Abstract
Objective: This study is to investigate the in vivo effects of 4-amino-2-trifluoromethyl-phenyl retinate (ATPR) on gastric carcinomas (GC). Methods: Adult male nude mice were subcutaneously injected with SGC-7901 human gastric cancer cells. Tumor cell cycle was analyzed with flow cytometry. The expression levels of cycloxygenase 2 (COX-2) and carcinoembryonic antigen (CEA) in xenograft tumors were detected with immunohistochemistry. The mRNA and protein expression levels of nuclear retinoic acid receptor β (RARβ) were detected with RT-PCR and Western blot analysis, respectively. Results: The mean survival time was dramatically increased in the ATPR treatment groups, in a dose-dependent manner. The in vivo results showed that, the xenograft tumor growth was significantly inhibited by the ATPR treatment. Moreover, the percentages of cells in the G0/G1 phase were significantly increased, while the percentages of cells in the S phase were significantly decreased, in the ATPR treatment groups. The serum levels of ALP and LDH were both dramatically decreased in the ATPR treatment groups. Furthermore, immunohistochemistry showed that, the expression levels of COX-2 and CEA were dramatically decreased in the ATPR treatment groups. Importantly, the mRNA and protein expression levels of RARβ in xenograft tumors were apparently increased by the ATPR treatment. Conclusion: ATPR could inhibit proliferation and induce differentiation of human gastric carcinoma xenografts via up-regulating RARβ expression. ATPR might be a potential effective antitumor agent for the treatment of GC.
Keywords: 4-amino-2-trifluoromethyl-phenyl retinate (ATPR), gastric carcinoma, antitumor effect, differentiation induction, retinoic acid receptor β (RARβ)
Introduction
Gastric carcinoma (GC) is a malignant tumor derived from gastric epithelial cells, and one of the most common causes of cancer-related death worldwide [1]. About 70% of new GC cases and deaths caused by GC occur in developing countries [2]. In China, GC is the second common cancer after lung cancer [3]. Surgery is the most effective means for the treatment of early GC. However, for patients with advanced GC, the current treatment and prognosis is poor [4]. These patients are treated with radiotherapy and chemotherapy which would kill normal cells while killing tumor cells. In 1980s, a novel concept named differentiation therapy has been raised [5]. Instead of directly killing tumor cells, the underlying mechanism of the differentiation therapy is to induce the differentiation of tumor cells into normal cells. Thus, the side effects of the conventional radiotherapy and chemotherapy such as bone marrow suppression and immune suppression can be avoided.
All-trans retinoic acid (ATRA) is a class of vitamin A that has the ability to induce cell differentiation in various cancer cells [6]. ATRA is believed to exert effects by binding to retinoic acid receptors (RARs) and retinoid X receptors (RXRs) [7,8]. However, clinical application of ATRA in the treatment of breast cancer and other solid tumors shows limited effects due to drug resistance [9]. Therefore, it is important to develop novel effective therapeutic strategies and to improve the structure and function of ATRA.
4-amino-2-trifluoromethyl-phenyl retinate (AT-PR), a novel retinoid derivative synthesized by our group, has a potent effect of inhibiting the proliferation and inducing the differentiation of SGC-7901 gastric cancer cells and many other tumor cells [10-13]. However, whether ATPR inhibits proliferation and induces differentiation of GC in vivo and the underlying mechanism is still unknown. In this study, the effects of ATPR on SGC-7901 human GC cells in vivo were investigated, and the underlying mechanisms were also analyzed.
Materials and methods
Cell line and culture
Human gastric cancer cell line SGC-7901 was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI 1640 medium (Gibco, Grand Island, NY, USA), supplemented with 10% fetal calf serum (FCS; Zhejiang Tianhang Biotechnology Co., Ltd., Hangzhou, Zhejiang, China) in a 37°C, 5% CO2 humidified incubator. Cells in the exponential growth phase were used in the following experiments.
Animal modeling and grouping
Totally 90 male BALB/c nude mice, 5-6-week old, were purchased from Slaccas Laboratory Animal Co., Ltd. (SCXK hu 2011-0005; Shanghai, China). The animal experimental procedures were approved by the Institutional Animal Care and Use Committee of the Anqing Hospital Affiliated to Anhui Medical University (KY-2011-002, 05 Apr 2011). Animals were housed in a barrier facility and acclimated to a 12-h light/dark cycle. For model establishment, 2 × 106 SGC-7901 cells were subcutaneously injected into the right anterior armpit of these nude mice. After one week, these mice developed visible tumors.
