Abstract
Currently available cyanide antidotes must be given by intravenous injection over 5–10 min, making them illsuited for treating many people in the field, as could occur in a major fire, an industrial accident, or a terrorist attack. These scenarios call for a drug that can be given quickly, e.g., by intramuscular injection. We have shown that aquohydroxocobinamide is a potent cyanide antidote in animal models of cyanide poisoning, but it is unstable in solution and poorly absorbed after intramuscular injection. Here we show that adding sodium nitrite to cobinamide yields a stable derivative (referred to as nitrocobinamide) that rescues cyanide-poisoned mice and rabbits when given by intramuscular injection. We also show that the efficacy of nitrocobinamide is markedly enhanced by coadministering sodium thiosulfate (reducing the total injected volume), and we calculate that ∼1.4 mL each of nitrocobinamide and sodium thiosulfate should rescue a human from a lethal cyanide exposure.
Introduction
Cyanide is a very potent and rapidly acting poison. Over one billion pounds are used each year in the United States in a variety of industries, including electroplating, paint manufacturing, and gold extraction from ore.1 Moreover, it is relatively easy to make from simple reagents, making it available for nefarious use. Thus, a large number of people could be exposed to cyanide in either a major industrial accident or a terrorist attack. In addition, cyanide may be as important as carbon monoxide as a cause of inhalational deaths in residential and industrial fires.2,3
Two treatments for cyanide poisoning are currently available in the United States: hydroxocobalamin (Cyanokit) and the combination of sodium nitrite and sodium thiosulfate (Nithiodote). Both treatments must be given intravenously. Even in the best of settings, starting an intravenous line can take several minutes, and in a clothed hypotensive subject, obtaining venous access can be particularly challenging. Moreover, both hydroxocobalamin and sodium nitrite/sodium thiosulfate are recommended to be given over 5–15 min. Thus, neither antidote is suitable for use in the field, particularly for a mass casualty scenario, and an antidote that can be given quickly and easily is urgently needed. The best approach would appear to be intramuscular injection using a prefilled syringe. This requires that the antidote (i) is sufficiently potent so that it can be administered in a small volume, (ii) is rapidly absorbed after intramuscular injection, and (iii) is sufficiently stable to be stored as a solution for long periods.
We have been developing the hydroxocobalmin analog cobinamide (see Experimental Section for nomenclature) as a cyanide antidote and have shown it is 3–10 times more potent than hydroxocobalamin in mouse, rabbit, and pig models of cyanide poisoning.4–7 Aquohydroxocobinamide (Supporting Information, Figure 1A) is poorly absorbed after intramuscular injection; however, we showed that placing a ligand on the cobalt atom markedly improved its absorption.4,6 The first ligand we tested was sulfite, but we subsequently found that sulfitocobinamide was not stable over time. We now show that nitrocobinamide (Supporting Information, Figure 1B) is very stable and well absorbed after intramuscular injection. The amount of nitrite in a nitrocobinamide preparation is subtherapeutic, and the nitrite is present only to improve absorption after intramuscular injection.
The combination of sodium thiosulfate and sodium nitrite was developed for cyanide poisoning because the two drugs act by different mechanisms and yield a synergistic effect. 8,9 Thiosulfate also synergizes with hydroxocobalamin, as well as with various experimental treatments of cyanide poisoning.10–13 The mechanism whereby thiosulfate potentiates other drugs is unknown, but we hypothesized thiosulfate might potentiate cobinamide, allowing a reduction of the cobinamide dose and thus injection volume. We now show that combining a small amount of thiosulfate with nitrocobinamide yields a potent cyanide antidote and that the two drugs can be given in small volumes by intramuscular injection. The major antidotal potency of the drug combination is derived from combining thiosulfate with the cobinamide in nitrocobinamide, not with the nitrite.
Results
Nitrite Binding to Aquohydroxocobinamide
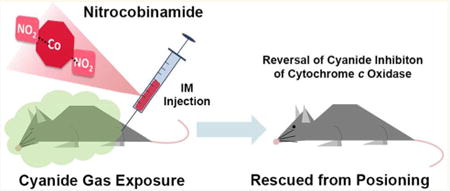
Nitrite has a reasonably high affinity for cobalamin,14 and we hypothesized that nitrite might bind tightly enough to cobinamide to allow the nitrocobinamide derivative to be absorbed after intramuscular injection (as described in the Experimental Section, we use the generic term “nitrocobinamide” to refer to cobinamide in the presence of nitrite, without specifying the number of bound nitrite groups). Ligand binding to cobinamide and cobalamin changes the molecules' ultraviolet–visible spectrum, and we found that adding one or two nitrite equivalents to a concentrated aquohydroxocobinamide solution yielded cobinamide derivatives with distinctly different spectra; the resulting derivatives were presumably mononitrocobinamide and dinitrocobinamide, respectively (Figure 1A). Both spectra were different from that of aquohydroxocobinamide (Figure 1A).
Figure 1.
Nitrite binding to aquohydroxocobinamide. (A) A 100 mM solution of aquohydroxocobinamide [Cbi(H2O)(OH), gray line], nitroaquocobinamide [Cbi(NO2)(H2O), dashed line], and Cbi(NO2)2 (black line) in water was scanned from 300 to 600 nm in a 0.01 mm path length cuvette. The nitroaquocobinamide and Cbi(NO2)2 were prepared by adding one and two molar equivalents, respectively, of sodium nitrite to the aquohydroxocobinamide solution; at 100 mM cobinamide, two nitrite equivalents saturate both ligand binding sites. (B, C) Increasing amounts of sodium nitrite were added to a 10 μM (B) and 500 μM (C) aquohydroxocobinamide solution at the following ratios of nitrite to aquohydroxocobinamide: 0.25×, 0.5×, 0.75×, 1×, 2×, 3×, 4×, 5×, 10×, and 20×. The spectra from 300 to 600 nm were recorded, with the aquohydroxocobinamide spectrum shown in black and the spectra with increasing nitrite concentrations shown in shades of gray from dark to light. Insets: The difference in absorption at 380 nm between the presence and absence of sodium nitrite was plotted, and a best fit equation was used to determine the binding affinity for the first and second binding sites.
Because of a negative trans effect, once a ligand binds to one of cobinamide's two free binding sites, affinity for the second ligand is reduced considerably.15 Thus, cobinamide's affinity for a first cyanide molecule is >1014 M−1 (and may approach 1019 M−1), whereas the affinity for a second cyanide molecule is ∼108 M−1. 15,16 It seemed likely, therefore, that cobinamide's affinity for a first nitrite ligand would be greater than for a second nitrite ligand, and we found that diluting a concentrated dinitrocobinamide solution in water changed the spectrum to that of mononitrocobinamide (Supporting Information, Figure 2A; the dinitrocobinamide solution was made with two moles of sodium nitrite per mole of cobinamide). This indicated that water or hydroxyl molecules competed effectively with, and thereby displaced, the second nitrite ligand but that they did not displace the first nitrite ligand. This suggested that if we increased the nitrite concentration relative to the cobinamide concentration, the second nitrite ligand should remain bound at lower cobinamide concentrations, since more nitrite molecules will be available to compete with the water and hydroxyl molecules. We therefore repeated the experiment at four molar equivalents of sodium nitrite per mole of cobinamide and found that cobinamide remained as dinitrocobinamide until relatively low concentrations (Supporting Information, Figure 2B).
