Abstract
Purpose
Bardoxolone methyl, a novel synthetic triterpenoid and antioxidant inflammation modulator, potently induces Nrf2 and inhibits NF-κB and Janus-activated kinase/STAT signaling. This first-in-human phase I clinical trial aimed to determine the dose-limiting toxicities (DLT), maximum tolerated dose (MTD), and appropriate dose for phase II studies; characterize pharmacokinetic and pharmacodynamic parameters; and assess antitumor activity.
Experimental Design
Bardoxolone methyl was administered orally once daily for 21 days of a 28-day cycle. An accelerated titration design was employed until a grade 2–related adverse event occurred. A standard 3 + 3 dose escalation was then employed until the MTD was reached. Single dose and steady-state plasma pharmacokinetics of the drug were characterized. Assessment of Nrf2 activation was examined in peripheral blood mononuclear cells (PBMC) by measuring NAD(P)H:quinone oxidoreductase (NQO1) mRNA levels. Immunohistochemical assessment of markers of inflammation, cell cycle, and apoptosis was carried out on tumor biopsies.
Results
The DLTs were grade 3 reversible liver transaminase elevations. The MTD was established as 900 mg/d. A complete tumor response occurred in a mantle cell lymphoma patient, and a partial response was observed in an anaplastic thyroid carcinoma patient. NQO1 mRNA levels increased in PBMCs, and NF-κB and cyclin D1 levels decreased in tumor biopsies. Estimated glomerular filtration rate (eGFR) was also increased.
Conclusions
Bardoxolone methyl was well tolerated with an MTD of 900 mg/d. The increase in eGFR suggests that bardoxolone methyl might be beneficial in chronic kidney disease. Objective tumor responses and pharmacodynamic effects were observed, supporting continued development of other synthetic triterpenoids in cancer.
Introduction
Bardoxolone methyl (RTA 402; CDDO-Me) is a novel synthetic triterpenoid and antioxidant inflammation modulator that potently activates Nrf2, a transcription factor that controls the expression of a large number of antioxidant and detoxification enzymes (1). Activation of Nrf2 reduces intracellular levels of reactive oxygen species (ROS) and attenuates inflammation (2–4). In cancer cell lines and clinical specimens, bardoxolone methyl inhibits constitutive and cytokine-induced activation of NF-κB and Janus-activated kinase (JAK)/STAT signaling (5–10). These effects are mediated in part by direct inhibition of upstream regulatory proteins, such as IKKβ and JAK1 (8, 10).
Bardoxolone methyl has been shown to induce differentiation, inhibit proliferation, and induce apoptosis in cancer cell lines (11–13). Potent single-agent activity has been observed in several animal models of cancer with significant inhibitory effects on tumor growth (1, 2, 14, 15), metastasis (1), angiogenesis (16), and tumor-associated myeloid-derived suppressor cells (MDSC; ref. 17). In preclinical toxicology studies carried out in non-human primates, bardoxolone methyl was found to be orally bioavailable and well tolerated (C. Meyer; unpublished data).
We report here the first-in-human phase I clinical trial of the crystalline formulation of bardoxolone methyl in patients with advanced solid tumors and lymphoma. The objectives of the study were to determine the dose-limiting toxicities (DLT), maximum tolerated dose (MTD), and appropriate dose for phase II studies; characterize the pharmacokinetic and pharmacodynamic parameters; and assess antitumor activity.
Patients and Methods
Patient selection
This study was to be restricted to adult patients (≥18 years) with a histologically confirmed advanced solid tumor or lymphoid malignancy refractory to standard therapy. Eligibility criteria included the following: Eastern Cooperative Group (ECOG) performance status of 2 or less; life expectancy of 12 weeks or more; absolute neutrophils of 1,500/μL or more; platelets of 100,000/μL or more, hemoglobin of 8.0 g/dL or more; total bilirubin of 1.5 mg/dL or less, aspartate aminotransferase and alanine amino-transferase of 2.5 or less times the institutional upper limit of normal (≤5 times for hepatic involvement), serum creatinine of 2.0 mg/dL or less or creatinine clearance of more than 60 mL/min; discontinuation of all prior anti-neoplastic therapies for 4 weeks or more; and no residual side effects of prior therapies. Patients were required to practice effective contraception. Measurable disease was not required, and there was no limit to the number of prior treatment regimens. Exclusion criteria included active brain metastases or primary central nervous system malignancies (stable brain metastases were eligible), pregnancy or breast feeding, a clinically significant illness or psychiatric condition, and concurrent use of any other investigational drugs.
The protocol was reviewed and approved by the Scientific Review and Human Protection Committees at each participating institution. A signed written informed consent document was obtained. Screening evaluations conducted within 1 month before beginning treatment included an electrocardiogram, chest X-ray, radiographic tumor measurement, biochemical tumor markers, an optional tumor core biopsy, and bone marrow biopsy/aspiration for lymphoid patients. In addition, a medical history; physical examination; pregnancy test (if applicable); performance status, complete blood count with platelet and differential counts, hemoglobin, hematocrit, and coagulation panel; a comprehensive serum chemistry profile; and urinalysis were carried out within 14 days of dosing.
Treatment plan
Bardoxolone methyl was supplied by Reata Pharmaceuticals, Inc. as gelatin capsules containing 5, 50, or 100 mg in a micronized crystalline preparation. The drug was administered orally once daily for 21 consecutive days of a 28-day cycle for up to 12 cycles. Patients ingested the capsules in the morning with a glass of water before any food intake. The amount of water taken with the capsules was not recorded. Antiemetics were not used on a preventative basis unless a patient began to experience grade 1 or more nausea or vomiting. Treatment with a subsequent cycle could be delayed for recovery from toxicity for up to 15 days after completing the prior cycle. Treatment was discontinued on the occurrence of a DLT, failure to adequately recover from toxicity within 15 days after completing a cycle of therapy, or tumor progression.
Toxicity evaluation
Hematology tests, serum chemistry profiles, and urinalyses were obtained at baseline (within 72 hours prior to beginning treatment), weekly during cycle 1, and biweekly thereafter. Physical examinations were carried out every 2 weeks, and a urinalysis was repeated before every cycle of therapy.
Adverse events (AE) were evaluated weekly in cycle 1, then every other week during subsequent cycles, and graded according to the Common Toxicity Criteria version 3.0 (http://ctep.info.nih.gov). DLT was defined as any of the following AEs attributable to the study drug that occurred during the first cycle of treatment: grade 3 or more non-hematologic toxicity, excluding alopecia and diarrhea, nausea/vomiting in the absence of optimal prophylactic/supporting treatment; grade 4 thrombocytopenia or (any grade) requiring transfusion; anemia lasting more than 7 days; febrile neutropenia (grade 3 or 4); grade 4 neutropenia lasting more than 7 days.
