Abstract
Backgrounds/Aims
Vitamin K may plays a role in controlling hepatocellular carcinoma (HCC) cell growth. In this study, we intended to present 5-year experience of 72 patients receiving oral vitamin K with or without sorafenib. Its end-point was to evaluate the safety of combination therapy using sorafenib and vitamin K.
Methods
An interim analysis was performed as a single-arm cross-sectional study, including 72 HCC patients who underwent liver resection or transplantation and administered oral vitamin K2 alone (n=47) or with sorafenib (n=25).
Results
In all patients, administration of vitamin K2 analog 45 mg/day did not show any noticeable adverse side-effect during vitamin K therapy of 23.3±10.6 months, except for one patient who experienced skin rash at the third day of vitamin K therapy. In 25 patients receiving sorafenib and vitamin K for 6 months or longer, any noticeable adverse side-effect suspected of vitamin K origin was not identified yet. A small proportion of patients showed unexpectedly favorable anti-tumor effects after use of vitamin K with or without sorafenib.
Conclusions
Because add-on of oral vitamin K did not increase the adverse side-effects of sorafenib, a combination therapy with these two agents appears to be worthy of further clinical trial with an expectation of synergistic therapeutic effects.
Keywords: Vitamin K, Hepatocellular carcinoma, Sorafenib, Synergy, Metastasis
INTRODUCTION
The most common primary liver cancer, hepatocellular carcinoma (HCC), accounts for 70-85% of all liver cancers. Cancer progression is associated with an abrogation of the normal controls that limit cell migration and invasion, eventually leading to metastasis. The epithelial-mesenchymal transition (EMT) is proposed as a crucial step in promoting cell migration, tumor invasiveness and metastasis. EMT is a transient and reversible switch from a polarized, epithelial phenotype to a fibroblastic or mesenchymal cellular phenotype, the latter exhibiting highly motile and invasive properties.1,2
Vitamin K has the ability to prevent the development of HCC in women with viral cirrhosis.3 A number of findings in vitro have indicated that vitamin K may play a role in controlling HCC growth.4,5 In the absence of vitamin K or in the presence of vitamin K antagonists, abnormal prothrombin (des-gamma-carboxy prothrombin [DCP]; or protein induced by vitamin K absence antagonist II [PIVKA-II]) is released into the blood. The precise mechanism by which HCC produces DCP remains still unclear, but it is suggested that HCC cells may shift to a DCP-producing phenotype when they gain migratory or invasive properties through EMT.6
Sorafenib is a multikinase inhibitor that targets several serine/threonine and tyrosine receptor kinases.7 Despite its therapeutic efficacy, sorafenib treatment in HCC patients causes DCP levels to increase, and paradoxically, the elevation of DCP may also indicate a highly therapeutic effect of sorafenib.8,9 This induction may represent tumor suppression due to the anti-angiogenic effects of sorafenib because hypoxia stimulation is known to impair vitamin K uptake and to induce DCP in HCC.10 These findings led us to hypothesize that a synergistic therapeutic effect against HCC could be achieved by combining sorafenib with vitamin K treatment.
Clinically, we had administered oral vitamin K2 analog for HCC patients with an expectation of some anti-tumor effects. We experienced favorable outcomes in a few patients with metastatic HCCs, but meta-analyses including a randomized controlled study failed to prove its preventive and therapeutic effects.11,12 Currently, sorafenib is the only effect-proven therapeutic agent for patients with HCC lesions which are not manageable with loco-regional treatments,13,14 but its therapeutic response is often disappointing despite its adverse side-effects.
We have carried out two separate studies, one experimental in vitro study and one single-arm clinical study. In the experimental studies, we already demonstrated that sorafenib and vitamin K can function synergistically to inhibit the migration and proliferation of HCC cells.15,16 In this clinical study, we presented our 5-year experience of 72 patients receiving oral vitamin K2 with or without sorafenib. Its end-point was to evaluate the safety of combination therapy using sorafenib and vitamin K.
MATERIALS AND METHODS
An interim analysis of single-surgeon's clinical experience (SH) was performed as a single-arm cross-sectional study. Vitamin K therapy was primarily indicated for HCC patients showing high DCP level in this study protocol.
After resection of primary HCC, oral vitamin K2 (menatetrenone: Glakay; its primary indication is osteoporosis) of 45 mg/day (15 mg three times per day) has been administered with a preventive intention since early 2008 along following indication criteria: preoperative DCP level ≥100 mAU/ml combined with any of following pathological findings (HCC with microvascular invasion and diameter ≥5 cm, macrovascular invasion, or bile duct tumor thrombus). Patients with recurrent HCC lesions after curative resection were also indicated if postoperative DCP level raised to be ≥100 mAU/ml. In contrast, post-transplant HCC recurrence in liver transplant recipients was indicated for oral vitamin K2 therapy regardless of DCP level. When various loco-regional treatments failed, sorafenib was administered to these HCC patients. When anticoagulation therapy had to be concurrently used, oral vitamin K2 therapy was ceased before use of warfarin and dropped out from study; sometimes rivaroxaban (Xarelto, Bayer) was used to continue vitamin K therapy.