The 4-Amino-2-trifluoromethyl-phenyl retinate (ATPR; with a purity of 99.66%) was synthesized by our laboratory (School of Pharmacy, Anhui Medical University, Hefei, Anhui, China) (Figure 1). All-trans retinoic acid (ATRA) was purchased from Sigma (St. Louis, MO, USA). The nude mice were randomly divided into the following groups (n = 15 per group): (1) the model group; (2) the vehicle control group, in which model mice were treated with the polyoxyethylene castor oil alone; (3) the ATPR treatment groups, in which the model mice were treated with 5, 10, and 20 mg/kg ATPR, respectively; and (4) the ATRA treatment group, in which model mice were treated with 10 mg/kg ATRA. ATPR and ATRA were first dissolved in the polyoxyethylene castor oil, and then intraperitoneally administered into these mice every other day over a 5-w period. After drug administration, 8 mice from each group were sacrificed. The tumor xenografts were removed, and the tumor weight and volume were measured and recorded.
Figure 1.
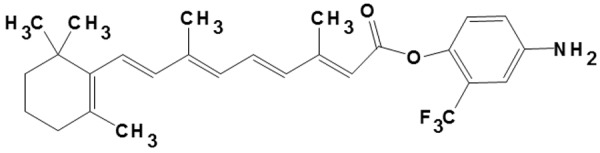
Structure of 4-amino-2-trifluoromethyl-phenyl retinate (ATPR).
Survival time analysis
The remaining 7 mice in each group were fed with a basal diet until death, and the survival time was recorded. The mean survival time (MST) was calculated for each group. The increased lifespan (ILS) was calculated as: ILS (%) = (MSTthe treatment group - MSTthe model group)/MSthe model group × 100%.
Flow cytometry
Cell cycle was analyzed using flow cytometry. Tumor tissues were triturated, and tumor cells were collected. After washing twice with ice-cold PBS, these cells were fixed with 70% alcohol overnight, and stained with 1 mg/mL propidium iodide (PI), in the presence of 1% RNase A, for 30 min. Cell populations in the G0/G1, S, and G2/M phases were measured by a flow cytometer (Becton Dickinson, Mountain view, CA, USA). Datas were analyzed with the Mod-FitLT software (Becton Dickinson).
Serum ALP and LDH level determination
Blood was collected into the tubes for the separation of serum. Serum levels of alkaline phosphatase (ALP) and lactate dehydrogenases (LDH) were assessed using commercially available kits (Jiancheng Bioengineering Research Institute, Nanjing, Jiangsu, China), according to the manufacturer’s instructions.
Immunohistochemistry
Expression levels of cycloxygenase 2 (COX-2) and carcinoembryonic antigen (CEA) in tumors were assessed with immunohistochemistry. The xenograft tumors were embedded in paraffin, and then cut into 4-μm sections. The sections were incubated with goat anti-human anti-COX-2 (ZSGB-BIO, Beijing, China) and goat anti-human anti-CEA (ZSGB-BIO) antibodies, respectively. The tissue sections were then incubated with rabbit anti-goat secondary antibody, and visualized with a microscope (Olympus, Tokyo, Japan). The optical densities were analyzed using the Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA).
Reverse transcription (RT) PCR
The mRNA expression levels of RARβ in the tumor tissues were detected with RT-PCR. Total RNA was isolated with the Trizol reagent, and reverse transcription was performed to synthesize the cDNA. The primer sequences were: RARβ, forward 5’-GGAACGCATTCGGAAGGCTT-3’ and reverse 5’-GGAAGACGGACTCGCAGTGT-3’; and GAPDH, forward 5’-GTGAAGGTCGGTGTCAACGGATTT-3’ and reverse 5’-CACAGTCTTCTGAGTGGCAGTGAT-3’. Amplification conditions were as follows: denaturation at 94°C for 5 min; 94°C for 40 s, 50°C (for RARβ) or 56°C (for GAPDH) for 1 min, and 72°C for 40 s, for totally 35 cycles; final extension at 72°C for 10 min. PCR products were separated on a 1.5% agarose gel, which was then semiquantitatively analyzed using the Image Master and Software Image Analyzer (Jieda; Suzhou, Jiangsu, China). Target mRNA levels were normalized to the internal control GAPDH.
Western blot analysis
The protein expression levels of RARβ in the tumor tissues were analyzed by the Western blot analysis. Total protein was extracted using the RIPA lysis buffer (Beyotime, Haimen, Jiangsu, China). 10 µg protein sample was separated by 12% SDS-PAGE, and then transferred onto a PVDF membrane (Millipore Corp., Billerica, MA, USA). The membrane was blocked with 5% non-fat milk (diluted with TBST) at 4°C overnight, and incubated with rabbit anti-human anti-RARβ (1:500 dilution; Santa Cruz, Santa Cruz, CA, USA) and rabbit anti-human anti-β-actin (1:1000 dilution; Santa Cruz) primary antibodies, respectively, at 4°C overnight. The membrane was then incubated with goat anti-rabbit secondary antibody at 25°C for 2 h. Protein bands were visualized using the ECL chemiluminescence kit (ECL-plus; Thermo Scientific, Hudson, NH, USA) with X-ray exposure.