Study design
The starting dose was 5 mg/d, which was one-tenth the dose that caused severe toxicity in rats. The dose was escalated between cohorts with an accelerated titration design. Decisions to escalate the dose to the next higher level were made no earlier than 7 days after each of the predetermined number of patients (initially a single patient) enrolled at a given dose level had received all 21 days of treatment. The dose was doubled (100%) between cohorts in single-patient cohorts until the first occurrence of grade of 2 or more drug-related toxicity. At this point, the cohort size was expanded to 3 or 4 patients and the dose escalation was reduced to 50% between cohorts. At the first occurrence of a grade 3 toxicity not classified as DLT, dose escalation between cohorts was decreased to 33%. The first occurrence of a DLT resulted in expanding the current cohort to 6 patients. Dose escalation at an increment of 25% proceeded if no more than 1 patient in a group of 6 patients evaluated at a given dose level experienced a DLT. Dose escalation was terminated when DLT occurred in 2 or more patients at a given dose level. The MTD was defined as the dose at which no more than one of 6 patients experienced a DLT during cycle 1. Once the MTD was defined, the cohort was expanded to 25 patients at that dose to further define the toxicity and response profiles.
Evaluation of response
Evaluation of malignant disease that was initially measured by physical examination, diagnostic imaging, biochemical markers, or bone marrow studies, as appropriate for the tumor type, was repeated every 2 cycles and 4 weeks after a tumor response for confirmation. Response and disease progression were defined by Response Evaluation Criteria in Solid Tumors 1.0 (RECIST) guidelines for solid tumors (18) and International Workshop Criteria for non-Hodgkins lymphoma (19).
Pharmacokinetic studies
Blood specimens (7 mL) were drawn from an arm vein into tubes containing sodium heparin before dosing and at 0.25, 0.50, 1.0, 2.0, 4.0, 6.0, 8.0, and 24 hours after taking the first and last doses during cycle 1. An additional sample was collected 48 hours after the final dose. Plasma was promptly harvested by centrifugation (1,300 g, 4°C, 10 minutes) and stored at −70°C or less until assayed. The concentration of bardoxolone methyl in plasma was determined by liquid chromatography/mass spectrometry. The ethyl ester analogue of bardoxolone methyl was used as the internal standard (IS) for the assay. Study samples were thawed in a refrigerator and vortexed. Plasma (100 μL) was spiked with IS working solution (5 μL, 0.40 μg/mL in methanol) and extracted with methyl tert-butyl ether (3.5 mL). After centrifuging the mixture (3,000 rpm, 5 minutes), the organic phase was evaporated under nitrogen and the extract reconstituted with 150 μL of methanol/water (3:1, v/v). This solution (100 μL) was loaded onto a Phenomenex Luna 5 μm C8 (2) analytical column (15 cm × 4.6 mm) using an autosampler. Gradient elution chromatography was carried out at 30°C with a binary mobile phase composed of acetonitrile and 25 mmol/L ammonium formate buffer, pH 3.75 delivered at 1.0 mL/min. The amount of acetonitrile was increased from 80% at the beginning of the run to 95% over 8 minutes, held at 95% for 3 minutes, then decreased to 80% for 3 minutes before the next injection. An Agilent Technologies (Palo Alto) single-quadrupole SL model MSD with an atmospheric pressure ionization-electrospray interface was used for detection. Nitrogen was used as the nebulizing gas (50 p.s.i.) and drying gas (12 L/min, 350°C). With a transfer capillary potential of 5,000 V, positive ions corresponding to the [M+H]+ ions for bardoxolone methyl at m/z 506.3 and the IS at m/z 520.3 were measured by selected ion monitoring (fragmentor potential, 175 V; dwell time, 114 milliseconds). Extracted ion chromatograms were integrated to provide peak areas.
Each study sample was assayed in duplicate, on different days, together with a set of 9 calibration standards containing bardoxolone methyl in plasma at concentrations ranging from 0.5 to 100 ng/mL, drug-free plasma prepared for analysis with and without IS, and quality control samples of bardoxolone methyl in plasma (1.5, 15, and 90 ng/mL). The relationship between the bardoxolone methyl/IS peak area ratio and known drug concentration in the calibration standards was analyzed by weighted linear regression. The slope and y-intercept of the best fit line was used to calculate the drug concentration in study samples. Specimens with concentrations of more than 100 ng/mL were reassayed upon dilution with blank plasma. The average of the 2 determinations of each study sample was calculated. Samples were reassayed in cases in which the individual determinations differed from their average by more than 10%.
The analytic method was validated to document specificity, recovery, accuracy and precision within and between days, and drug stability in the final sample solution and in human plasma upon long-term storage and after multiple freeze-thaw cycles as recommended (20). Retention times (mean ± SD) were 5.09 ± 0.03 minutes for bardoxolone methyl and 5.91 ± 0.31 minutes for the IS. Peaks that interfered with either compound were not evident in chromatograms of blank plasma from several anonymous donors and plasma obtained shortly before dosing from subjects enrolled in this clinical trial. Calibration curves exhibited excellent linearity with correlation coefficients more than 0.99. During application of the assay to samples from this clinical trial, the lowest drug concentration in the calibration curves (0.5 ng/mL) was assayed with a between-day accuracy of 93.3% and 13.7% precision. Quality control samples were assayed with a between-day accuracy of 99.4% to 105.6% and a precision of 7.1% to 8.3%.
Individual patient plasma concentration–time data for each dose of bardoxolone methyl were analyzed by standard noncompartmental methods with WinNonlin Professional version 5.0.1 software (Pharsight Corp.). The maximum concentration of drug in plasma (Cmax) was based directly upon the actual assayed values of the study samples. The slope of the terminal disposition phase (λz) for the day 21 dose was determined by logarithmic linear regression and used to calculate the terminal phase half-life (t1/2,z) as 0.693/λz. Area under the plasma concentration-time curve from time zero to 24 hours after dosing (AUCτ) was estimated with the logarithmic linear trapezoidal algorithm. Apparent oral clearance (CL/F) was calculated as the daily dose divided by AUCτ for the day 21 dose, under the assumption that steady-state conditions had been achieved for the administration schedule. The accumulation factor for repeated dosing (X) was calculated by dividing AUCτ for the day 21 dose by that for the first dose. Pharmacokinetic parameters are reported as the geometric mean ± SD of values for individual patients at each dose level (21).
Real-time PCR for analysis of Nrf2 activation
Activation of Nrf2 was assessed in peripheral blood mononuclear cells (PBMC) by measuring NAD(P)H:quinone oxidoreductase (NQO1) mRNA levels using real-time quantitative PCR (qPCR). PBMCs were isolated from blood, and RNA was extracted with RNeasy with on-column DNase digestion (QIAGEN). cDNA was synthesized with the High-Capacity cDNA Archive Kit (Applied Biosystems). TaqMan Gene Expression Assays (Applied Biosystems) were used to detect NQO1 (Hs00168547_m1) and the control gene, ABL1 (Hs00245445_m1). PCR amplification was carried out with using the ABI Prism 7500 Sequence Detection System (Applied Biosystems) and standard cycling conditions. The amount of NQO1 transcript was normalized to corresponding ABL1 levels.
