- 7.Tomaselli GF, Harty MB, Horton K, Schoeberl M. The American Heart Association and the Million Hearts Initiative: a presidential advisory from the American Heart Association. Circulation. 2011;124:1795–9. doi: 10.1161/CIR.0b013e3182327084. [DOI] [PubMed] [Google Scholar]
- 8.Turner BJ, Hollenbeak CS, Weiner M, Have TT, Tang SS. Effect of unrelated comorbid conditions on hypertension management. Ann Intern Med. 2008;148:578–86. doi: 10.7326/0003-4819-148-8-200804150-00002. [DOI] [PubMed] [Google Scholar]
- 9.Petersen LA, Woodard LD, Henderson LM, Urech TH, Pietz K. Will hypertension performance measures used for pay-for-performance programs penalize those who care for medically complex patients? Circulation. 2009;119:2978–85. doi: 10.1161/CIRCULATIONAHA.108.836544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkins K, Campbell NRC, Joffres MR, et al. Blood pressure in Canadian adults. Health Reports. 2010;21:37–46. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevension . National Health and Nutrition Examination survey data. US Department of Health and Human Services, CDC; Hyattsville, MD: [Accessed December 2, 2011]. 2010. Available at: http://www.cdc.gov/nchs/nhanes.htm. [Google Scholar]
- 12.McAlister FA, Wilkins KM, Joffres M, et al. Changes in hypertension awareness, treatment, and control rates in Canada over the past two decades. CMAJ. 2011;183:1007–13. doi: 10.1503/cmaj.101767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers MG, McInnis NH, Fodor GJ, et al. Comparison between an automated and manual sphygmomanometer in a population survey. Am J Hypertens. 2008;21:280–3. doi: 10.1038/ajh.2007.54. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO) Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of the WHO/IDF Consultation. WHO Press; Geneva, Switzerland: 2006. pp. 1–46. [Google Scholar]
- 16.D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 17.Bhatt DL, Steg PG, Ohman EM, et al. REACH Registry Investigators International prevalence, recognition, and treatment for cardiovascular risk factor in outpatients with atherothrombosis. JAMA. 2006;295:180–9. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald S, Joffres MR, Stachenko S, Horlick L, Fodor G. Multiple cardiovascular disease risk factors in Canadian adults. CMAJ. 1992;146:2021–9. [PMC free article] [PubMed] [Google Scholar]
- 19.McAlister FA, Teo KK, Lewanczuk RZ, Wells G, Montague TJ. Contemporary practice patterns in the management of newly diagnosed hypertension. Can Med Assoc J. 1997;157:23–30. [PMC free article] [PubMed] [Google Scholar]
- 20.Stamler J, Stamler R, Neaton JD, et al. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: Findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282:2012–8. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 21.Petrella RJ, Merikle E. A retrospective analysis of the prevalence and treatment of hypertension and dyslipidemia in Southwestern Ontario, Canada. Clin Ther. 2008;30:1145–54. doi: 10.1016/j.clinthera.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Thoenes M, Neuberger HR, Volpe M, Khan BV, Kirch W, Bohm M. Antihypertensive drug therapy and blood pressure control in men and women: an international perspective. J Hum Hypertens. 2010;24:336–44. doi: 10.1038/jhh.2009.76. [DOI] [PubMed] [Google Scholar]
- 23.Belletti D, Zacker C, Wogen J. Effect of cardiometabolic risk factors on hypertension management: a cross-sectional study among 28 physician practices in the United States. Cardiovasc Diabetology. 2010;9:7–11. doi: 10.1186/1475-2840-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303:2043–50. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 25.Lindholm L, Ejlertsson G, Schersten B. High risk of cerebro-cardiovascular morbidity in well treated male hypertensives: a retrospective study of 40-59 year old hypertensives in a Swedish primary care district. Acta Med Scand. 1984;216:251–9. doi: 10.1111/j.0954-6820.1984.tb03802.x. [DOI] [PubMed] [Google Scholar]
- 26.Andersson OK, Almgren T, Persson B, Samuelsson O, Hedner T, Wilhelmsen L. Survival in treated hypertension: follow up study after two decades. BMJ. 1998;317:167–71. doi: 10.1136/bmj.317.7152.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudmundsson LS, Johannsson M, Thorgeirsson G, et al. Risk profiles and prognosis of treated and untreated hypertensive men and women in a population-based longitudinal study: the Reykavik Study. J Hum Hypertension. 2004;18:615–22. doi: 10.1038/sj.jhh.1001725. [DOI] [PubMed] [Google Scholar]
- 28.Gu Q, Burt VL, Paulose-Ram R, Yoon S, Gillum RF. High blood pressure and cardiovascular disease mortality risk among US adults: The Third National Health and Nutrition Examination Survey mortality follow-up study. Ann Epidemiol. 2008;18:302–9. doi: 10.1016/j.annepidem.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Benetos A, Thomas F, Bean KE, Guize L. Why cardiovascular mortality is higher in treated hypertensives versus subjects of the same age, in the general population. J Hypertens. 2003;21:1635–40. doi: 10.1097/00004872-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Lee DS, Chiu M, Manuel DG, et al. Trends in risk factors for cardiovascular disease in Canada: temporal, socio-demographic and geographic factors. CMAJ. 2009;181:e55–66. doi: 10.1503/cmaj.081629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuyuki RT, McLean DL, McAlister FA. Management of hypertension in elderly long-term-care residents. Can J Cardiol. 2008;24:912–5. doi: 10.1016/s0828-282x(08)70698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger JS, Jordan CO, Lloyd-Jones D, Blumenthal RS. Screening for cardiovascular risk in asymptomatic patients. J Am Coll Cardiol. 2010;55:1169–77. doi: 10.1016/j.jacc.2009.09.066. [DOI] [PubMed] [Google Scholar]
- 33.Shay CM, Ning H, Allen NB, et al. Status of cardiovascular health in US adults: Prevalence estimates from the National Health and Nutrition Examination Survey (NHANES) 2003-2008. Circulation. 2012;125:45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of “ideal cardiovascular health” in a community-based population: the Heart Strategies Concentrating on Risk Evaluation (Heart Score) study. Circulation. 2011;123:850–7. doi: 10.1161/CIRCULATIONAHA.110.980151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daskalopoulou SS, et al. The 2012 Canadian hypertension education program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol. 2012;28:270–87. doi: 10.1016/j.cjca.2012.02.018. [DOI] [PubMed] [Google Scholar]