Statistical analysis
Data was expressed as the mean ± SD. Statistical analysis was performed using the SPSS 11.5 software. One-way analysis of variance (ANOVA) and Student-Newman-Keul’s test were performed for the group comparison. Survivor functions were estimated using the Kaplan-Meier method, and the log-rank test was used for the comparison. P < 0.05 was considered statistically significant.
Results
ATPR treatment increases the survival time of xenograft mouse models
To investigate the effects of ATPR on the survival of the nude mice inoculated with SGC-7901 cells, the survival time of the remaining mice in each group after drug administration were recorded and analyzed. Our results showed that, the mean survival time (MST) was significantly elevated in the 10 mg/kg ATRA treatment group, compared with the vehicle control group (P < 0.05) (Figure 2). Moreover, MST was also dramatically increased in the ATPR treatment groups, in a dose-dependent manner, and significant differences were observed in the 10 and 20 mg/kg ATPR treatment groups (both P < 0.05) (Figure 2). The increased lifespan (ILS) for the ATRA treatment group, and the 5, 10, and 20 mg/kg ATPR treatment groups were 9.8%, 2.0%, 4.24%, and 11.02%, respectively. These results showed that ATPR treatment could significantly increase the survival time of the xenograft mouse models.
Figure 2.
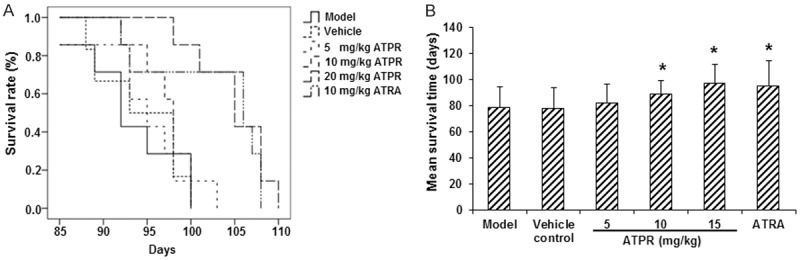
ATPR increased the survival time of xenograft mouse models. A. The Kaplan-Meier curve for survival. B. Mean survival time in of the xenograft mouse models. n = 8. Compared with the vehicle control group, *P < 0.05.
ATPR treatment inhibits the growth of xenograft tumor in mouse models
To evaluate the effects of ATPR on the in vivo growth of SGC-7901 gastric cancer cells, the tumor weight and volume were measured at 5 w after inoculation and drug administration. Our results showed that, compared with the vehicle control group, the xenograft tumor growth was significantly inhibited by the ATPR treatments, in a dose-dependent manner (P < 0.05 for the 5 and 10 mg/kg groups, and P < 0.01 for the 20 mg/kg group) (Figure 3). The tumor growth was also dramatically declined in the ATRA group (P < 0.05) (Figure 3). These findings suggested that the ATPR treatment could significantly inhibit the growth of xenograft tumors in nude mice.
Figure 3.
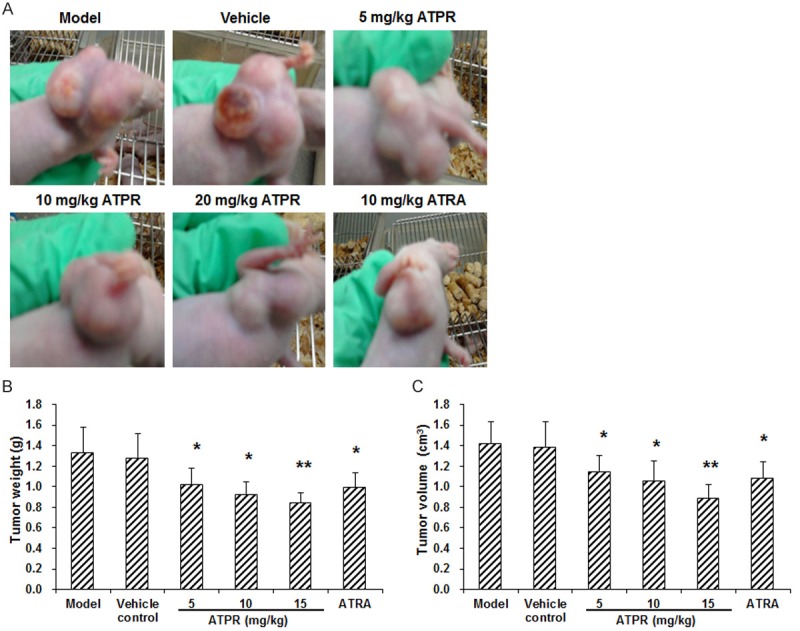
ATPR inhibited the growth of xenograft tumors in mouse models. (A) Representative photos of tumors in each group at 5 w after modeling. (B, C) Statistical analyses of the weight (B) and volume (C) of the xenograft tumors in mouse models. n = 8. Compared with the vehicle control group, *P < 0.05, **P < 0.01.