Tumor biopsies
Optional core biopsies (18 gauge needles) were carried out in consenting patients at baseline (within 28 days before treatment start) and at the end of the treatment period of cycle 1. Formalin-fixed, paraffin-embedded tissue was sectioned (5 μm) and used for immunohistochemical assessment of NF-κB, STAT3, pSTAT3, cyclin D1, p21, active caspase-3, VEGF, and HIF1-α protein levels. Primary mouse monoclonal antibodies against NF-κB p65, STAT3, p-STAT3, and VEGF were obtained from Santa Cruz Biotechnology. Primary mouse monoclonal antibodies against CD68 and p21 were obtained from Dako North America. Primary mouse monoclonal antibodies against active caspase-3 and HIF-1α were obtained from BD Biosciences. Cyclin D1 mouse monoclonal antibodies were obtained from Thermo Fisher Scientific. Positive reactions were visualized by incubating the slides with diaminobenzidine/hydrogen peroxide (DAKO) as a substrate. Nonspecific staining was not observed in samples incubated with secondary antibody alone. DNA fragmentation was monitored by in situ terminal deoxynucleotidyl transferase–dUTP nick end labeling (TUNEL) assay (Boehringer-Mannheim). To assess protein levels or DNA fragmentation, positive and negative cells were counted in 10 random high power fields (hpf; × 400) and averaged. The level of these proteins was assessed in tumor cells only, which were defined as CD68-negative cells. All staining experiments were carried out in duplicate.
Results
Patient characteristics
Forty-seven patients were enrolled across 3 study centers in the United States between April 2006 and March 2008. Demographics and clinical characteristics at study entry are summarized in Table 1. The most common cancer types were melanoma (16 patients, 34%), colorectal (8 patients, 17%), renal cell (5 patients, 11%), anaplastic thyroid (4 patients, 9%), and non-Hodgkin’s lymphoma (3 patients, 6%). The median number of prior therapies was 3 (range = 0–7).
Table 1.
Patient characteristics (N = 47)
| Gender, n (%) | |
| Male | 34 (72) |
| Female | 13 (28) |
| Race, n (%) | |
| White | 46 (98) |
| Other | 1 (2) |
| Median age, y (range) | 60 (24–81) |
| ECOG, n (%) | |
| 0 | 21 (45) |
| 1 | 23 (49) |
| 2 | 3 (6) |
| Diagnosis, n (%) | |
| Melanoma | 16 (34) |
| Colorectal | 8 (17) |
| Renal cell | 5 (11) |
| Anaplastic thyroid | 4 (9) |
| Non-Hodgkins lymphoma | 3 (6) |
| Other | 11 (23) |
| Median number of prior anticancer therapy regimensa, n (range) | 3 (0–7) |
Including immunotherapy.
Safety
All 47 patients received at least 1 dose of bardoxolone methyl and were evaluated for safety. Eighty-one percent of patients (n = 38) experienced at least 1 AE that was considered at least possibly related to study drug. Of these patients, 31 (82%) experienced grade 2 or less, and 7 (18%) experienced grade 3, toxicities. There were no grade 4 toxicities attributed to treatment with bardoxolone methyl. The most common drug-related AEs were fatigue (n = 19; 40%); nausea (n = 16; 34%), and anorexia (n = 14; 30%; Table 2).
Table 2.
Drug-related AEs occurring in more than 3% of patients
| Dose, mg/d patients treatedb | 5 (n = 2)
|
10 (n = 1)
|
20 (n = 1)
|
40 (n = 1)
|
80 (n = 1)
|
150 (n = 3)
|
300 (n = 5)
|
600 (n = 6)
|
900a (n =25)
|
1,300 (n =9)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCI-CTCAE grade | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 |
| Blood/bone marrow | ||||||||||||||||||||
| Thrombocytopenia | 1 | 1 | ||||||||||||||||||
| Anemia | 2 | |||||||||||||||||||
| Hemoglobin | 1 | 1 | ||||||||||||||||||
| Gastrointestinal | ||||||||||||||||||||
| Nausea | 1 | 2 | 9 | 1 | 3 | |||||||||||||||
| Diarrhea | 1 | 1 | 1 | 6 | 3 | |||||||||||||||
| Vomiting | 1 | 1 | 6 | 2 | ||||||||||||||||
| Dysgeusia | 1 | 2 | 4 | 3 | ||||||||||||||||
| Constipation | 2 | 2 | 1 | |||||||||||||||||
| Oral mucosal exfoliation | 1 | 1 | 1 | 2 | 1 | 1 | ||||||||||||||
| Abdominal distention | 1 | 1 | 1 | |||||||||||||||||
| Dry mouth | 2 | 1 | ||||||||||||||||||
| Stomatitis/oral mucosal blistering | 1 | 1 | 1 | |||||||||||||||||
| Abdominal pain | 1 | 1 | ||||||||||||||||||
| Constitutional symptoms | ||||||||||||||||||||
| Fatigue | 1 | 1 | 12 | 3 | 2 | |||||||||||||||
| Anorexia/decreased appetite | 1 | 1 | 7 | 1 | 4 | |||||||||||||||
| Weight loss | 2 | 2 | ||||||||||||||||||
| Myalgia | 1 | 1 | 2 | |||||||||||||||||
| Headache | 1 | 1 | ||||||||||||||||||
| Night sweats/hot flash | 1 | 1 | 1 | |||||||||||||||||
| Metabolic/laboratory | ||||||||||||||||||||
| Elevated AST | 1 | 8 | 3 | |||||||||||||||||
| Elevated ALT | 1 | 7 | 1 | 1 | 2 | |||||||||||||||
| Hypomagnesemia | 3 | 1 | ||||||||||||||||||
| Other | ||||||||||||||||||||
| Rash/psoriasis/pruritus | 1 | 4 | ||||||||||||||||||
| Muscle spasm | 1 | 1 | 4 | 2 | ||||||||||||||||
NOTE: Drug-related is defined as possibly, probably, or definitely related to bardoxolone methyl. Abbreviation: NCI-CTCAE, National Cancer Institute-Common Terminology Criteria for Adverse Events.
A total of 900 mg/d is the recommended phase II dose for bardoxolone methyl administered orally once a day for 21 consecutive days during a 28-day treatment cycle.
Number of patients treated at each dose level is greater than the total number of patients enrolled due to dose adjustments during the study.