Until the end of 2013, a total of 72 patients received oral vitamin K2 therapy for 6 months or longer. Vitamin K therapy was used with a preventive intention (start just after liver resection) in 35 liver resection patients and with a therapeutic intention (start after HCC recurrence) in 22 liver resection patients and 15 liver transplant recipients. Of them, 25 patients concurrently administered sorafenib due to failure of loco-regional treatments (18 resection patients and 7 transplant recipients).
Their medical records were prospectively collected according to the study protocol. The specific data regarding on adverse side-effects from vitamin K2 therapy were analyzed at June 2014 according to the end-point of this study. The study protocol was approved by the Institutional Review Board of our institution.
RESULT
The mean age of 72 patients were 54.3±6.3 years and 61 patients were male. Their primary background liver diseases were viral hepatitis B in 56, viral hepatitis C in 7, alcoholic liver disease in 4 and others in 5. All patients received antiviral treatment if indicated. They were regularly followed up every 2 or 3 months for surveillance of HCC recurrence.
In all patients, administration of vitamin K2 analog 45 mg/day did not show any noticeable adverse side-effect during vitamin K therapy of 23.3±10.6 months, except for one patient who experienced skin rash at the third day of vitamin K therapy thus being dropped out. In 25 patients receiving sorafenib and vitamin K for 6 months or longer, any noticeable adverse side-effect suspected of vitamin K origin was not identified yet.
In patients with HCC recurrence, DCP level was dramatically decreased close to the baseline values after vitamin K administration and maintained stably unless the recurrent HCC lesions progressed very aggressively.
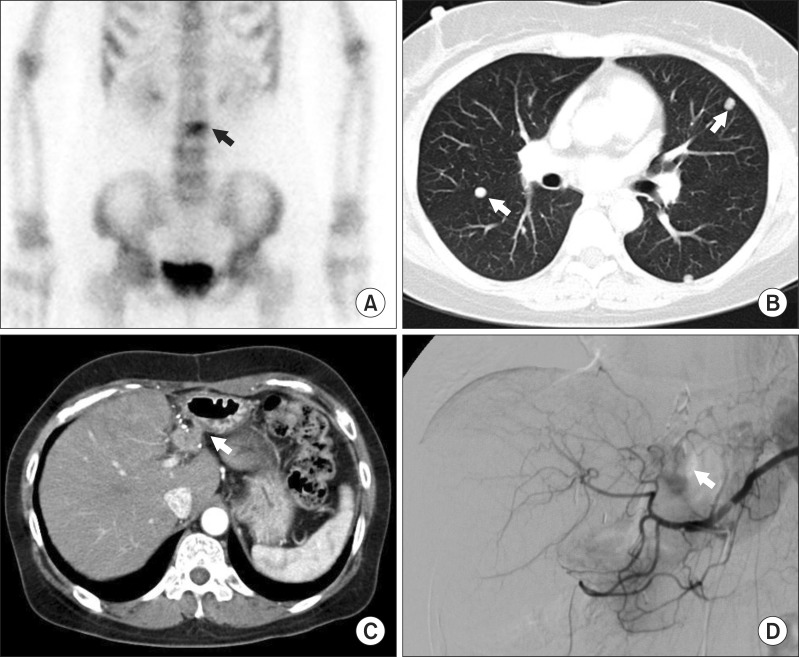
As being an interim analysis focused on only vitamin K-associated side-effects, the HCC recurrence and survival outcomes of these patients were not analyzed yet; instead we briefly presented two selected patients demonstrating definite anti-tumor effect of vitamin K alone (Fig. 1) and with sorafenib (Fig. 2).
Fig. 1. A liver resection patient demonstrating vitamin K-associated anti-tumor effect on multiple HCC metastasis. A 50 year-old female patient showed lumbar spine metastasis 4 months after resection of two small HCC lesions. (A) Despite radiotherapy, bone metastasis aggravated. Multiple lung and intrahepatic metastases occurred with high rise of HCC tumor markers (AFP 2070 ng/ml and DCP 610 mAU/ml). (B, C) Transarterial chemoembolization was performed to the intrahepatic recurrence. (D) After oral vitamin K was administered for 6 month, intrahepatic metastasis disappeared, lung metastasis were regressed and bone metastasis was stationary, with normalization of all tumor markers.