ATPR treatment alters tumor cell cycle in xenograft mouse models
To evaluate the effects of ATPR on tumor cell cycle, flow cytometry was performed. This analysis revealed that, compared with the vehicle control group, the percentages of cells in the G0/G1 phase were significantly increased, while the percentages of cells in the S phase were significantly decreased, in the ATPR treatment groups, with statistical significance for the 10 mg/kg (P < 0.05) and 20 mg/kg (P < 0.01) groups (Figure 4). Similar results were observed for the ATRA treatment group (P < 0.05) (Figure 4). These results demonstrated that the ATPR treatment could arrest the tumor cell cycle at the G0/G1 phase.
Figure 4.
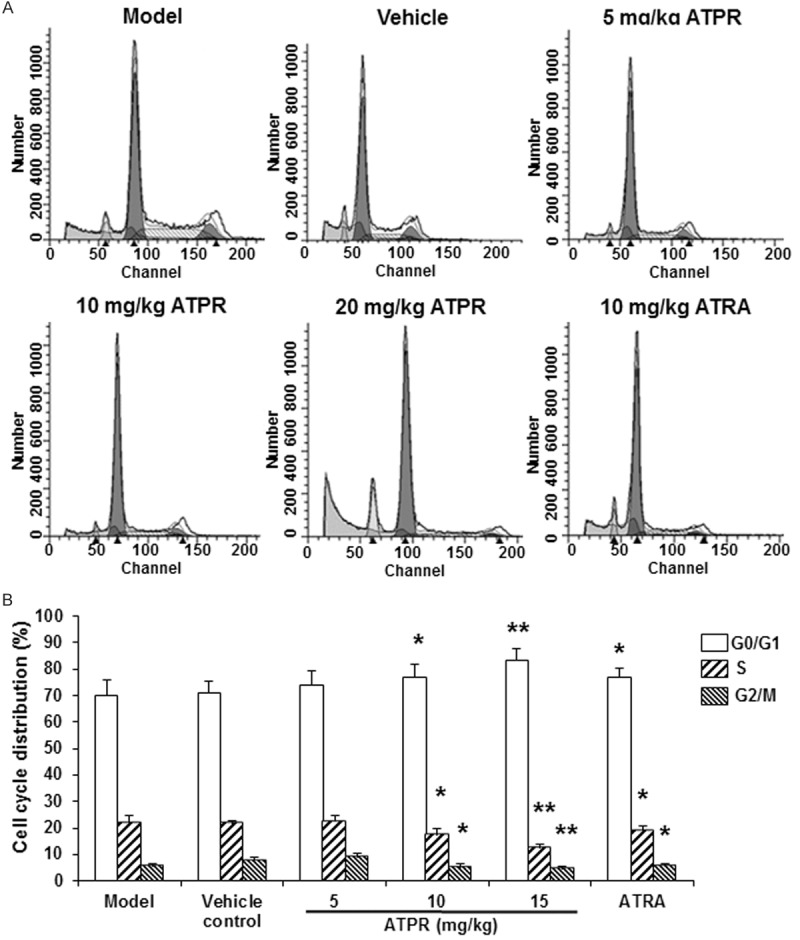
ATPR changed the tumor cell cycle in xenograft mouse models. A. Representative flow cytometric histograms for tumor cell cycle analysis. B. Statistical analysis of the cell proportions of the tumor cells. n = 8. Compared with the vehicle control group, *P < 0.05, **P < 0.01.
ATPR treatment induces the differentiation of xenograft tumors in vivo
ALP and LDH have been used to evaluate the differentiation inducing effects of anticancer drugs [14]. To explore the differentiation inducing effect of ATPR on xenograft gastric tumors, the serum levels of ALP and LDH in these nude mice were detected. Our results showed that, the serum levels of ALP and LDH were 45.81 ± 3.79 King unit/100 mL and 6734.36 ± 133.89 U/L, respectively, in the vehicle control group, which were both dramatically decreased in the ATPR treatment groups, with statistical significance for the 20 mg/kg ATPR group (serum ALP level was 34.55 ± 2.23 King unit/100 mL, and serum LDH level was 5953.17 ± 230.79 U/L; P < 0.05) (Figure 5). The ATRA treatment also decreased the serum levels of ALP and LDH in these xenograft mouse models (P < 0.05) (Figure 5). These results suggested that the ATPR treatment may induce the differentiation of human gastric carcinoma xenografts in vivo.