Starting at 5 mg/d, dose escalation by accelerated titration at increments of approximately 100% of the previous dose (as formulation would allow) continued for 8 dose levels to 600 mg/d. Although there was no evidence of DLT in the 5 patients treated at 600 mg/d, the next dose level was increased by 50% to 900 mg/d in consideration of the magnitude of the dose and concern that the true MTD could be underestimated. Three patients were enrolled at 900 mg/d without any DLTs. The first occurrence of a DLT (grade 3 ALT elevation) was observed at the 1,300 mg/d dose level. Accordingly, the cohort was expanded to 6 evaluable patients. Nine patients were enrolled at the 1,300 mg/d dose level; however, 3 patients terminated the study prematurely due to disease progression and were deemed nonevaluable for DLT assessment. Two of the 6 evaluable patients treated at 1,300 mg/d experienced grade 3 ALT elevation. The first patient experienced the DLT on day 8 of cycle 1 and spontaneously resolved after discontinuing treatment. The patient restarted treatment at the 900 mg/d dose level during the next cycle and remained on study without recurrence of grade 3 ALT elevation through the end of cycle 8. Following the occurrence of ALT elevation in the second patient (day 8), the dose was reduced to 900 mg/d. There were no other indicators of hepatotoxicity in either patient. Accordingly, the 900 mg/d dose level cohort was expanded to 25 patients. No further DLTs were observed. Therefore, 900 mg/d was established as the MTD and recommended phase II dose.
Pharmacokinetics
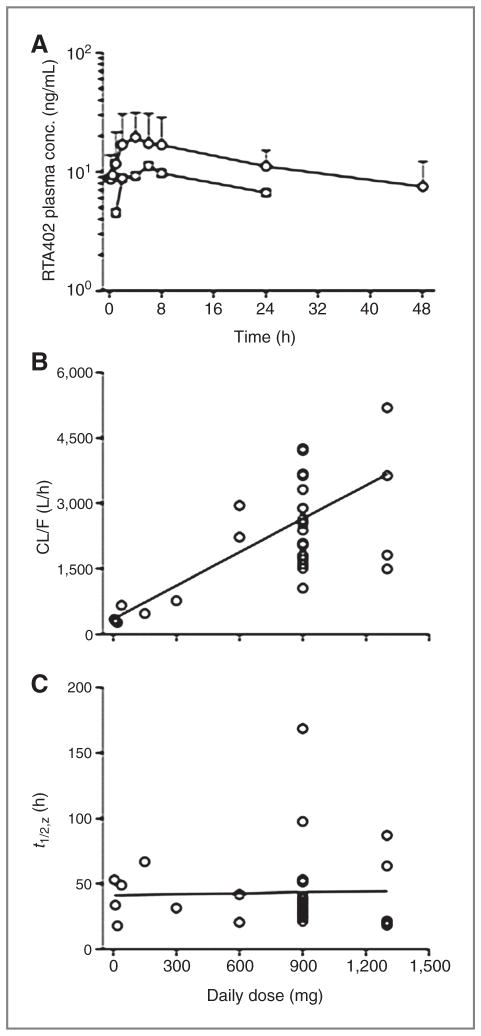
Mean plasma concentration-time profiles of bardoxolone methyl for patients treated with the first (n = 23) and last (n = 19) daily dose of 900 mg during cycle 1 are shown in Fig. 1A. The median time of the observed maximum concentration of drug in plasma was 4 hours for both the initial dose (range, 1–24 hours) and the dose given on day 21 (range, 0.25–24 hours). Thereafter, bardoxolone methyl plasma levels decreased very slowly, with an apparent t1/2,z of 39 ± 20 hours in patients treated with 900 mg/d. It should be recognized that the decline in drug levels may not have been monitored for a sufficiently long period following administration of the final dose to provide an accurate estimate of the t1/2,z. Nevertheless, the drug remained in plasma at significant concentrations for more than 24 hours after dosing, and the mean accumulation factor for dosing once daily was 1.6 ± 0.9. Mean steady-state minimum and maximum concentrations of bardoxolone methyl in plasma provided by daily administration of a 900 mg dose were 8.8 ± 4.3 ng/mL and 24.7 ± 13.3 ng/mL, with a peak-to-trough ratio of only 2.8 ± 1.6.
Figure 1.
Pharmacokinetics of bardoxolone methyl. A, mean plasma concentration-time profiles for the first (circles) and last (squares) dose of bardoxolone methyl in the cohort of patients receiving 900 mg/d. Error bars depict the SD of the mean concentration of bardoxolone methyl in plasma for the day 21 dose. B, relationship between the apparent oral clearance (CL/F) of bardoxolone methyl and dose. C, relationship between the biological half-life (t1/2,z) of bardoxolone methyl and dose. B and C, data points are the observed values in individual patients and the solid lines were generated by linear regression analysis of the data.
Mean values of the steady-state pharmacokinetic parameters for the cohorts evaluated at each dose level are presented in Table 3. Plots of the Cmax, the concentration of drug in plasma 24 hours after dosing, and AUCτ as a function of dose all exhibited less than proportionate increases for both the initial and final doses of bardoxolone methyl, suggestive of saturable drug absorption (not shown). The existence of a highly significant correlation (r = 0.69, P < 0.01) between CL/F and dose was also consistent with saturable absorption (Fig. 1B). This is further supported by the absence of a significant correlation (r = 0.03, P = 0.86) between the t1/2,z and dose (Fig. 1C), which suggests that the dose-dependent pharmacokinetic behavior is not attributable to an effect associated with changes in drug elimination. Interpatient variability in the mean pharmacokinetic parameters ranged from 64% to 77% for the first dose and 39% to 54% for the last dose among patients at the 900 mg dose level.
Table 3.
Steady-state pharmacokinetic parameters of bardoxolone methyl
| Dose (mg/d) | No. of patients | Cmin (ng/mL) | tmax (h) | Cmax (ng/mL) | t1/2,z | (h) AUCτ (ng·h/mL) | CL/F (L/h) | X |
|---|---|---|---|---|---|---|---|---|
| 5 | 1 | 0.7 | 1.0 | 1.0 | 53 | 15 | 342 | 1.5 |
| 10 | 1 | 1.3 | 4.0 | 2.5 | 34 | 33 | 306 | 1.8 |
| 20 | 1 | 1.2 | 8.0 | 4.6 | 18 | 75 | 266 | 4.0 |
| 40 | 1 | 1.5 | 2.0 | 3.2 | 49 | 60 | 663 | 1.4 |
| 80 | 0 | |||||||
| 150 | 1 | 9.7 | 6.0 | 16.6 | 67 | 314 | 477 | 2.3 |
| 300 | 1 | 18.9 | 1.0 | 22.7 | 32 | 389 | 771 | 2.9 |
| 600a | 2 | 6.3 ± 0.7 | 4.0 ± 0.0 | 14.0 ± 0.3 | 29 ≥ | 15 235 ± 47 | 2,557 ± 515 | 3.7 ± 3.3 |
| 900b | 19 | 8.8 ± 4.3 | 4.1 ± 3.4 | 24.7 ± 13.3 | 39 ≥ | 20 370 ± 145 | 2,430 ± 956 | 1.6 ± 0.9 |
| 1,300b | 5 | 14.3 ± 8.9 | 2.4 ± 3.6 | 28.8 ± 14.6 | 34 ≥ | 24 411 ± 257 | 3,161 ± 2,030 | 2.6 ± 1.7 |
Abbreviations: Cmin, concentration in plasma at time zero before dosing; tmax, time of the maximum concentration in plasma; Cmax, maximum concentration in plasma; t1/2,z, apparent terminal phase half-life; AUCτ, area under the plasma concentration-time curve from time zero to 24 hours after dosing; CL/F, apparent oral clearance; X, accumulation factor for repeated dosing.