Fig. 2. A liver transplant recipient demonstrating vitamin K-associated anti-tumor effect on isolated pulmonary metastasis. A 58 year-old female recipient had isolated lung metastasis 18 months after transplantation, thus sorafenib and vitamin K were administered for 30 months. (A) Since lung mass progressed slowly, sorafenib was discontinued due to disease progression and only vitamin K was administered for more than 12 months. (B) The patient is currently doing well without any serious symptom despite very slow tumor progression.
DISCUSSION
The escape of carcinoma cells from a solid tumor can be due to de-differentiation of epithelial cells via the loss of cell-to-cell contacts and the concomitant gain of migratory and invasive abilities.17 This phenotypic conversion of cells, collectively designated as EMT, has been described in different types of carcinoma cells including HCC.18,19 Accumulating evidence suggests that EMT plays a pivotal role in the dissemination of malignant hepatocytes during HCC progression. A recent study by Dooley et al.19 clarified the role of hepatocytes in hepatic fibrosis and demonstrated that primary mouse hepatocytes lose their epithelial phenotype in response to TGF-β through the loss of E-cadherin and upregulation of SNAI1 which represents a potent transcriptional repressor of E-cadherin. This switching loss of E-cadherin expression is a critical step in EMT, and E-cadherin is therefore emerging as one of the caretakers of the epithelial phenotype.20
Our precedent experimental studies demonstrated that E-cadherin expression increases in HCC cells in response to hepatocyte growth factor (HGF) in a concentration-dependent manner.15,16 Concurrent treatment of these cells with HGF and vitamins K1/K2 inhibits cadherin switching. These findings indicate that there is a synergism between vitamin K and sorafenib in HGF-induced cell scattering by enhancing the expression of E-cadherin and reducing the expression of N-cadherin, both of which play crucial roles in EMT. Combination of sorafenib and vitamin K also enhances inhibition of HGF-induced cell proliferation and migration/invasion in vitro to a greater extent than either agent alone. We demonstrated that vitamin K1/K2 or sorafenib alone decreases p-MET and p-ERK levels, but vitamin K at low concentrations in combination with sorafenib produces a greater reduction of p-MET and p-ERK levels. Vitamin K or sorafenib alone can mediate a modest inhibition of phosphorylation of MET and ERK, but a clear and significant synergistic effect is evident from combination treatment with these agents.15,16 The mechanism by which combination of sorafenib and vitamin K1/K2 synergistically inhibits HGF-stimulated HepG2 cells appears to be complex. Downregulation of the c-MET and RAF/MEK/ERK pathways has been suggested to hold great promise for preventing tumor cell growth.21 We suggested that the synergistic effect of EMT inhibition by combining sorafenib with vitamin K is, at least in part, attributable to inhibition of the c-MET signaling pathway.
Positive clinical trials have now established that sorafenib improves survival in advanced HCC patients compared with a placebo. However, sorafenib monotherapy offers only limited survival benefits in a small proportion of HCC patients. Especially after liver transplantation, multiple aggressive pattern of HCC recurrence is very common, thus systemic chemotherapy is often indicated.22 But, sorafenib causes multiple human toxicities, including anorexia, gastrointestinal bleeding and hand-foot syndrome.13,14 Modulation of its actions to reduce its toxicity levels is a desirable goal. Therefore, development of more efficacious combination therapies involving sorafenib and vitamin K are a potentially attractive approach for HCC treatment.
In this clinical study, we have performed a clinical trial involving combination therapy with sorafenib and vitamin K2 in HCC patients expecting beneficial effects similar to those demonstrated in this study. At this time, it is not possible for us to estimate the survival benefit objectively, but an interim analysis of 72 patients revealed that there are nearly no adverse side-effects after intake of vitamin K2 analogue. This safety of vitamin K intake in HCC patients is already well known because this agent has been widely used for treatment of osteoporosis.11,12 We also found that vitamin K add-on to sorafenib did not increase the adverse side-effect of sorafenib. Interestingly, we observed exceptionally favorable outcomes in a small proportion of patients with HCC metastasis after vitamin K therapy alone or with sorafenib. Solitary administration of oral vitamin K to patients with metastatic HCC has sporadically led to a favorable outcome, but meta-analyses including a randomized controlled study failed to prove its anti-tumor effects.11,12
In conclusion, the results of our clinical study combined with precedent experimental studies support our assumption that supplementing sorafenib with vitamin K may produce synergistic therapeutic effects against HCC. Because add-on of oral vitamin K did not increase the adverse side-effects of sorafenib, a combination therapy with these two agents appears to be worthy of further clinical trial with an expectation of synergistic therapeutic effects.