Figure 5.
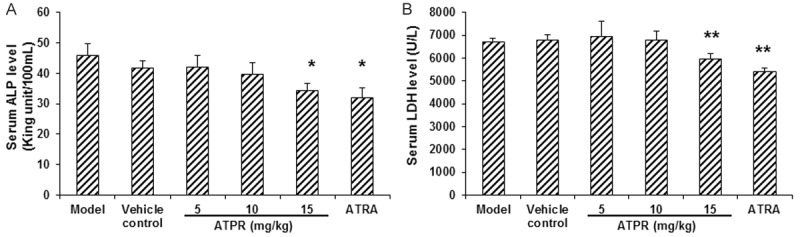
ATPR decreased the serum levels of ALP and LDH in xenograft mouse models. Serum levels of ALP (A) and LDH (B) were detected in the mouse models. n = 8. Compared with the vehicle control group, *P < 0.05, **P < 0.01.
ATPR treatment decreases COX-2 and CEA expression levels in xenograft tumors
To determine the effects of ATPR on the expression levels of COX-2 and CEA, immunohistochemistry analysis was carried out in xenograft gastric tumors. Our results showed that, compared with the vehicle control group, the expression levels of COX-2 and CEA were dramatically decreased in the ATPR treatment groups, in a dose-dependent manner, with statistical significance for the 20 mg/kg treatment group (P < 0.01 for both COX-2 and CEA expression levels) (Figure 6). Similar reducing effects were observed for the ATRA treatment group (P < 0.05) (Figure 6). These results indicate that the ATPR treatment could significantly decrease the expression levels of COX-2 and CEA in the xenograft mouse models.
Figure 6.
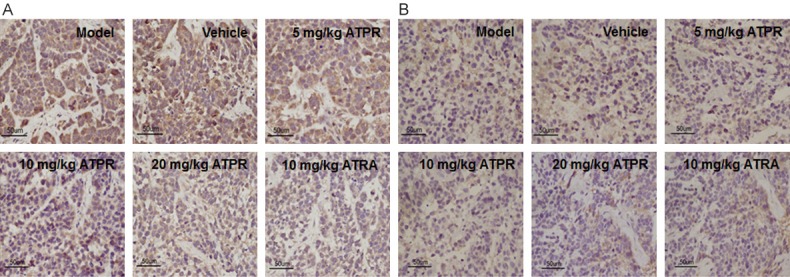
ATPR decreased the expression levels of COX-2 and CEA in xenograft tumors. The expression levels of COX-2 (A) and CEA (B) in xenograft tumors were detected with immunohistochemistry (×400).
RARβ mediates the proliferation inhibiting and differentiation inducing effects of ATPR on xenograft tumors
To determine whether the proliferation inhibiting and differentiation inducing effects of ATPR on human gastric carcinoma xenografts were mediated by RARβ, the mRNA and protein expression levels of RARβ in xenograft tumor tissues were assessed by RT-PCR and Western blot analysis, respectively. Our results from RT-PCR showed that, compared with the vehicle group, the mRNA expression levels of RARβ in the xenograft tumor tissues were apparently increased in the 20 mg/kg ATPR treatment group (P < 0.01) and the ATRA treatment group (P < 0.05) (Figure 7). Similar results were observed with the Western blot analysis, in which the 20 mg/kg ATPR treatment and the ATRA treatment apparently increased the protein expression levels of RARβ in the xenograft tumor tissues (P < 0.01 for the 20 mg/kg ATPR treatment, and P < 0.05 for the ATRA treatment). These results indicated that the proliferation inhibiting and differentiation inducing effects of ATPR on human gastric carcinoma xenografts might be mediated by RARβ.
Figure 7.