Values reported as the average ± SD.
Values reported as the geometric mean ± SD.
Tumor biopsies and immunohistochemistry analysis
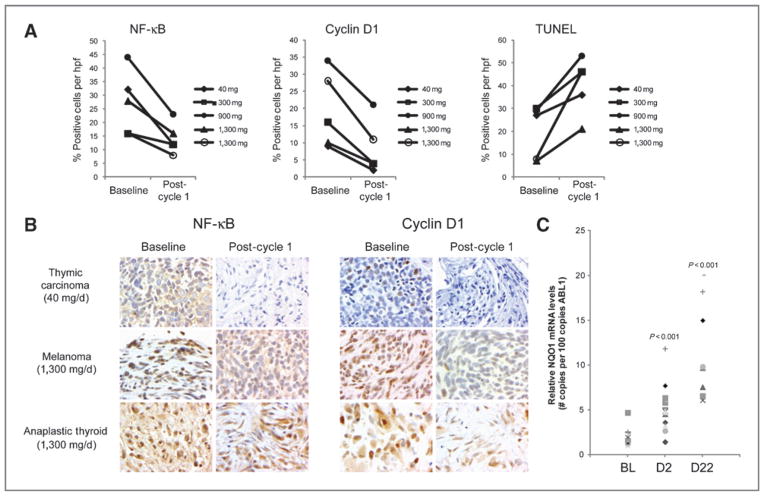
Optional paired biopsies were collected from 5 patients (Fig. 2A and B). Two patients at the 1,300 mg and 1 patient each at the 40, 300, and 900 mg dose levels underwent biopsies predose and on day 22. Neoplastic cells from all 5 samples showed a decrease in NF-κB (19.4% ± 4.8% positive cells vs. 10.0% ± 2% positive cells; P = 0.038) and cyclin D1 levels (18.8% ≥4.5% positive cells vs. 5.8% ± 1.6% positive cells; P = 0.015) on day 22 relative to baseline. A marked increase in DNA fragmentation as assessed by the TUNEL assay (17.2% ± 4.8% positive cells vs. 33.4% ± 5.8% positive cells; P = 0.025) was also observed on day 22 relative to baseline. Statistically significant differences in levels of STAT3, p-STAT3, p21, active caspase-3, VEGF, and HIF1-α were not observed in these samples.
Figure 2.
Pharmacodynamics of bardoxolone methyl. A, levels of NF-κB, cyclin D1, and TUNEL activity were monitored by immunohistochemistry in core biopsies from consenting patients. The average number of positively staining tumor cells per hpf at baseline and after 1 cycle of treatment is shown for 5 patients. The dose of bardoxolone methyl each patient received is indicated. B, representative images of NF-κB and cyclin D1 staining in tumor biopsies from 3 patients. C, NQO1 transcript levels were monitored using qPCR in PBMCs isolated from patients at baseline (BL), day 2 (D2), and day 22 (D22). P-values were determined using the Student’s t-test and indicate statistical significance relative to baseline (BL).
Assessment of Nrf2 activation
To assess pharmacologic activation of Nrf2 by bardoxolone methyl, mRNA levels of NQO1, a transcriptional target of Nrf2, were measured by qPCR in PBMCs obtained from 14 patients enrolled at 9 different dose levels. Samples were obtained at baseline, day 2 (before dosing) and day 22. Significant increases in mean relative NQO1 mRNA levels were observed on day 2 (5.22 ± 0.11 vs. 1.97 ± 0.07; P = 0.0001) and day 22 (10.99 ± 0.22 vs. 1.97 ± 0.07; P < 0.0001; Fig. 2C).
Efficacy
Efficacy was assessed at the end of every 2 cycles of treatment. Two patients withdrew consent, and one patient was removed from the study due to an unrelated AE before receiving posttreatment evaluation. Fourteen of the remaining 44 patients terminated the study before the first assessment (end of cycle 2) due to clinical disease progression. Tumor measurements were obtained at study termination for 8 of these patients; posttreatment tumor measurements were not obtained for the other 6 patients. The remaining 30 patients completed at least 2 cycles of treatment, and both pre- and posttreatment tumor measurements were obtained.
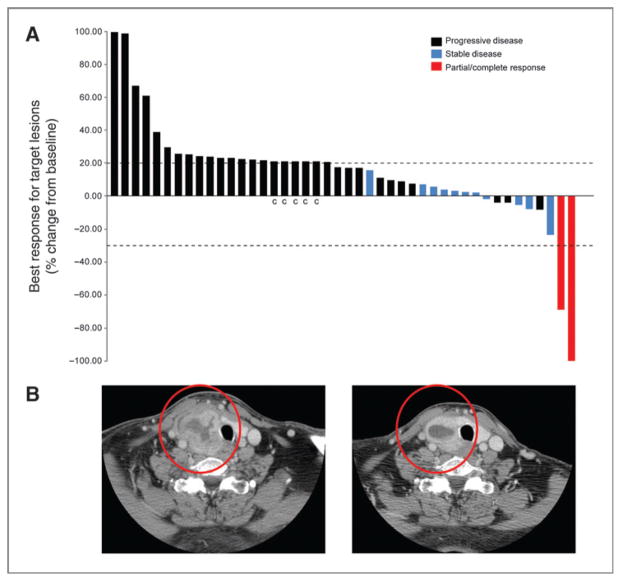
Best overall responses (n = 44) are shown in Fig. 3A. One patient with mantle cell lymphoma who had failed autologous peripheral stem cell transplant was treated with 900 mg/d during cycle 1 and 600 mg/d secondary to elevated transaminases for the remaining cycles and achieved a complete response following cycle 4. This patient received a total of 7 cycles of therapy and was then removed from the study in preparation for stem cell transplantation. A second patient with an anaplastic thyroid carcinoma achieved a durable partial response (−69%) lasting 18 months before progressing (Fig. 3B). In addition, 10 patients (5 melanoma, 2 renal cell carcinoma, and 1 each thyroid, colorectal, and lymphoma) experienced disease stabilization for 4 to 10 months.
Figure 3.
A, waterfall plot of best response of 44 patients who received bardoxolone methyl. Best overall response was categorized as progressive disease (black bars), stable disease (blue bars), partial response or complete response (both red bars). “c” represents patients who exhibited clinical progression but were not restaged by RECIST/WHO. B, images of a patient with anaplastic thyroid cancer who responded to bardoxolone methyl after cycle 2 (best response −69%) and stayed on protocol for 18 months. Left panel is the image taken at baseline; right panel is the image of the same patient taken after two cycles of treatment.