We then determined the Ka for the first and second nitrite ligands of cobinamide and found they were 1 × 105 and 2 × 103 M−1, respectively (Figure 1B and Figure 1C). The Ka for the first nitrite ligand was determined at a cobinamide concentration of 10 μM (to minimize interference by binding of the second nitrite ligand), and the Ka for the second nitrite ligand was determined at a cobinamide concentration of 500 μM (to minimize competition by water and hydroxyl molecules).
Nitrocobinamide Converts Readily to Dicyanocobinamide and Effectively Scavenges Cyanide in Cultured Cells
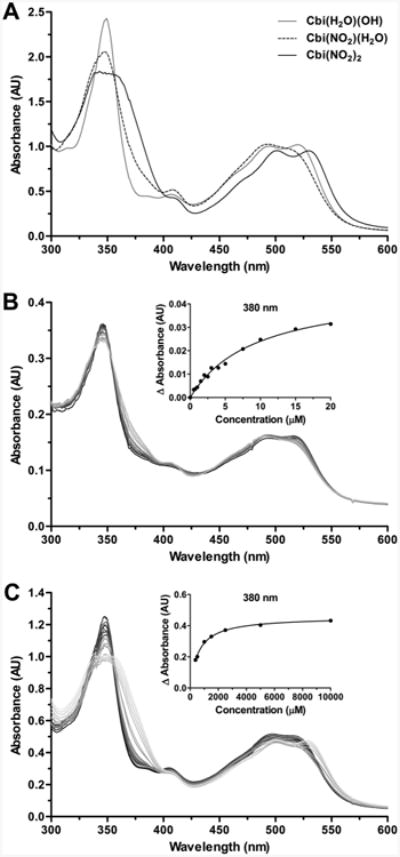
For nitrocobinamide to be an effective cyanide scavenger, cyanide needs to displace the nitrite ligands efficiently. We assessed cyanide binding to nitrocobinamide in two sets of experiments. First, we showed that adding two molar equivalents of cyanide to a nitrocobinamide solution immediately changed the spectrum to that of dicyanocobinamide17 [Figure 2A]; the data shown are for cobinamide in the presence of four molar equivalents of sodium nitrite (designated subsequently as Cbi(NO2)4), but similar results were obtained with cobinamide in the presence of two molar equivalents of sodium nitrite (designated subsequently as Cbi(NO2)2). And second, we showed that both Cbi(NO2)2 and Cbi(NO2)4 rapidly and completely reversed cyanide-induced inhibition of cellular oxygen consumption (Figure 2B). The rate of reversal was the same as for aquohydroxocobinamide (Figure 2B), indicating no interference by the nitrite ligand. These data also indicate that cobinamide effectively removes cyanide from mitochondrial cytochrome c oxidase, the primary intracellular cyanide target. Cobinamide was more effective than hydroxocobalamin at reversing cyanide-induced inhibition of oxygen consumption, and neither sodium nitrite nor sodium thiosulfate counteracted cyanide's inhibition (Figure 2B). The last two agents were tested because nitrite can generate nitric oxide, which can displace cyanide from cytochrome c oxidase, and thiosulfate is a substrate for rhodanese, a mitochondrial cyanide-detoxifying enzyme.18–22 The lack of an effect by nitrite suggests that either nitrite did not generate sufficient nitric oxide under the conditions tested or that nitrite detoxifies cyanide through other mechanisms, i.e., methemoglobin generation.23 We conclude that cyanide readily displaces nitrite from nitrocobinamide and that nitrocobinamide efficiently reverses cyanide-induced inhibition of cellular oxygen consumption.
Figure 2.
Nitrocobinamide conversion to dicyanocobinamide and reversal of cyanide inhibition of mitochondrial respiration. (A) To a 50 mM Cbi(NO2)2 solution was added KCN to a final concentration of 100 mM. The UV–visible spectra from 300 to 650 nm were recorded immediately before (gray line) and after adding the cyanide (black line). (B) Oxygen consumption rates were measured in COS-7 monkey kidney fibroblasts using a Seahorse Bioscience XF24 extracellular flux analyzer. At the indicated time (vertical line at 19 min), KCN at a final concentration of 1 mM was added to all of the cells except control cells (black circles, dashed line), and 18 min later the following agents were added to the final concentrations noted: XF assay buffer (gray circles, dashed line), 10 mM sodium thiosulfate (Na2S2O3, black diamonds, solid line), 2 mM sodium nitrite (NaNO2, gray diamonds, solid line), 0.5 mM hydroxocobalamin [Cbl(OH), black squares, dashed line], 0.5 mM aquohydroxocobinamide [Cbi(H2O)(OH), gray squares, dashed line], 0.5 mM Cbi(NO2)2 (black triangles, solid line), and 0.5 mM Cbi(NO2)4 (gray triangles, solid line). Error bars are omitted for clarity, but the standard deviation about each value was <10%.
Nitrocobinamide Stability
Delivering an antidote from a prefilled syringe is much faster and easier than mixing the contents of two vials (one containing solid antidote and the other containing solvent) and then drawing up the dissolved antidote in a syringe. Thus, for treating mass casualties in the field, a prefilled syringe would be preferred, but the antidote must have a reasonably long shelf life as a solution. We therefore tested the stability of nitrocobinamide solutions and found that both Cbi(NO2)2 and Cbi(NO2)4 were stable for at least 45 days under the accelerated degradation condition of 50 °C and 5 months at room temperature. The accelerated degradation data can be extrapolated to a nitrocobinamide solution being stable for at least 1 year at room temperature.21,24
Nitrocobinamide Pharmacokinetics after Intramuscular Injection
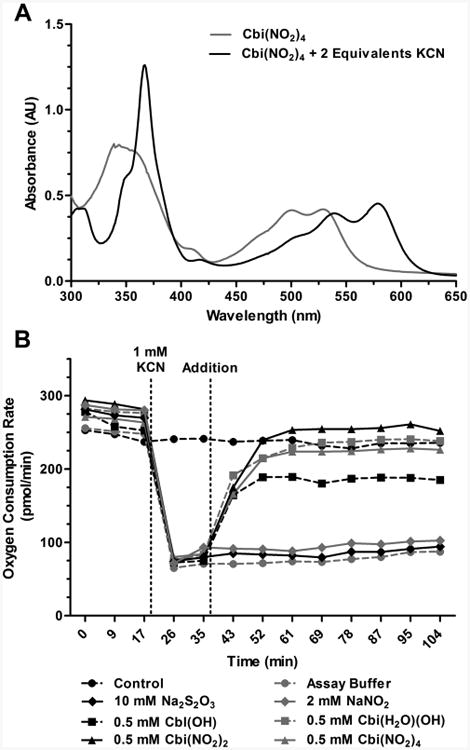
We measured the total plasma cobinamide concentration at various times after intramuscular injection of nitrocobinamide into rabbits. We found that Cbi(NO2)2 yielded a similar maximal plasma concentration as Cbi(NO2)4 but that the time to maximal concentration (Tmax) was markedly different at ∼250 and 45 min, respectively (Figure 3A). Moreover, the difference between the two nitrocobinamide formulations in the time to half maximal plasma concentration (T1/2max) was even more dramatic: T1/2max for Cbi(NO2)4 and Cbi(NO2)4 was ∼4 and ∼90 min, respectively (Figure 3A). Because of cobinamide's red color, the difference in absorption was observed on visually inspecting the plasma (Figure 3B). The more rapid absorption of Cbi(NO2)4 is likely from more complete saturation of cobinamide's binding sites with nitrite and therefore possibly less binding of cobinamide to large negatively charged molecules in the extracellular matrix.
Figure 3.