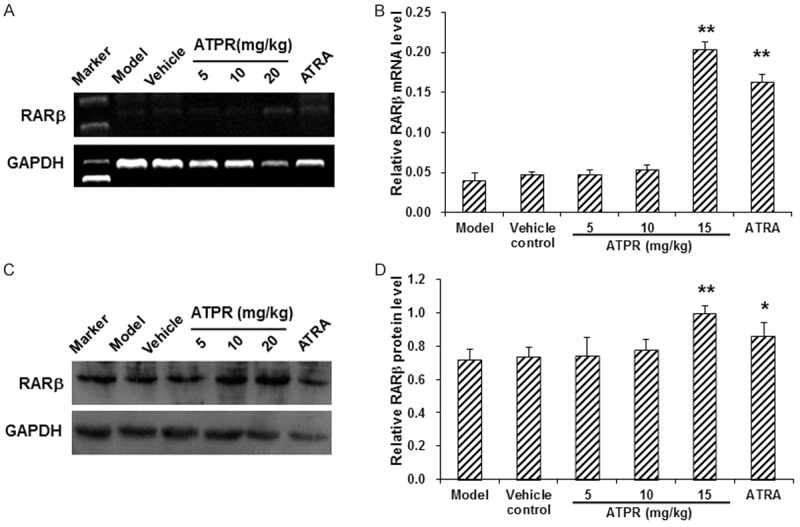
ATPR increased the expression levels of RARβ in xenograft tumors. The mRNA (A) and protein (B) expression levels of RARβ in xenograft tumors were detected with RT-PCR and Western blot analysis, respectively. n = 3. Compared with the vehicle control group, *P < 0.05, **P < 0.01.
Discussion
Our previous in vitro studies have investigated the effects of ATPR on the growth of gastric cancer cell lines SGC-7901, AGS, MKN-74, and SC-M1 [10,12,13]. We have found that ATPR inhibits the growth of these cell lines, arrests the cell cycle, and increases the percentage of SGC-7901, AGS, and MKN-74 cells at the G0/G1 phase. These results demonstrate that ATPR could effectively inhibit the proliferation and induce the differentiation of gastric cancer cells in vitro. However, whether ATPR could exert the similar effects on gastric tumors in vivo has not been known. In this study, xenograft models in BALB/c nude mice inoculated with SGC-7901 cells were established, and the therapeutic effects of ATPR on GC in vivo were evaluated. Our results showed that ATPR treatments decreased the weight and volume of xenograft tumors, elongated the survival time of mouse models, and arrested the tumor cell cycle at the G0/G1 phase, suggesting that ATPR could significantly inhibit the growth of xenograft tumors in these nude mice.
ALP and LDH are usually expressed at relatively low levels or even absent in normal cells. However, their expression levels will be dramatically increased once malignant transformation occurs [15,16]. Thus, ALP and LDH can be used as indicators to reflect the extent of the differentiation of malignant tumors [15]. Our results showed that, ATPR treatment notably decreased the expression levels of these enzymes in xenograft tumors, which revealed that ATPR could induce the differentiation of xenograft tumors.
COX-2 is frequently expressed in primary gastric cancers, and high expression of COX-2 has been shown in the lymph node metastasis of the cancer, which may be involved in the pathogenesis of carcinogenesis, as well as tumor invasion and metastasis [15,17]. As a potential marker of tumor progression, COX-2 has always been used in the disease diagnosis and prognosis, and the development of the therapeutic tools for gastric cancers. On the other hand, the glycoprotein CEA is only expressed in tumors, but not in normal tissues. CEA has been widely used in the diagnosis and prognosis of cancers of the digestive system in clinic [18]. Our results from immunohistochemistry showed that ATPR treatments decreased both the expression levels of COX-2 and CEA, suggesting that ATPR could definitely inhibit the malignant proliferation of xenograft tumor cells.
RARs are retinoic acid-dependent transcriptional activators in the heterodimeric form with RXRs, which regulate the process of ligand binding and phosphorylation [19]. It has been shown that, the differentiation inducing effects of retinoids are primarily mediated by RAR/RXR heterodimers [20]. Among the RARs, RARβ is well known for its tumor-suppressive effects in epithelial cells [21,22]. Numerous studies have demonstrated that the absence of RARβ gene expression is a feature for human malignant tumors, which may be involved in the retinoid resistance in neoplasias [23,24]. Zhang et al. [25] have reported that the up-regulation of RARβ expression levels induced by all-trans retinoic acid or other retinoids contribute to the growth inhibition of breast cancer cells. RARβ may be a tumor suppressor in malignant tumors, and retinoids can induce apoptosis in neoplasias through regulating RARβ expression. Our results from RT-PCR and Western blot analysis showed that ATPR treatments significantly up-regulated both the mRNA and protein expression levels of RARβ in xenograft tumors. Up-regulated RARβ may further alter the expression levels of the downstream genes and signaling pathways. Song et al. [26] have found that RAR-β2, an isoform of RARβ, could suppress the expression level of COX-2 via increasing retinoid receptor-induced gene-1 (RRIG1). Moreover, the down-regulation of COX-2 by RAR-β2 has been shown to be associated with the suppression of EGFR and the subsequent decline in ERK1/2 phosphorylation and AP-1 expression. Based on these results, we presume that the decreased expression of COX-2 might be correlated to the increased expression of RARβ, and RARβ could exert antitumor effects via inhibiting COX-2.