Improvement in estimated glomerular filtration rate
One interesting observation, revealed in a retrospective analysis, was the improvement of patients’ estimated glomerular filtration rate (eGFR), while on study (Supplementary Table S1). There was an overall 26% increase of eGFR for all patients (P < 0.0001). At 900 mg/d, the dose at which the greatest number of patients were treated (n = 20), there was a statistically significant increase in eGFR (33.9% ± 4.2%; P < 0.0001) on day 21 compared with baseline. Patients with baseline eGFR values of less than 60 mL/min/1.73 m2 showed a greater improvement in eGFR than patients with baseline eGFR values of 60 mL/min/1.73 m2 or more (35.6% ± 6.8% vs. 22.9% ± 3.5%). This trend did not reach statistical significance (P = 0.08) due to the small number of patients (N = 10) with baseline eGFR values less than 60 mL/min/1.73 m2. The increase in eGFR was sustained in patients (N = 9) that were treated with bardoxolone methyl for at least 6 months (Supplementary Fig. S1).
Discussion
This first-in-human study of bardoxolone methyl, a novel synthetic triterpenoid, showed that this agent had minimal toxicity and evidence of antitumor activity. DLT was confined to patients receiving 1,300 mg/d and consisted of grade 3 serum transaminase (specifically ALT) elevations. The increase in transaminase levels was transient and not associated with increases in other indicators of hepatotoxicity, such as bilirubin or alkaline phosphatase. Seven and 9 patients experienced drug-related grade of 2 or less elevations in ALT and AST, respectively. Similar increases in transaminases were observed in preclinical toxicology studies in cynomolgus monkeys treated with bardoxolone methyl for 12 months and were not associated with adverse liver histopathology (C. Meyer; unpublished data). Genetic activation of Nrf2 in animal models results in increased serum ALT activity (22). Conversely, animals lacking the Nrf2 gene have lower basal levels of serum ALT and AST activity (23). Recently, Nrf2 has been shown to bind to the promoter of ALT2, suggesting that the increased transaminase levels observed in patients treated with bardoxolone methyl may be due to transcriptional regulation of these enzymes by Nrf2 (22–24). There was an overall lack of toxicity attributable to bardoxolone methyl at the MTD and lower doses. The nausea and anorexia experienced by some may have been related to the number of capsules that had to be taken for each daily dose. A new formulation of the drug has since been developed and is currently being tested in clinical trials.
The pharmacokinetic behavior of the tested formulation of bardoxolone methyl is characterized by slow absorption after oral administration, a relatively long terminal phase half-life, nonlinearity at high doses, and high interpatient variability. The maximum concentration of the drug in plasma is not achieved until 4 hours after dosing, on average, although the time varied greatly (from 15 minutes to 24 hours) in different patients. Once daily repeated dosing schedule is appropriate for future studies in consideration of the 39-hour mean biological half-life of bardoxolone methyl. The mean peak-to-trough ratio for the concentration of drug in plasma during steady state was only 2.8, indicating that the once daily dosing regimen effectively maintains plasma levels of the drug within a relatively narrow range in individual patients. The apparent oral clearance of bardoxolone methyl was significantly correlated with the daily dose, whereas the terminal phase half-life was independent of the dose, strongly suggestive of an inverse relationship between the extent of absorption and dose. This finding prompted efforts to improve the oral dosage form of the drug with the objective of increasing the rate and extent of absorption, which would hopefully minimize the dose-dependent behavior as well.
Concentrations of the drug achieved in the group of patients receiving bardoxolone methyl at 900 mg/d, the recommended dose for phase II oncology clinical trials, were within the range required to elicit in vitro antiproliferative and antiangiogenic activity. In these patients, the maximum concentration of bardoxolone methyl in plasma during steady state was 20 to 133 nmol/L and the corresponding minimum concentration was 8 to 40 nmol/L. Effective concentrations of the drug required to inhibit the growth of the more sensitive types of human cancer cell lines ranged from 5 to 500 nmol/L (12, 13). Bardoxolone methyl inhibits the growth of endothelial cells in monolayer cultures and suppresses neovascular morphogenesis in 3-dimensional cultures at concentrations ranging from 50 to 200 nmol/L. Evidence of antiangiogenic activity was also shown when given to mice at doses as low as 3 μg/kg. In addition, bardoxolone methyl at concentrations of 25 to 100 nmol/L in vitro also abrogates the immunosuppressive effect of MDSCs, one of the major factors responsible for immune suppression in cancer (17).
Objective tumor responses were observed in 2 patients. The first was a complete clinical response in a mantle cell lymphoma patient. Mantle cell lymphoma is characterized by a chromosomal translocation that leads to upregulation of cyclin D1, resulting in dysregulation of the cell cycle (25). Bardoxolone methyl has been shown to inhibit NF-κB and JAK/STAT signaling and to reduce cyclin D1 levels (5, 9). Furthermore, in this study, significant downregulation of these pathways was observed in several tumor biopsies following treatment, showing that these pathways are also targeted in human tumors (Fig. 2A and B). Cyclin D1 levels could not be measured in the mantle cell lymphoma patient, as a biopsy was not obtained. The second objective response occurred in an anaplastic thyroid tumor patient who exhibited a partial response (69% reduction) that was durable for 18 months. NF-κB is a common point of convergence for many oncogenic signaling pathways involved in thyroid carcinogenesis, and the NF-κB pathway has been shown to be constitutively active in primary human anaplastic thyroid cancers (26, 27). The potent inhibitory effect of bardoxolone methyl on the NF-κB pathway may have contributed to the partial response observed. Modulation of the NF-κB and cyclin D1 were seen in the 40 mg dose implying that even at the 40 mg dose there is biologic activity, which is consistent with the improvement in eGFR seen in doses as low as 25 mg/d in later studies in chronic kidney disease. One limitation of the study was the optional nature of the biopsies, which limited the number of samples.
Prolonged stable disease of 4 or more months was observed in 10 patients, including 5 melanoma patients and 2 renal cell carcinoma patients. The prolonged period of stable disease in these patients may be attributed to the inhibitory effect of bardoxolone methyl on NF-κB and its downstream target iNOS (28). Constitutively activated NF-κB induces iNOS in melanoma cell line (29), and iNOS has been implicated as an indicator of poor prognosis in melanoma and a possible target for therapy (30). Nitric oxide (NO) and ROS also play an important role in tumor-mediated immune suppression (31). In this study, NQO1 levels in PBMCs were significantly increased after treatment with bardoxolone methyl, showing that the Nrf2 pathway was activated and thus bardoxolone methyl has the potential to reduce ROS and NO and to restore immune surveillance. In this regard, bardoxolone methyl has been shown to abrogate the tumor-suppressive activity of MDSCs and inhibit tumor growth in mice in a manner that is dependent on the immune system (17). Therefore, in addition to suppression of NF-κB and JAK/STAT signaling, inhibition of ROS and NO may contribute to the prolonged stable disease observed in several patients.