Pharmacokinetics of nitrocobinamide after intramuscular injection. Equal volumes (80 μL) of 200 mM Cbi(NO2)2 or Cbi(NO2)4 were injected into the pectoral muscle of anesthetized rabbits, and blood was collected at the following times: at the time of injection (“0 min”) and at the following times after injection, 1, 2.5, 4, 5, 7.5, 10, 15, 30, 45, 60, 90, 120, 180, and 240 min. (A) The plasma cobinamide concentration was measured by HPLC: gray circles, [Cbi(NO2)2]; black squares, [Cbi(NO2)4]. The data are the mean from two independent experiments (four rabbits total). (B) Plasma samples are shown for one rabbit injected with Cbi(NO2)2 (upper photograph) and one with Cbi(NO2)4 (lower photograph).
We also measured the total plasma cobinamide concentration in mice 5 min after an intramuscular injection of either Cbi(NO2)2 or Cbi(NO2)4 and found concentrations of 75.5 ± 2.6 and 167.3 ± 76.61 μM, respectively (mean ± SD, n = 4 in each group; p < 0.05 for differences between the two groups). Thus, Cbi(NO2)4 is better absorbed than Cbi(NO2)2 in two different species.
Muscle Analysis after Intramuscular Injection of Nitrocobinamide
For intramuscular injection to be a usable method for administering nitrocobinamide, it would need to cause minimal amount of muscle damage at the injection site. We therefore injected 200 μmol/kg (234 mg/kg) nitrocobinamide in mouse gastrocnemius muscle and examined the injected site over the ensuing 24 h. We found no evidence of edema or necrosis at the injection site, and at 24 h, the mice were euthanized and a histopathological examination of the muscle was performed. We found that Cbi(NO2)2 caused a moderate amount of muscle injury but that Cbi(NO2)4 caused only minimal muscle injury: injury scores of 2.75 and 1.58, respectively (the scoring scale is defined in the Experimental Section) (Figure 4). The muscle injury was resolved fully by 1 week after injection for both nitrocobinamide formulations. These data indicate that both Cbi(NO2)2 and Cbi(NO2)4 could be administered by intramuscular injection but that the latter formulation would be preferred.
Figure 4.
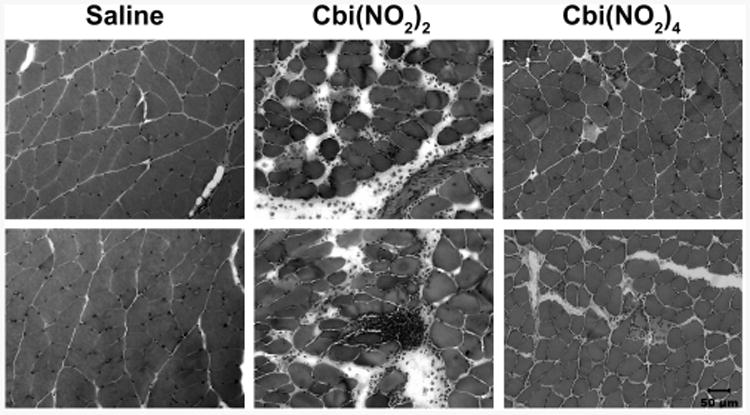
Muscle analysis after intramuscular injection of nitrocobinamide. Mice were injected in the gastrocnemius muscle with 50 μL of saline or 50 μL of 200 μmol/kg (216 mg/kg) Cbi(NO2)2 or Cbi(NO2)4. At 24 h after the injection, the mice were euthanized, the injected muscle was harvested, and cryosections were stained with hematoxylin and eosin. The experiments were repeated three times, with two representative areas for each condition shown. The small cells in the Cbi(NO2)2-injected muscle are polymorphonuclear leukocytes. Bar in lower right image is 50 μm for all images.
Efficacy of Nitrocobinamide as a Cyanide Antidote
The more rapid and efficient absorption of both Cbi(NO2)4 than of Cbi(NO2)2 suggested the former would be a better cyanide antidote than the latter. We therefore compared these two nitrocobinamide formulations in an inhaled mouse model of cyanide poisoning. As expected, intramuscular injection of aquohydroxocobinamide did not rescue animals and appeared similar to saline-injected control animals, presumably because little of the drug was absorbed (Figure 5A). Nitrocobinamide, however, did rescue animals, and we found a significant difference between Cbi(NO2)2 and Cbi(NO2)4: the former at 50 μmol/kg (54 mg/kg) yielded 50% survival, whereas the latter at 37.5 μmol/kg (40.6 mg/kg) yielded 100% survival (Figure 5A). The difference between aquohydroxocobinamide and nitrocobinamide was not from the nitrite present in the latter, because 100 μmol/kg (6.9 mg/kg) sodium nitrite (the amount of nitrite present in the 50 μmol/kg Cbi(NO2)2) did not rescue any animals and 150 μmol/kg (10.4 mg/kg) sodium nitrite (the amount of nitrite present in the 37.5 μmol/kg Cbi(NO2)4) had only a minimal effect (Figure 5A). In a separate set of experiments, we found the protection index of Cbi(NO2)4 to be 3.6 at 135 μmol/kg (158 mg/kg) [the protection index was calculated as the LD50 of cyanide in the presence of Cbi(NO2)4 divided by the LD50 of cyanide alone]; the dose corresponds to a likely human dose, since it rescues 50 kg pigs from a lethal cyanide exposure.7 We conclude that Cbi(NO2)4 is an effective cyanide antidote when administered by intramuscular injection, much more effective than aquohydroxocobinamide or an equivalent amount of sodium nitrite.
Figure 5.
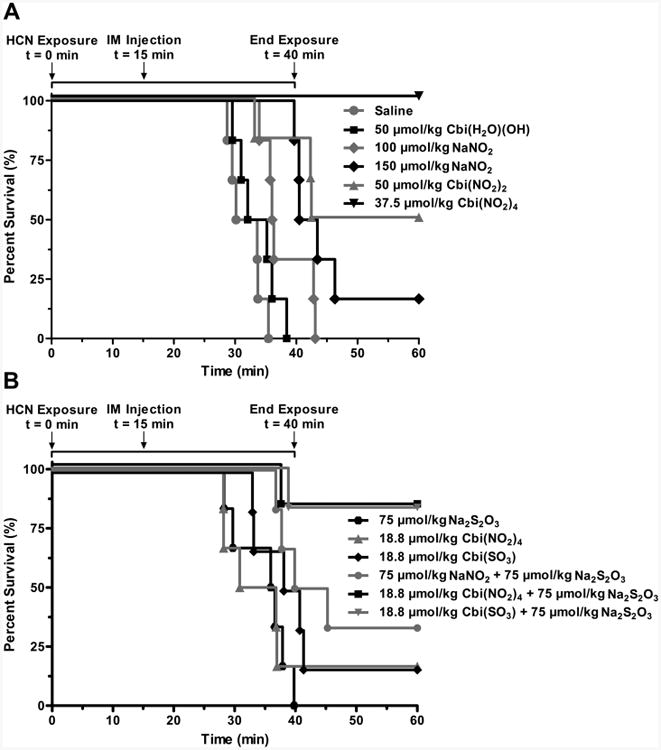
Cobinamide as a cyanide antidote in mice without and with sodium thiosulfate. C57BL/6J mice (6 animals per condition) were exposed to 587 ppm of cyanide gas (HCN) for a total of 40 min in a sealed Plexiglas chamber. After 15 min of cyanide exposure, they were removed from the chamber and received an intramuscular (IM) injection of the indicated agent(s). They were placed back in the chamber for an additional 25 min to complete the 40 min of cyanide exposure, and then they were removed from the chamber. (A) Animals were injected with saline (gray circles), 50 μmol/kg aquohydroxocobinamide [Cbi(H2O)(OH), black squares], 100 μmol/kg sodium nitrite [(NO2), gray diamonds], 150 μmol/kg sodium nitrite (black diamonds), 50 μmol/kg Cbi(NO2)2 (gray triangles), or 37.5 μmol/kg Cbi(NO2)4 (black inverted triangles. (B) Animals were injected with 75 μmol/kg sodium thiosulfate [(S2O3), black hexagons], 18.8 μmol/kg Cbi(NO2)4 (gray triangles), 18.8 μmol/kg sulfitocobinamide [Cbi(SO3), black diamonds], 75 μmol/kg sodium thiosulfate plus 75 μmol/kg sodium nitrite (gray circles), 75 μmol/kg sodium thiosulfate plus 18.8 μmol/kg Cbi(NO2)4 (black squares), or 18.8 μmol/kg sulfitocobinamide plus 75 μmol/kg sodium thiosulfate (inverted gray triangles). The data were divided into two graphs for ease of interpretation only; thus, any condition in each panel could have been shown in the other panel, e.g., the saline-injected controls shown in panel A could also have been shown in panel B.