References
- 1.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 2.Grünert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4:657–665. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- 3.Habu D, Shiomi S, Tamori A, Takeda T, Tanaka T, Kubo S, et al. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. JAMA. 2004;292:358–361. doi: 10.1001/jama.292.3.358. [DOI] [PubMed] [Google Scholar]
- 4.Ozaki I, Zhang H, Mizuta T, Ide Y, Eguchi Y, Yasutake T, et al. Menatetrenone, a vitamin K2 analogue, inhibits hepatocellular carcinoma cell growth by suppressing cyclin D1 expression through inhibition of nuclear factor kappaB activation. Clin Cancer Res. 2007;13:2236–2245. doi: 10.1158/1078-0432.CCR-06-2308. [DOI] [PubMed] [Google Scholar]
- 5.Kaneda M, Zhang D, Bhattacharjee R, Nakahama K, Arii S, Morita I. Vitamin K2 suppresses malignancy of HuH7 hepatoma cells via inhibition of connexin 43. Cancer Lett. 2008;263:53–60. doi: 10.1016/j.canlet.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Murata K, Suzuki H, Okano H, Oyamada T, Yasuda Y, Sakamoto A. Cytoskeletal changes during epithelial-to-fibroblastoid conversion as a crucial mechanism of des-gamma-carboxy prothrombin production in hepatocellular carcinoma. Int J Oncol. 2009;35:1005–1014. doi: 10.3892/ijo_00000415. [DOI] [PubMed] [Google Scholar]
- 7.Dal Lago L, D'Hondt V, Awada A. Selected combination therapy with sorafenib: a review of clinical data and perspectives in advanced solid tumors. Oncologist. 2008;13:845–858. doi: 10.1634/theoncologist.2007-0233. [DOI] [PubMed] [Google Scholar]
- 8.Kuzuya T, Asahina Y, Tsuchiya K, Tanaka K, Suzuki Y, Hoshioka T, et al. Early decrease in α-fetoprotein, but not des-γ-carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology. 2011;81:251–258. doi: 10.1159/000334454. [DOI] [PubMed] [Google Scholar]
- 9.Ueshima K, Kudo M, Takita M, Nagai T, Tatsumi C, Ueda T, et al. Des-γ-carboxyprothrombin may be a promising biomarker to determine the therapeutic efficacy of sorafenib for hepatocellular carcinoma. Dig Dis. 2011;29:321–325. doi: 10.1159/000327570. [DOI] [PubMed] [Google Scholar]
- 10.Murata K, Suzuki H, Okano H, Oyamada T, Yasuda Y, Sakamoto A. Hypoxia-induced des-gamma-carboxy prothrombin production in hepatocellular carcinoma. Int J Oncol. 2010;36:161–170. [PubMed] [Google Scholar]
- 11.Yoshida H, Shiratori Y, Kudo M, Shiina S, Mizuta T, Kojiro M, et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology. 2011;54:532–540. doi: 10.1002/hep.24430. [DOI] [PubMed] [Google Scholar]
- 12.Zhong JH, Mo XS, Xiang BD, Yuan WP, Jiang JF, Xie GS, et al. Postoperative use of the chemopreventive vitamin K2 analog in patients with hepatocellular carcinoma. PLoS One. 2013;8:e58082. doi: 10.1371/journal.pone.0058082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 14.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 15.Ha TY, Hwang S, Hong HN, Choi YI, Yoon SY, WO YJ, et al. Synergistic effect of sorafenib and vitamin K on suppression of hepatocellular carcinoma cell migration and metastasis. Anticancer Res. [in press] [PubMed] [Google Scholar]
- 16.Ha TY, Hwang S, Moon KM, Won YJ, Song GW, Kim N, et al. Sorafenib inhibits migration and invasion of hepatocellular carcinoma cells through suppression of matrix metalloproteinase expression. Anticancer Res. [in press] [PubMed] [Google Scholar]
- 17.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 18.Gotzmann J, Mikula M, Eger A, Schulte-Hermann R, Foisner R, Beug H, et al. Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat Res. 2004;566:9–20. doi: 10.1016/s1383-5742(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 19.Dooley S, Hamzavi J, Ciuclan L, Godoy P, Ilkavets I, Ehnert S, et al. Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology. 2008;135:642–659. doi: 10.1053/j.gastro.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 20.Imhof BA, Vollmers HP, Goodman SL, Birchmeier W. Cell-cell interaction and polarity of epithelial cells: specific perturbation using a monoclonal antibody. Cell. 1983;35:667–675. doi: 10.1016/0092-8674(83)90099-5. [DOI] [PubMed] [Google Scholar]
- 21.You H, Ding W, Dang H, Jiang Y, Rountree CB. c-Met represents a potential therapeutic target for personalized treatment in hepatocellular carcinoma. Hepatology. 2011;54:879–889. doi: 10.1002/hep.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon DH, Ryoo BY, Ryu MH, Lee SG, Hwang S, Suh DJ, et al. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010;40:768–773. doi: 10.1093/jjco/hyq055. [DOI] [PubMed] [Google Scholar]