A retrospective analysis of measurements of serum creatinine, which were collected as a standard safety measure, indicated that eGFR improved in many patients. Despite excluding patients with serum creatinine of more than 2.0 mg/dL, the average eGFR at enrollment was modestly below normal. Surprisingly, the observed reductions in serum creatinine suggested a possible beneficial effect of bardoxolone methyl on measures of kidney function. Many factors that contribute to the development of chronic kidney disease, such as hyperglycemia and activation of the renin-angiotensin system (RAS), stimulate proinflammatory signaling pathways resulting in the production of ROS (32–34). Markers of oxidative stress have been shown to positively correlate with chronic kidney disease stage, suggesting that the production of ROS contributes to renal pathology (35–37). Bardoxolone methyl activates the Nrf2 pathway in cultured renal cells and in mouse kidney tissues (38); therefore, induction of antioxidant enzymes by Nrf2 may reduce ROS in renal tissue and improve renal function. Based on the eGFR effects observed in this trial, 2 additional trials were designed to assess the effect of bardoxolone methyl in patients with type 2 diabetes and chronic kidney disease. Significant increases in eGFR were observed following treatment with bardoxolone methyl in both trials (39, 40).
In conclusion, the crystalline formulation of bardoxolone methyl was well tolerated at 900 mg/d, with the primary DLT being reversible elevation of serum ALT. The pharmacokinetic activity of bardoxolone methyl is characterized by slow oral absorption, a relatively long biological half-life, nonlinearity, and high interpatient variability. Pharmacodynamic analyses showed that bardoxolone methyl activates Nrf2 (NQO1) in PBMCs and inhibits NF-κB and cyclin D1 in tumor biopsies. The improvement in creatinine clearance suggests a possible role for bardoxolone methyl in treating chronic kidney disease in addition to cancer. Clinical activity was observed in a variety of tumor types, supporting continued study to evaluate the activity of other synthetic triterpenoids.
Supplementary Material
Translational Relevance.
Bardoxolone methyl (RTA 402; CDDO-Me) is a novel synthetic triterpenoid and antioxidant inflammation modulator. Bardoxolone methyl potently induces Nrf2, reducing oxidative stress, and inhibits constitutive and cytokine-induced activation of NF-κB and Janus-activated kinase/STAT signaling, thereby suppressing inflammation. This first-in-human phase I trial aimed to determine the dose-limiting toxicities and maximum tolerated dose, characterize the pharmacokinetics and pharmacodynamics, and assess antitumor activity of bardoxolone methyl. Bardoxolone methyl was found to be well tolerated, and objective tumor activity was observed in patients with mantle cell lymphoma (complete response) and anaplastic thyroid carcinoma (partial response). NQO1 mRNA levels, indicative of Nrf2 activation, were increased in peripheral blood mononuclear cells, and NF-κB and cyclin D1 levels were decreased in tumor biopsy samples. These data show that bardoxolone methyl modulates antioxidant and anti-inflammatory targets and exhibits anticancer activity in patients. In addition, an interesting effect on renal function was incidentally observed as estimated glomerular filtration rate was noted to improve. This observation has led to development of bardoxolone methyl in patients with Stage 4 chronic kidney disease.
Acknowledgments
The authors thank Joann Aaron for editing the manuscript and the participating patients, their families, and study investigators for their invaluable contribution.
Grant Support
This work was supported by REATA Pharmaceuticals, Inc. (Irving, TX) and by the Leukemia and Lymphoma Society (R6149-07 01) to M. Konoplev, and the Haas Chair in Genetics (M. Andreeff).
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
D.S. Hong and J.G. Supko have a commercial research grant from Reata Pharmaceuticals. C.J. Meyer, D.A. Ferguson, M. Konopleva, M. Andreeff and D.W. Kufe have an ownership interest (including patents) in Reata Pharmaceuticals. M. Konopleva, M. Andreeff, and D.W. Kufe are consultants/advisory board members of Reata Pharmaceuticals. No potential conflicts of interest were disclosed by the other authors.
This article was presented, in part, at the 43rd Annual Meeting of the American Society of Clinical Oncology (ASCO), June, 2008, Chicago, IL.
Authors’ Contributions
Conception and design: D.S. Hong, R. Kurzrock, J.G. Supko, J.J. Wheler, J.P. Eder, C.J. Meyer, S. Konoplev, M. Andreeff, B.J. Dezube
Development of methodology: D.S. Hong, J.G. Supko, X. He, C.J. Meyer, M. Konopleva, S. Konoplev, M. Andreeff
Acquisition of data: D.S. Hong, J.G. Supko, X. He, A. Naing, D.P. Lawrence, J.W. Mier, S. Konoplev, H. Lazarus, G.I. Shapiro, B.J. Dezube
Analysis and interpretation of data: D.S. Hong, J.G. Supko, J.G. Supko, D. P. Lawrence, C.J. Meyer, D.A. Ferguson, M. Konopleva, S. Konoplev, M. Andreeff, H. Lazarus, G.I. Shapiro, B.J. Dezube
Writing, review, and/or revision of the manuscript: D.S. Hong, R. Kurzrock, J.G. Supko, A. Naing, J.J. Wheler, D.P. Lawrence, J.P. Eder, C.J. Meyer, D.A. Ferguson, M. Konopleva, S. Konoplev, M. Andreeff, D.W. Kufe, H. Lazarus, G.I. Shapiro, B.J. Dezube
Administrative, technical, or material support: D.S. Hong, J.G. Supko, S. Konoplev, B.J. Dezube
Study supervision: D.S. Hong, G.I. Shapiro, B.J. Dezube
References
- 1.Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A. 2005;102:4584–9. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, et al. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351:883–9. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin W, Zhu L, Guan Q, Chen G, Wang QF, Yin HX, et al. Influence of Nrf2 genotype on pulmonary NF-kappaB activity and inflammatory response after traumatic brain injury. Ann Clin Lab Sci. 2008;38:221–7. [PubMed] [Google Scholar]
- 4.Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–9. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling X, Konopleva M, Zeng Z, Ruvolo V, Stephens LC, Schober W, et al. The novel triterpenoid C-28 methyl ester of 2-cyano-3, 12-dioxoolen-1, 9-dien-28-oic acid inhibits metastatic murine breast tumor growth through inactivation of STAT3 signaling. Cancer Res. 2007;67:4210–8. doi: 10.1158/0008-5472.CAN-06-3629. [DOI] [PubMed] [Google Scholar]