Combination of Nitrocobinamide and Thiosulfate for Treating Cyanide Poisoning
Mouse Studies
Having shown that Cbi(NO2)4 was an effective cyanide antidote, we asked if combining it with thiosulfate would allow a reduction in the Cbi(NO2)4 dose and thus injection volume. Because of the steep dose–response curve with cyanide, we found that reducing the Cbi(NO2)4 dose by only half [from 37.5 to 18.8 μmol/kg (20.3 mg/kg)]) reduced survival from 100% to only 16.7% (compare Figure 5A and Figure 5B). Not surprisingly, sodium thiosulfate at a low subtherapeutic dose [75 μmol/kg (12.6 mg/kg)] did not rescue any animals (Figure 5B; when converted to the human equivalent dose, the amount of thiosulfate was ∼1 mg/kg, which is 0.56% of the sodium thiosulfate dose in Nithiodote). However, this low amount of thiosulfate combined with 18.8 μmol/kg Cbi(NO2)4 increased survival 5-fold to 83% (Figure 5B). This marked improvement in survival was not due to the combination of thiosulfate with the nitrite in Cbi(NO2)4 because combining this same dose of sodium thiosulfate with the corresponding amount of sodium nitrite (75 μmol/kg) yielded only a modest 33.3% survival rate (Figure 5B). Thus, combining sodium thiosulfate with Cbi(NO2)4 clearly improved survival over either agent alone. Moreover, the data indicate that the major improvement in survival was from combining thiosulfate with the cobinamide in Cbi(NO2)4 and not the nitrite. To evaluate this interpretation of the data further, we tested sulfitocobinamide, which we previously showed is well absorbed after intramuscular injection in mice and rabbits and rescues animals from lethal cyanide concentrations.6,25 We found that 18.8 μmol/kg sulfitocobinamide yielded the same 16.7% survival rate as the identical dose of Cbi(NO2)4 (Figure 5B) and that on adding sodium thiosulfate, we once again observed a 83% survival rate (Figure 5B). Since no nitrite was present in these experiments, and we have previously shown that sulfite has no antidotal effect against cyanide, we conclude that thiosulfate augments cobinamide.25
Rabbit Studies
To determine if the combination of nitrocobinamide and sodium thiosulfate was effective in other species, we used a well-established lethal rabbit model of cyanide poisoning.5,6,26 We found that the combination of 4 μmol/kg (4.88 mg/kg) Cbi(NO2)4 and 60 μmol/kg (14.9 mg/kg) sodium thiosulfate rescued 80% of animals (four of five animals), whereas five of five saline-injected animals died (difference between the two groups was significant at p < 0.01).
In addition to assessing survival, we measured the mean arterial pressure (MAP) and cytochrome c oxidase redox state in the animals. Because the animals were anesthetized and ventilated, one would expect neither normal blood pressure nor cytochrome c oxidation, and for comparison, we used anesthetized ventilated rabbits that had received a continuous intravenous infusion of saline, instead of sodium cyanide, over the same time period. We found that the MAP and cytochrome c oxidation decreased by ∼20 mmHg and 0.3 μM, respectively, in these control non-cyanide-poisoned animals (Figure 6A and Figure 6B). In the cyanide-poisoned animals, the MAP and cytochrome c oxidation were significantly lower than in the control animals and, in the animals that received an intramuscular injection of saline, decreased further until all the animals had died by 15 min after injection (Figure 6A and Figure 6B). The MAP in the Cbi(NO2)4-thiosulfate-treated animals decreased by ∼8 mmHg within the first 5 min after antidote injection and then stabilized, remaining at ∼40 mmHg until the animals were euthanized at 90 min (Figure 6A); as described in Experimental Section, the animals continued to receive intravenous cyanide for 30 min after antidote injection. The initial decrease in MAP in the Cbi(NO2)4-thiosulfatetreated animals was likely due to several minutes being required for the Cbi(NO2)4 to be absorbed (Figure 3) and thus to counter cyanide's hypotensive effects. The cytochrome c oxidase redox state rose in the Cbi(NO2)4-thiosulfate-treated animals, approaching that of non-cyanide-poisoned animals (Figure 6B).
Figure 6.
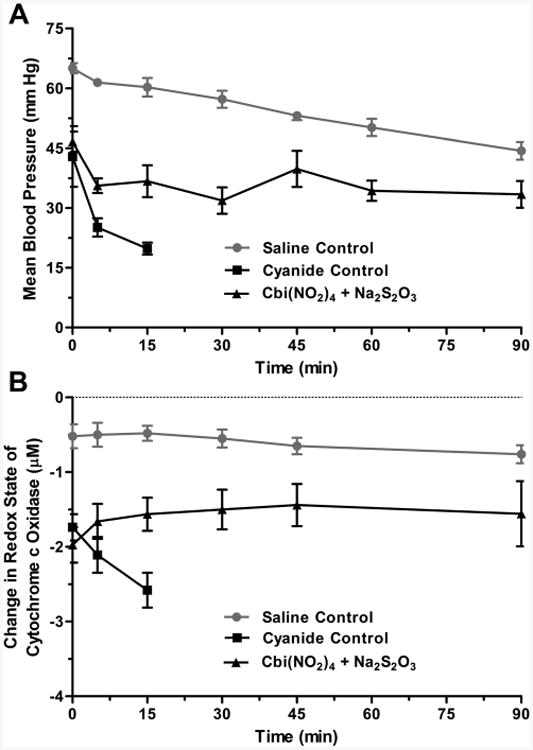
Nitrocobinamide and thiosulfate as a cyanide antidote in rabbits. New Zealand white rabbits were anesthetized and received a continuous intravenous infusion of sodium cyanide. When their MAP was <70% of precyanide infusion values, generally after ∼30 min of cyanide infusion, the animals received an intramuscular injection of saline (cyanide control, black squares) or antidote [Cbi(NO2)4 plus Na2S2O3, black triangles]. The time of injection is defined as zero time. In separate experiments, rabbits were anesthetized and treated similarly, except they received a continuous intravenous infusion of saline (saline control, gray circles) instead of sodium cyanide and they did not receive an intramuscular injection. (A) MAP is plotted versus time. (B) Cytochrome c oxidase redox state is plotted versus time. The data are the mean ± SD of three independent experiments.
In the cyanide-poisoned animals, the methemoglobin concentration was normal in both the saline- and Cbi(NO2)4-thiosulfate-treated groups, ranging from 2% to 4% with no more than a 2% variation throughout the studies in any animal.