In conclusion, our results showed that ATPR increased the survival time of xenograft mouse models, and inhibited the growth of xenograft tumors in these models. Moreover, ATPR altered the tumor cell cycle and induced the differentiation of xenograft tumors in the mouse models. Furthermore, ATPR decreased the expression levels of COX-2 and CEA in xenograft tumors. Importantly, the proliferation inhibiting and differentiation inducing effects of ATPR on xenograft tumors was mediated by RARβ. These results suggest that ATPR might be a potential effective antitumor agent for the treatment of GC.
Acknowledgements
This work was supported by the National Major Scientific and Technological Special Project for Significant New Drugs Development (No. 2011ZX09401).
Disclosure of conflict of interest
All authors declare no financial and non-financial competing interests.
References
- 1.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013;63:151–166. doi: 10.3322/caac.21173. [DOI] [PubMed] [Google Scholar]
- 2.Qu Y, Dang S, Hou P. Gene methylation in gastric cancer. Clin Chim Acta. 2013;424:53–65. doi: 10.1016/j.cca.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Zhao P, Dai M, Chen W, Li N. Cancer trends in China. Jpn J Clin Oncol. 2010;40:281–285. doi: 10.1093/jjco/hyp187. [DOI] [PubMed] [Google Scholar]
- 4.Xiong B, Ma L, Cheng Y, Zhang C. Clinical effectiveness of neoadjuvant chemotherapy in advanced gastric cancer: an updated meta-analysis of randomized controlled trials. Eur J Surg Oncol. 2014;40:1321–1330. doi: 10.1016/j.ejso.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Sachs L. Growth, differentiation and the reversal of malignancy. Sci Am. 1986;254:40–47. doi: 10.1038/scientificamerican0186-40. [DOI] [PubMed] [Google Scholar]
- 6.Choi Y, Kim SY, Kim SH, Yang J, Park K, Byun Y. Inhibition of tumor growth by biodegradable microspheres containing all-trans-retinoic acid in a human head-and-neck cancer xenograft. Int J Cancer. 2003;107:145–148. doi: 10.1002/ijc.11354. [DOI] [PubMed] [Google Scholar]
- 7.Stehlin-Gaon C, Willmann D, Zeyer D, Sanglier S, Van Dorsselaer A, Renaud JP, Moras D, Schule R. All-trans retinoic acid is a ligand for the orphan nuclear receptor ROR beta. Nat Struct Biol. 2003;10:820–825. doi: 10.1038/nsb979. [DOI] [PubMed] [Google Scholar]
- 8.Ye X, Wu Q, Liu S, Lin X, Zhang B, Wu J, Cai J, Zhang M, Su W. Distinct role and functional mode of TR3 and RARalpha in mediating ATRA-induced signalling pathway in breast and gastric cancer cells. Int J Biochem Cell Biol. 2004;36:98–113. doi: 10.1016/s1357-2725(03)00143-2. [DOI] [PubMed] [Google Scholar]
- 9.Dutta A, Sen T, Banerji A, Das S, Chatterjee A. Studies on Multifunctional Effect of All-Trans Retinoic Acid (ATRA) on Matrix Metalloproteinase-2 (MMP-2) and Its Regulatory Molecules in Human Breast Cancer Cells (MCF-7) J Oncol. 2009;2009:627840. doi: 10.1155/2009/627840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu KW, Pan XH, Chen FH, Qin R, Wu LM, Zhu HG, Wu FR, Ge JF, Han WX, Yin CL, Li HJ. A novel retinoic acid analog, 4-amino-2-trifluoromethyl-phenyl retinate, inhibits gastric cancer cell growth. Int J Mol Med. 2014;33:415–422. doi: 10.3892/ijmm.2013.1574. [DOI] [PubMed] [Google Scholar]
- 11.Wang B, Yan Y, Zhou J, Zhou Q, Gui S, Wang Y. A novel all-trans retinoid acid derivatives inhibits the migration of breast cancer cell lines MDA-MB-231 via myosin light chain kinase involving p38-MAPK pathway. Biomed Pharmacother. 2013;67:357–362. doi: 10.1016/j.biopha.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Gui SY, Chen FH, Zhou Q, Wang Y. New insights into 4-amino-2-tri-fluoromethyl-phenyl ester inhibition of cell growth and migration in the A549 lung adenocarcinoma cell line. Asian Pac J Cancer Prev. 2013;14:7265–7270. doi: 10.7314/apjcp.2013.14.12.7265. [DOI] [PubMed] [Google Scholar]