- 6.Liby K, Risingsong R, Royce DB, Williams CR, Yore MM, Honda T, et al. Prevention and treatment of experimental estrogen receptor-negative mammary carcinogenesis by the synthetic triterpenoid CDDO-methyl ester and the rexinoid LG100268. Clin Cancer Res. 2008;14:4556–63. doi: 10.1158/1078-0432.CCR-08-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan Z, Ames RY, Ryan M, Hornicek FJ, Mankin H, Seiden MV. CDDO-Me, a synthetic triterpenoid, inhibits expression of IL-6 and Stat3 phosphorylation in multi-drug resistant ovarian cancer cells. Cancer Chemother Pharmacol. 2009;63:681–9. doi: 10.1007/s00280-008-0785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad R, Raina D, Meyer C, Kufe D. Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)–>signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer Res. 2008;68:2920–6. doi: 10.1158/0008-5472.CAN-07-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shishodia S, Sethi G, Konopleva M, Andreeff M, Aggarwal BB. A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin Cancer Res. 2006;12:1828–38. doi: 10.1158/1078-0432.CCR-05-2044. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006;281:35764–9. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 11.Zou W, Liu X, Yue P, Zhou Z, Sporn MB, Lotan R, et al. c-Jun NH2-terminal kinase-mediated up-regulation of death receptor 5 contributes to induction of apoptosis by the novel synthetic triterpenoid methyl-2-cyano-3,12-dioxooleana-1, 9-dien-28-oate in human lung cancer cells. Cancer Res. 2004;64:7570–8. doi: 10.1158/0008-5472.CAN-04-1238. [DOI] [PubMed] [Google Scholar]
- 12.Kim KB, Lotan R, Yue P, Sporn MB, Suh N, Gribble GW, et al. Identification of a novel synthetic triterpenoid, methyl-2-cyano-3,12-dioxooleana-1,9-dien-28-oate, that potently induces caspase-mediated apoptosis in human lung cancer cells. Mol Cancer Ther. 2002;1:177–84. [PubMed] [Google Scholar]
- 13.Konopleva M, Tsao T, Ruvolo P, Stiouf I, Estrov Z, Leysath CE, et al. Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood. 2002;99:326–35. doi: 10.1182/blood.v99.1.326. [DOI] [PubMed] [Google Scholar]
- 14.Hyer ML, Shi R, Krajewska M, Meyer C, Lebedeva IV, Fisher PB, et al. Apoptotic activity and mechanism of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic-acid and related synthetic triterpenoids in prostate cancer. Cancer Res. 2008;68:2927–33. doi: 10.1158/0008-5472.CAN-07-5759. [DOI] [PubMed] [Google Scholar]
- 15.Liby K, Risingsong R, Royce DB, Williams CR, Ma T, Yore MM, et al. Triterpenoids CDDO-methyl ester or CDDO-ethyl amide and rexinoids LG100268 or NRX194204 for prevention and treatment of lung cancer in mice. Cancer Prev Res (Phila Pa) 2009;2:1050–8. doi: 10.1158/1940-6207.CAPR-09-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vannini N, Lorusso G, Cammarota R, Barberis M, Noonan DM, Sporn MB, et al. The synthetic oleanane triterpenoid, CDDO-methyl ester, is a potent antiangiogenic agent. Mol Cancer Ther. 2007;6:3139–46. doi: 10.1158/1535-7163.MCT-07-0451. [DOI] [PubMed] [Google Scholar]
- 17.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–23. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors., European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 20.Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, et al. Bioanalytical method validation—a revisit with a decade of progress. Pharm Res. 2000;17:1551–7. doi: 10.1023/a:1007669411738. [DOI] [PubMed] [Google Scholar]
- 21.Lacey LF, Keene ON, Pritchard JF, Bye A. Common noncompartmental pharmacokinetic variables: are they normally or log-normally distributed? J Biopharm Stat. 1997;7:171–8. doi: 10.1080/10543409708835177. [DOI] [PubMed] [Google Scholar]
- 22.Osburn WO, Yates MS, Dolan PD, Chen S, Liby KT, Sporn MB, et al. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol Sci. 2008;104:218–27. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang YK, Yeager RL, Tanaka Y, Klaassen CD. Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet. Toxicol Appl Pharmacol. 2010;245:326–34. doi: 10.1016/j.taap.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010;38:5718–34. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez V, Hartmann E, Ott G, Campo E, Rosenwald A. Pathogenesis of mantle-cell lymphoma: all oncogenic roads lead to dysregulation of cell cycle and DNA damage response pathways. J Clin Oncol. 2005;23:6364–9. doi: 10.1200/JCO.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Pacifico F, Leonardi A. Role of NF-kappaB in thyroid cancer. Mol Cell Endocrinol. 2010;321:29–35. doi: 10.1016/j.mce.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Pacifico F, Mauro C, Barone C, Crescenzi E, Mellone S, Monaco M, et al. Oncogenic and anti-apoptotic activity of NF-kappa B in human thyroid carcinomas. J Biol Chem. 2004;279:54610–9. doi: 10.1074/jbc.M403492200. [DOI] [PubMed] [Google Scholar]
- 28.Suh N, Honda T, Finlay HJ, Barchowsky A, Williams C, Benoit NE, et al. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res. 1998;58:717–23. [PubMed] [Google Scholar]
- 29.Uffort DG, Grimm EA, Ellerhorst JA. NF-kappaB mediates mitogen-activated protein kinase pathway-dependent iNOS expression in human melanoma. J Invest Dermatol. 2009;129:148–54. doi: 10.1038/jid.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekmekcioglu S, Ellerhorst JA, Prieto VG, Johnson MM, Broemeling LD, Grimm EA. Tumor iNOS predicts poor survival for stage III melanoma patients. Intl J Cancer. 2006;119:861–6. doi: 10.1002/ijc.21767. [DOI] [PubMed] [Google Scholar]
- 31.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–53. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 32.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–51. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 33.Marrero MB, Fulton D, Stepp D, Stern DM. Angiotensin II-induced signaling pathways in diabetes. Curr Diabetes Rev. 2005;1:197–202. doi: 10.2174/1573399054022802. [DOI] [PubMed] [Google Scholar]
- 34.Farmer DG, Kennedy S. RAGE, vascular tone and vascular disease. Pharmacol Ther. 2009;124:185–94. doi: 10.1016/j.pharmthera.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Cottone S, Mule G, Guarneri M, Palermo A, Lorito MC, Riccobene R, et al. Endothelin-1 and F2-isoprostane relate to and predict renal dysfunction in hypertensive patients. Nephrol Dial Transplant. 2009;24:497–503. doi: 10.1093/ndt/gfn489. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi H, Karasawa R, Inn H, Saitou T, Ueno M, Nishi S, et al. An electron microscopic study of glomeruli in Japanese patients with non-insulin dependent diabetes mellitus. Kidney Int. 1992;41:749–57. doi: 10.1038/ki.1992.117. [DOI] [PubMed] [Google Scholar]
- 37.Dounousi E, Papavasiliou E, Makedou A, Ioannou K, Katopodis KP, Tselepis A, et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis. 2006;48:752–60. doi: 10.1053/j.ajkd.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Wu QQ, Wang Y, Senitko M, Meyer C, Wigley WC, Ferguson DA, et al. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPAR{gamma}, and HO-1. Am J Physiol Renal Physiol. 2011;300:F1180–92. doi: 10.1152/ajprenal.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pergola PE, Krauth M, Huff JW, Ferguson DA, Ruiz S, Meyer CJ, et al. Effect of bardoxolone methyl on kidney function in patients with T2D and stage 3b-4 CKD. Am J Nephrol. 2011;33:469–76. doi: 10.1159/000327599. [DOI] [PubMed] [Google Scholar]
- 40.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–36. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.