Discussion and Conclusions
A large number of agents have shown efficacy as cyanide antidotes in various animal models. These agents can be divided into at least six different classes: (i) sulfur donors, e.g., cysteine, sodium thiosulfate, and sodium tetrathionate; (ii) methemoglobin generators, e.g., amyl nitrite, sodium nitrite, and 4-dimethylaminophenol; (iii) cyanide scavengers, e.g., hydroxocobalamin, dicobalt ethylenediaminetetracetic acid, and 5,10,15,20-tetrakis(4-sulfonatophenyl)porphinato iron encapsulated in β-cyclodetrin dimers; (iv) cyanohydrin formers, e.g., α-ketoglutarate, oxaloacetate, and pyruvate; (v) antioxidants, e.g., ascorbic acid, glutathione, and melatonin, and (vi) miscellaneous compounds, e.g., chlorpromazine, pyridoxal phosphate, and riboflavin.10,11,23,27–34 The only ones that have been used in humans are sodium thiosulfate, amyl nitrite, sodium nitrite, 4-dimethylaminophenol, and hydroxocobalamin. As mentioned earlier, sodium thiosulfate, sodium nitrite, and hydroxocobalamin are given intravenously over 5–10 min. Amyl nitrite is inhaled, but it is not very effective.35 The only one that is given by intramuscular injection is 4-dimethylaminophenol, but it is limited by toxicity due to generating unpredictable concentrations of methemoglobin.28 Thus, no safe and effective cyanide antidote is currently available that can be administered by intramuscular injection, as would be needed for treating mass casualties.
We have been developing cobinamide as a cyanide antidote and found it was extremely effective at rescuing animals from lethal cyanide exposures when given by intravenous or intraperitoneal injection.4,5 We were disappointed to find that aquohydroxocobinamide was absorbed poorly after intramuscular injection and hypothesized that it was binding, and thus becoming tethered, to negatively charged macromolecules in the extracellular matrix such as collagen, heparan sulfate, and chondroitin sulfate; the water molecule is easily displaced, and the hydroxyl group is readily converted to a water molecule. We reasoned that placing a ligand on cobinamide that had a reasonably high affinity for the cobalt would prevent binding to anions in the extracellular matrix. We first tested sulfitocobinamide because we knew that sulfite binds tightly to cobinamide and found that sulfitocobinamide was well absorbed after intramuscular injection and effectively rescued animals from lethal exposures to cyanide.4,6,25 However, we found that sulfitocobinamide was not stable over time because of a slow redox reaction between the sulfite and cobinamide, and in a preinvestigator's new drug application meeting with the Food and Drug Administration, the agency expressed concern about possible allergic reactions to sulfite. We therefore sought a different ligand and tested a very large number of potential ligands. Because of poor solubility, limited stability, low binding affinity, or unacceptable toxicity, all but nitrite were eliminated. Nitrite salts are highly water-soluble, deoxygenated nitrite solutions are stable for long periods of time, and as reported here, nitrite has a reasonably high affinity for cobinamide. Moreover, we found that Cbi(NO2)4 is well absorbed after intramuscular injection and rescues animals from cyanide poisoning. Nitrite has been used for almost a century to treat cyanide poisoning and more recently is being used to treat cardiovascular diseases.36–38 It has two potentially toxic effects: (i) generation of methemoglobin (which is largely how it functions as a cyanide antidote, since cyanide binds to methemoglobin) but methemoglobin does not bind oxygen and (ii) generation of nitric oxide (which is how it functions for treating cardiovascular diseases) but excess nitric oxide can lead to hypotension.20,21,23,36–39 As calculated below, the amount of nitrite in the projected human dose of Cbi(NO2)4 for treating cyanide poisoning would be considerably less than the amount of nitrite in Nithiodote, the approved form of nitrite used for cyanide poisoning. Furthermore, we found no evidence of methemoglobin production or hypotension in Cbi(NO2)4-treated rabbits. Thus, at the doses nitrite would be used, it appears to be an excellent cobinamide ligand.
We now show in both mice and rabbits that Cbi(NO2)4 was better absorbed after intramuscular injection than Cbi(NO2)2. We also showed that on diluting a Cbi(NO2)4 solution, two nitrite groups remain bound to cobinamide more effectively than on diluting a Cbi(NO2)2 solution, and this could reduce cobinamide binding to anions in the extracellular matrix as the nitrocobinamide is diluted in the interstitial fluid. Less cobinamide binding to the extracellular matrix could potentially reduce tissue injury, and this could explain why Cbi(NO2)4 caused less muscle inflammation and injury in mice than Cbi(NO2)2. With either nitrocobinamide formulation, the muscle injury reversed fully within 1 week. We should note that the amount of nitrocobinamide injected in these studies of muscle injury was 5 times the amount required to rescue animals from cyanide toxicity. Thus, nitrocobinamide may cause little muscle damage at a therapeutic dose.
Because of the small size of mice, scaling a drug dose from a mouse to a human is less likely to be accurate than scaling from a larger animal, such as a rabbit. The 4.88 mg/kg Cbi(NO2)4 and 14.9 mg/kg sodium thiosulfate that rescued 80% of rabbits from a lethal exposure of cyanide translate to 1.57 and 4.8 mg/kg human equivalent doses of Cbi(NO2)4 and sodium thiosulfate, respectively.40 Sodium nitrite comprises 18.8% of the molecular mass of Cbi(NO2)4, and hence the amount of nitrite delivered to a human would be 0.29 mg/kg. This is ∼15% of the amount of nitrite administered in Nithiodote (4.2 mg/kg sodium nitrite), and this small amount of nitrite likely explains the lack of efficacy of the sodium nitrite by itself. It also likely explains why we observed no methemoglobin generation or change in blood pressure by the Cbi(NO2)4. The 4.8 mg/kg human equivalent dose of sodium thiosulfate is also much less than the amount of sodium thiosulfate administered in Nithiodote (178 mg/kg). Thus, the amounts of sodium nitrite and sodium thiosulfate in the Cbi(NO2)4–thiosulfate combination that we found to be efficacious are low and well within a tolerable range.
By injection of relatively concentrated solutions of Cbi(NO2)4 and sodium thiosulfate (200 mM and 3 M, respectively), an injection volume of 1.4 mL of each of the two solutions should rescue a human from a lethal cyanide exposure, based on the human equivalent doses of Cbi(NO2)4 and sodium thiosulfate calculated above. The total volume of 2.8 mL could be administered by injection into the vastus lateralis muscle, a common site for intramuscular injection. We found that nitrocobinamide is stable in solution, and sodium thiosulfate is well-known to be stable. Thus, both drugs could be administered via prefilled autoinjectors, and to ensure that both drugs are administered to a patient, the autoinjectors could be bound together, with the two drugs injected simultaneously. Refinement of the Cbi(NO2)4 and sodium thiosulfate doses and injection volumes may occur as we study larger animals, but we conclude that the combination of nitrocobinamide with thiosulfate is an effective cyanide antidote that can be administered by intramuscular injection and could therefore be used in the field to treat victims of cyanide poisoning.
A new cyanide antidote would be approved by the FDA via the “Animal Rule Pathway”, which is used when studies cannot be done ethically on humans.41 Phase I safety studies of the drug are, of course, still required, but well-controlled animal efficacy studies replace standard phase II and III clinical trials. Thus, no human efficacy studies will be performed, and the studies reported here will form the basis for future animal efficacy studies.