- 13.Wang N, Ge JF, Pan CX, Peng XQ, Chen HH, Wang XQ, Tang J, Hu W, Chen FH. Anti-tumor effect of 4-Amino-2-Trifluoromethyl-Phenyl Retinate on human breast cancer MCF-7 cells via up-regulation of retinoid receptor-induced gene-1. Biomed Pharmacother. 2013;67:687–692. doi: 10.1016/j.biopha.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Zhao AG, Shao J, Mu XY, Jiang L, Liu JW. The effects of PTBP3 silencing on the proliferation and differentiation of MKN45 human gastric cancer cells. Life Sci. 2014;114:29–35. doi: 10.1016/j.lfs.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 15.Chen RC, Su JH, Yang SM, Li J, Wang TJ, Zhou H. Effect of isoverbascoside, a phenylpropanoid glycoside antioxidant, on proliferation and differentiation of human gastric cancer cell. Acta Pharmacol Sin. 2002;23:997–1001. [PubMed] [Google Scholar]
- 16.Zhang Y, Zhang X, Wang X, Gan L, Yu G, Chen Y, Liu K, Li P, Pan J, Wang J, Qin S. Inhibition of LDH-A by lentivirus-mediated small interfering RNA suppresses intestinal-type gastric cancer tumorigenicity through the downregulation of Oct4. Cancer Lett. 2012;321:45–54. doi: 10.1016/j.canlet.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Ren C, Fan Z, Jin G, Du J, Liu L, Zhu C, Lu F, Ding Y, Deng B, Hu Z, Xu Y, Shen H. A genetic variant in 3’-untranslated region of cyclooxygenases-2 gene is associated with risk of gastric cancer in a Chinese population. DNA Cell Biol. 2012;31:1252–1257. doi: 10.1089/dna.2012.1615. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu Y, Takeuchi H, Sakakura Y, Saikawa Y, Nakahara T, Mukai M, Kitajima M, Kitagawa Y. Molecular detection of sentinel node micrometastases in patients with clinical N0 gastric carcinoma with real-time multiplex reverse transcription-polymerase chain reaction assay. Ann Surg Oncol. 2012;19:469–477. doi: 10.1245/s10434-011-2122-4. [DOI] [PubMed] [Google Scholar]
- 19.Brtko J. Retinoids, rexinoids and their cognate nuclear receptors: character and their role in chemoprevention of selected malignant diseases. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151:187–194. doi: 10.5507/bp.2007.033. [DOI] [PubMed] [Google Scholar]
- 20.Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 21.Connolly RM, Nguyen NK, Sukumar S. Molecular pathways: current role and future directions of the retinoic acid pathway in cancer prevention and treatment. Clin Cancer Res. 2013;19:1651–1659. doi: 10.1158/1078-0432.CCR-12-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez M, David CS. Demyelination induced by Theiler’s virus: influence of the H-2 haplotype. J Immunol. 1985;135:2145–2148. [PubMed] [Google Scholar]
- 23.Ben Ayed-Guerfali D, Benhaj K, Khabir A, Abid M, Bayrouti MI, Sellami-Boudawara T, Gargouri A, Mokdad-Gargouri R. Hypermethylation of tumor-related genes in Tunisian patients with gastric carcinoma: clinical and biological significance. J Surg Oncol. 2011;103:687–694. doi: 10.1002/jso.21875. [DOI] [PubMed] [Google Scholar]
- 24.Berrada N, Amzazi S, Ameziane El Hassani R, Benbacer L, El Mzibri M, Khyatti M, Chafiki J, Abbar M, Al Bouzidi A, Ameur A, Attaleb M. Epigenetic alterations of adenomatous polyposis coli (APC), retinoic acid receptor beta (RARbeta) and survivin genes in tumor tissues and voided urine of bladder cancer patients. Cell Mol Biol (Noisy-le-grand) 2012;(Suppl 58):OL1744–1751. [PubMed] [Google Scholar]
- 25.Zhang XK, Liu Y, Lee MO. Retinoid receptors in human lung cancer and breast cancer. Mutat Res. 1996;350:267–277. doi: 10.1016/0027-5107(95)00102-6. [DOI] [PubMed] [Google Scholar]
- 26.Song S, Lippman SM, Zou Y, Ye X, Ajani JA, Xu XC. Induction of cyclooxygenase-2 by benzo[a] pyrene diol epoxide through inhibition of retinoic acid receptor-beta 2 expression. Oncogene. 2005;24:8268–8276. doi: 10.1038/sj.onc.1208992. [DOI] [PubMed] [Google Scholar]