Experimental Section
Nomenclature
The term “cobinamide” is used generically, without specifying the ligand(s) bound to the cobalt atom. “Aquohydroxocobinamide” refers to cobinamide with a water and hydroxyl group coordinated to the cobalt atom, without designating which group is in the lower (α) or upper (β) axial position (Supporting Information, Figure 1). “Dicyanocobinamide” refers to cobinamide with two bound cyanide molecules, and “sulfitocobinamide” refers to cobinamide with one bound sulfite group.
Nitrite binds to cobalamin via the nitrogen atom and not via one of the two oxygen atoms.42,43 Assuming the same binding mechanism occurs for cobinamide, the proper names for cobinamide with one bound nitrite group is mononitrocobinamide (in aqueous solution, the other axial ligand would be a water molecule or hydroxyl group, depending on the pH), and with two bound nitrite groups, dinitrocobinamide. As described in the Results, the number of nitrite groups bound to cobinamide is dependent on the cobinamide concentration. We therefore use the generic term “nitrocobinamide” to describe cobinamide in the presence of nitrite, without specifying whether one or two nitrites are bound; nitrocobinamide generated by adding two or four molar equivalents of sodium nitrite to cobinamide is designated Cbi(NO2)2 and Cbi(NO2)4, respectively.
Materials
The sodium nitrite, sodium sulfite, and sodium thiosulfate were from Sigma-Aldrich and were all >99% pure. Potassium cyanide was from Fisher Chemical and was 99.4% pure. All other chemicals and reagents were of the highest grade available. The cobinamide was synthesized from hydroxocobalamin by base hydrolysis using cerium hydroxide and was >96% pure as determined by HPLC at 370 nm and by comparison to a cobinamide malate reference standard.44,45 The certificates of analysis for the nitrocobinamide and cobinamide malate are provided in the Supporting Information.
Nitrite Binding to Cobinamide
Sodium nitrite was added to 10 and 500 μM solutions of aquohydroxocobinamide to final concentrations ranging from 0.25 to 20 times the aquohydroxocobinamide concentration. Spectra from 300 to 600 nm were recorded on a Kontron 860 spectrophotometer. The binding affinity (Ka) of nitrite for cobinamide was determined by plotting the difference between absorbance at 380 nm in the presence of sodium nitrite from absorbance in the absence of sodium nitrite at various nitrite concentrations using Prism 4 software (Carlsbad, CA). In some experiments, two molar equivalents of sodium cyanide were added to Cbi(NO2)4, and the spectrum was recorded immediately.
Measurement of Cellular Oxygen Consumption
Cyanide inhibits mitochondrial cytochrome c oxidase, and because mitochondrial respiration accounts for >90% of cellular oxygen consumption,46,47 we studied the effects of cyanide on cellular oxygen consumption using an XF extracellular flux analyzer (Seahorse Bioscience). This instrument allows real-time measurement of oxygen consumption without disturbing cells and sequential addition of drugs to cells.
COS-7 monkey kidney fibroblasts were plated in 24-well XF tissue culture plates, and 24 h later the cells were washed and left in XF assay medium. After obtaining baseline readings, potassium cyanide was added to a final concentration of 1 mM, and 18 min later known cyanide antidotes were added to the cells.
Nitrocobinamide Stability
Solutions of Cbi(NO2)2 and Cbi(NO2)4 were incubated at 25 and 50 °C for 1–4 months. At various times during the incubation, samples were removed and analyzed by HPLC. Purity was determined by comparison to known standards.
Nitrocobinamide Pharmacokinetics after Intramuscular Injection
The total plasma cobinamide concentration was measured in New Zealand white rabbits and C57/BL/6J mice after injecting Cbi(NO2)2 and Cbi(NO2)4. In the rabbits, 80 μL of 200 mM nitrocobinamide solutions were injected into the pectoral muscle of anesthetized animals, and blood was collected at various times prior to and after injection. In the mice, an amount of 50 μL of 100 mM nitrocobinamide solutions was injected into the gastrocnemius muscle, and blood was collected 5 min after injection. In both cases, a severalfold excess of potassium cyanide (over the predicted cobinamide concentration) was added to plasma to generate dicyanocobinamide, and the samples were heated at 80 °C for 15 min. Acetonitrile/methanol (90/10) was added to the samples, which were centrifuged, and the clear deproteinized supernatant was analyzed by HPLC, measuring absorption at 366 nm and comparing to dicyanocobinamide standards.
Muscle Analysis after Intramuscular Injection of Nitrocobinamide
C57BL/6J adult male mice were injected in the gastrocnemius muscle with 50 μL of saline, 50 μL of 80 mM Cbi(NO2)2, or 50 μL of 80 mM Cbi(NO2)4. The mice were euthanized 24 h later, and the injected muscle was harvested. Immediately following collection, muscle specimens were flash frozen in isopentane precooled in liquid nitrogen; cryosections (8 μm) were cut and stained with hematoxylin and eosin. Three injected muscles for each condition were reviewed in a blinded fashion by a veterinary pathologist, who has special expertise in muscle pathology. The pathologist used a scale of 0 to 4 to evaluate the muscle histology: 0, normal muscle; +1, minimally abnormal by evidence of some edema and few polymorphonuclear leukocytes but no muscle necrosis; +2, mildly abnormal by evidence of many polymorphonuclear leukocytes and mild muscle necrosis; +3, moderately abnormal by evidence of extensive polymorphonuclear leukocyte infiltration and moderate muscle necrosis; +4, severely abnormal by evidence of extensive muscle necrosis.
Nitrocobinamide as a Cyanide Antidote in Mice
We developed an inhalational model that allows mice to be exposed to cyanide gas, injected with an antidote, and then re-exposed to the gas.25 We use a custom-made gas chamber and can vary the gas exposure periods: we have chosen 15 min of cyanide gas exposure, inject the animals with test antidote, and then re-expose them to cyanide for 25 min; thus, total cyanide exposure time is 40 min. This model simulates a real-life situation of people being exposed to cyanide such as in an airport or subway station, with 15 min required for emergency medical personnel to arrive and another 25 min required to treat and evacuate the victims from the cyanide-contaminated area. The mice are anesthetized fully throughout the cyanide exposure as required by our Institutional Animal Care and Use Committee (IACUC): we inject isoflurane into the chamber to a final concentration of 2%, which rapidly vaporizes and anesthetizes the mice. The cyanide gas is generated in the chamber by injecting KCN into a beaker of sulfuric acid; we have shown that the HCN concentration reaches equilibrium within 5 min of injection of the KCN and that the HCN concentration remains stable throughout the 40 min exposure period.4 We used adult male C57BL/6J mice and tested Cbi(NO2)2 and Cbi(NO2)4 with and without sodium thiosulfate; for comparison, we tested sulfitocobinamide.
Nitrocobinamide as a Cyanide Antidote in Rabbits
New Zealand white rabbits weighing 3.5–4.5 kg were anesthetized with ketamine and xylazine and mechanically ventilated with room air at 20 respirations per minute and tidal volume of 50 cc. After a 10 min baseline equilibration period, intravenous sodium cyanide was started at 0.33 mg/min. When the mean arterial blood pressure (MAP, measured using an intra-arterial transducer) decreased to <70% of baseline (generally after ∼30 min), the animals were injected in the pectoral muscle with either 80 μL of saline (control group) or two separate simultaneous injections of 80 μL of 200 mM Cbi(NO2)4 and 80 μL of 3.0 M sodium thiosulfate (experimental group). Cyanide infusion was continued for 30 min after saline or antidote injection; animals that survived an additional 60 min after the cyanide infusion was stopped were euthanized.
Cyanide renders tissues unable to extract oxygen, resulting in direct reduction of optically active metal centers such as cytochrome c oxidase. The extent of cytochrome c oxidase reduction can be followed using diffuse optical spectroscopy with the cytochrome c oxidase redox state calculated as oxidized cytochrome c – reduced cytochrome c oxidase.5,6,48
Measurement of Methemoglobin
Oxidation of hemoglobin to methemoglobin leads to a marked increase in absorption in the red region of the visible spectrum (600–650 nm), with a strong absorption peak at 630 nm. We took advantage of this increased absorption and measured the tissue methemoglobin concentration in vivo using broadband diffuse optical spectroscopy.49
Institutional Animal Care and Use Committee Approval
All mouse studies were reviewed and approved by the University of California, San Diego IACUC, and all rabbit studies were reviewed and approved by the University of California, Irvine IACUC.
Data Analysis
All experiments were performed at least three times unless indicated otherwise. In the case of UV–visible spectra, a representative spectrum is shown, and for other data the mean value is shown. The data were plotted and analyzed using Prism 4 software.
Supplementary Material
Acknowledgments
This work was supported by the CounterACT Program, Office of the Director, National Institutes of Health (OD) and the National Institute of Neurological Disorders and Stroke (NINDS), Grant U01 NS058030, and by United States Army Grant AMRMC W81XWH-12-2-0098. J.J. was supported by National Institute of Diabetes and Digestive and Kidney Diseases Training Grant T32DK069263. H.H.P. was supported by grants from the National Heart, Lung Blood Institute (Grant HL107200) and Veterans Administration Merit (Grant BX001963).
Abbreviations
- Cbi(NO2)2
cobinamide with 2 equiv of sodium nitrite
- Cbi(NO2)4
cobinamide with 4 equiv of sodium nitrite
Footnotes
Supporting Information: Figures 1 and 2 showing cobinamide structures and UV–visible spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes: The authors declare no competing financial interest.
References
- 1.Dzomback DA, Ghosh RS, Wong-Chong GM. Cyanide in Water and Soil: Chemistry, Risk, and Management. CRC Press; Boca Raton, FL: 2006. [Google Scholar]
- 2.Baud FJ, Barriot P, Toffis V, Riou B, Vicaut E, Lecarpentier Y, Bourdon R, Astier A, Bismuth C. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med. 1991;325:1761–1766. doi: 10.1056/NEJM199112193252502. [DOI] [PubMed] [Google Scholar]
- 3.Alarie Y. Toxicity of fire smoke. Crit Rev Toxicol. 2002;32:259–289. doi: 10.1080/20024091064246. [DOI] [PubMed] [Google Scholar]
- 4.Chan A, Balasubramanian M, Blackledge W, Mohammad OM, Alvarez L, Boss GR, Bigby TD. Cobinamide is superior to other treatments in a mouse model of cyanide poisoning. Clin Toxicol. 2010;48:709–717. doi: 10.3109/15563650.2010.505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner M, Mahon SB, Lee J, Kim J, Mukai D, Goodman S, Kreuter KA, Ahdout R, Mohammad O, Sharma VS, Blackledge W, Boss GR. Comparison of cobinamide to hydroxocobalamin in reversing cyanide physiologic effects in rabbits using diffuse optical spectroscopy monitoring. J Biomed Opt. 2010;15:017001. doi: 10.1117/1.3290816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner M, Kim JG, Mahon SB, Lee J, Kreuter KA, Blackledge W, Mukai D, Patterson S, Mohammad O, Sharma VS, Boss GR. Intramuscular cobinamide sulfite in a rabbit model of sublethal cyanide toxicity. Ann Emerg Med. 2009;55:352–362. doi: 10.1016/j.annemergmed.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bebarta V, Tanen D, Boudrea S, Castaneda M, Zarzabal L, Vargas T, Boss G. Intravenous cobinamide versus hydrocobalamin for acute treatment of severe cyanide poisonong in a swine (Suc Scroba) model. Ann Emerg Med. 2014;64:612–619. doi: 10.1016/j.annemergmed.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen KK, Rose CL. Nitrite and thiosulfate therapy in cyanide poisoning. JAMA, J Am Med Assoc. 1952;149:113–119. doi: 10.1001/jama.1952.02930190015004. [DOI] [PubMed] [Google Scholar]
- 9.Frankenberg L, Sorbo B. Effect of cyanide antidotes on the metabolic conversion of cyanide to thiocyanate. Arch Toxicol. 1975;33:81–89. doi: 10.1007/BF00353233. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz C, Morgan RL, Way LM, Way JL. Antagonism of cyanide intoxication with sodium pyruvate. Toxicol Appl Pharmacol. 1979;50:437–441. doi: 10.1016/0041-008x(79)90396-x. [DOI] [PubMed] [Google Scholar]
- 11.Way JL, Burrows G. Cyanide intoxication: protection with chlorpromazine. Toxicol Appl Pharmacol. 1976;36:93–97. doi: 10.1016/0041-008x(76)90029-6. [DOI] [PubMed] [Google Scholar]
- 12.Hall AH, Rumack BH. Hydroxycobalamin/sodium thiosulfate as a cyanide antidote. J Emerg Med. 1987;5:115–121. doi: 10.1016/0736-4679(87)90074-6. [DOI] [PubMed] [Google Scholar]
- 13.Isom G, Way JL. Cyanide intoxication: protection with cobaltous chloride. Toxicol Appl Pharmacol. 1973;24:449–456. doi: 10.1016/0041-008x(73)90051-3. [DOI] [PubMed] [Google Scholar]
- 14.Firth RA, Hill HAO, Pratt JM, Thorp RG, Williams RJP. The chemistry of vitamin B12. Part XI. Some further formation constants. J Chem Soc A. 1969:381–386. [Google Scholar]
- 15.Hayward GC, Hill HAO, Pratt JM, Vanston NJ, Williams ARW. The chemistry of vitamin B(12). Part IV.1 The thermodynamic trans-effect. J Chem Soc. 1965:6485–6493. [PubMed] [Google Scholar]
- 16.Ma J, Dasgupta PK, Zelder FH, Boss GR. Cobinamide chemistries for photometric cyanide determination. A merging zone liquid core waveguide cyanide analyzer using cyanoaquacobinamide. Anal Chim Acta. 2012;736:78–84. doi: 10.1016/j.aca.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma J, Dasgupta PK, Blackledge W, Boss GR. Temperature dependence of Henry's law constant for hydrogen cyanide. Generation of trace standard gaseous hydrogen cyanide. Environ Sci Technol. 2010;44:3028–3034. doi: 10.1021/es1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce LL, Bominaar EL, Hill BC, Peterson J. Reversal of cyanide inhibition of cytochrome c oxidase by the auxiliary substrate nitric oxide: an endogenous antidote to cyanide poisoning? J Biol Chem. 2003;278:52139–52145. doi: 10.1074/jbc.M310359200. [DOI] [PubMed] [Google Scholar]
- 19.Leavesley HB, Li L, Prabhakaran K, Borowitz JL, Isom GE. Interaction of cyanide and nitric oxide with cytochrome c oxidase: implications for acute cyanide toxicity. Toxicol Sci. 2008;101:101–111. doi: 10.1093/toxsci/kfm254. [DOI] [PubMed] [Google Scholar]
- 20.Feelisch M, Fernandez BO, Bryan NS, Garcia-Saura MF, Bauer S, Whitlock DR, Ford PC, Janero DR, Rodriguez J, Ashrafian H. Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide-generating and -scavenging systems. J Biol Chem. 2008;283:33927–33934. doi: 10.1074/jbc.M806654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Kundu TK, Zweier JL. Characterization of the magnitude and mechanism of aldehyde oxidase-mediated nitric oxide production from nitrite. J Biol Chem. 2009;284:33850–33858. doi: 10.1074/jbc.M109.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorbo BH. Chrystalline rhodanese. II. The enzyme catalysed reaction. Acta Chem Scand. 1953;7:1137–1143. [Google Scholar]
- 23.Way JL. Cyanide intoxication and its mechanism of antagonism. Annu Rev Pharmacol Toxicol. 1984;24:451–481. doi: 10.1146/annurev.pa.24.040184.002315. [DOI] [PubMed] [Google Scholar]
- 24.Socarras S, Magari RT. Modeling the effects of storage temperature excursions on shelf life. J Pharm Biomed Anal. 2009;49:221–226. doi: 10.1016/j.jpba.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Chan A, Crankshaw DL, Monteil A, Patterson SE, Nagasawa HT, Briggs JE, Kozocas JA, Mahon SB, Brenner M, Pilz RB, Bigby TD, Boss GR. The combination of cobinamide and sulfanegen is highly effective in mouse models of cyanide poisoning. Clin Toxicol. 2011;49:366–373. doi: 10.3109/15563650.2011.584879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JC, Lee J, Mahon SB, Mukai D, Patterson SE, Boss GR, Tromberg BJ, Brenner M. Noninvasive monitoring of treatment response in a rabbit cyanide toxicity model reveals differences in brain and muscle metabolism. J Biomed Opt. 2012;17:105005. doi: 10.1117/1.JBO.17.10.105005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okolie NP, Iroanya CU. Some histologic and biochemical evidence for mitigation of cyanide-induced tissue lesions by antioxidant vitamin administration in rabbits. Food Chem Toxicol. 2003;41:463–469. doi: 10.1016/s0278-6915(02)00224-7. [DOI] [PubMed] [Google Scholar]
- 28.Rodgers GC, Jr, Condurache CT. Antidotes and treatments for chemical warfare/terrorism agents: an evidence-based review. Clin Pharmacol Ther. 2010;88:318–327. doi: 10.1038/clpt.2010.152. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe K, Kitagishi H, Kano K. Supramolecular ferric porphyrins as cyanide receptors in aqueous solution. ACS Med Chem Lett. 2011;2:943–947. doi: 10.1021/ml200231x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatch RC, Laflamme DP, Jain AV. Effects of various known and potential cyanide antagonists and a glutathione depletor on acute toxicity of cyanide in mice. Vet Hum Toxicol. 1990;32:9–16. [PubMed] [Google Scholar]
- 31.Reiter RJ, Paredes SD, Korkmaz A, Jou MJ, Tan DX. Melatonin combats molecular terrorism at the mitochondrial level. Interdiscip Toxicol. 2008;1:137–149. doi: 10.2478/v10102-010-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maduh EU, Johnson JD, Ardelt BK, Borowitz JL, Isom GE. Cyanide-induced neurotoxicity: mechanisms of attenuation by chlorpromazine. Toxicol Appl Pharmacol. 1988;96:60–67. doi: 10.1016/0041-008x(88)90247-5. [DOI] [PubMed] [Google Scholar]
- 33.Bhattacharya R, Tulsawani R. In vitro and in vivo evaluation of various carbonyl compounds against cyanide toxicity with particular reference to alpha-ketoglutaric acid. Drug Chem Toxicol. 2008;31:149–161. doi: 10.1080/01480540701688865. [DOI] [PubMed] [Google Scholar]
- 34.Nath AK, Roberts LD, Liu Y, Mahon SB, Kim S, Ryu JH, Werdich A, Januzzi JL, Boss GR, Rockwood GA, MacRae CA, Brenner M, Gerszten RE, Peterson RT. Chemical and metabolomic screens identify novel biomarkers and antidotes for cyanide exposure. FASEB J. 2013;27:1928–1938. doi: 10.1096/fj.12-225037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavon O, Bentur Y. Does amyl nitrite have a role in the management of pre-hospital mass casualty cyanide poisoning? Clin Toxicol. 2010;48:477–484. doi: 10.3109/15563650.2010.505573. [DOI] [PubMed] [Google Scholar]
- 36.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, III, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 37.Alef MJ, Tzeng E, Zuckerbraun BS. Nitric oxide and nitrite-based therapeutic opportunities in intimal hyperplasia. Nitric Oxide. 2012;26:285–294. doi: 10.1016/j.niox.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Nossaman VE, Nossaman BD, Kadowitz PJ. Nitrates and nitrites in the treatment of ischemic cardiac disease. Cardiol Rev. 2010;18:190–197. doi: 10.1097/CRD.0b013e3181c8e14a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gracia R, Shepherd G. Cyanide poisoning and its treatment. Pharmacotherapy. 2004;24:1358–1365. doi: 10.1592/phco.24.14.1358.43149. [DOI] [PubMed] [Google Scholar]
- 40.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Office of Training and Communications, Food and Drug Administration; Rockville, MD: 2005. pp. 1–27. [Google Scholar]
- 41.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER) Guidance for Industry: Product Development under the Animal Rule. Office of Communication, Food and Drug Administration; Silver Spring, MD: 2014. pp. 1–48. [Google Scholar]
- 42.Perry CB, Fernandes MA, Brown KL, Zou X, Valente EJ, Marques HM. Probing the nature of the CoIII ion in cobalamins—spectroscopic and structural investigations of the reactions of aquacobalamin (vitamin B12. with ambident nucleophiles. Eur J Inorg Chem. 2003:2095–2107. [Google Scholar]
- 43.Garau G, Geremia C, Marzilli RG, Randaccio L, Tauzher J. Crystal chemistry and binding of NO2, SCN and SeCN to Co in cobalamins. Acta Crystallogr. 2003;859:51–59. doi: 10.1107/s0108768102019353. [DOI] [PubMed] [Google Scholar]
- 44.Renz P. Some intermediates in the biosynthesis of vitamin B12. Methods Enzymol. 1971;18c:82–86. [Google Scholar]
- 45.Broderick KE, Singh V, Zhuang S, Kambo A, Chen JC, Sharma VS, Pilz RB, Boss G. R Nitric oxide scavenging by the cobalamin precursor cobinamide. J Biol Chem. 2005;280:8678–8685. doi: 10.1074/jbc.M410498200. [DOI] [PubMed] [Google Scholar]
- 46.van Buuren KJ, Nicholis P, van Gelder BF. Biochemical and biophysical studies on cytochrome aa 3. VI. Reaction of cyanide with oxidized and reduced enzyme. Biochim Biophys Acta. 1972;256:258–276. doi: 10.1016/0005-2728(72)90057-6. [DOI] [PubMed] [Google Scholar]
- 47.Babcock GT, Wikstrom M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992;356:301–309. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- 48.Lee J, Kim JG, Mahon SB, Mukai D, Yoon D, Boss GR, Patterson SE, Rockwood G, Isom G, Brenner M. Noninvasive optical cytochrome c oxidase redox state measurements using diffuse optical spectroscopy. J Biomed Opt. 2014;19:055001. doi: 10.1117/1.JBO.19.5.055001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J, El-Abaddi N, Duke A, Cerussi AE, Brenner M, Tromberg BJ. Noninvasive in vivo monitoring of methemoglobin formation and reduction with broadband diffuse optical spectroscopy. J Appl Physiol. 2006;100:615–622. doi: 10.1152/japplphysiol.00424.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


