Abstract
Background: Ulcerative colitis (UC) can be viewed as an autoimmune disease. Bone marrow-derived mesenchymal stem cells (MSCs) with its regenerative, cellular multi-lineage and immunomodulatory abilities can influence the repair of damaged tissues in UC. This study investigated the effects of MSCs transplantation on the mice intestinal barrier in response to oxidative stress injury. Methods: Colitis was induced by daily consecutive administration of 5% dextran sulfate sodium (DSS) solution for 7 days. Male murine MSCs were isolated and transplanted into female mice via injection in the tail vein. Serum and colon specimens were collected at 12 h, 24 h, 3 d, 7 d and 14 d after injection. Serum levels of D-lactate (D-LAC), diamine oxidase (DAO), colonic levels of malondialdehyde (MDA) and superoxide dismutase (SOD) were quantified. The SRY protein of the male sex determinant gene expression and E-cadherin were also ascertained intracellularly. Results: Three days after receiving male MSCs transplantation, SRY protein expression was detected. The quantity increased on successive days. Serum levels of D-LAC and DAO, colonic MDA and SOD normalized in a shorter time period compared to controls (p<0.05). Not surprisingly, histological regeneration of tissue and E-cadherin expression in the colon of MSCs transplanted mice also occurred in a shorter time period than controls. Conclusions: Transplanted MSCs restored mucosal permeability, and minimized oxidative stress related injury.
Keywords: Mesenchymal stem cell, ulcerative colitis, intestinal barrier, cell transplantation, mice
Introduction
Ulcerative Colitis (UC) is an inflammatory disease in colonorectum of undefined etiology and mechanism. The annual onset of UC varies from 1.2 to 20.3 per 100,000 with the highest prevalence found in in Europe and North America: 7.6 to 246.0 per 100,000 [1,2]. The onset of UC is likely to be multifactorial, e.g. immune dysregulation, environmental factors, hereditary susceptibility, etc [3,4]. Pathophy-siologically, alterations in the mucosal permeability of the intestinal barrier results in bowel bacteria invasion and endotoxin exposure. This in turn activates the innate immune system. The consequent release and persistence of proinflammatory cytokines and chemokines lead to chronic inflammation. Severe UC may induce complications such as intestinal bleeding, toxic megacolon, intestinal epithelial dysplasia and colonic carcinogenesis [5].
A variety of drugs are used to treat UC including 5-aminosalicylic acid, immunosuppressants, monoclonal antibodies, and glucocorticoids, each with different mechanisms of action and these decrease the levels of proinflammatory cytokines. However, the long term administration of these agents is accompanied by adverse effects [6].
Stem cell transplantation therapy for inflammatory bowel diseases (IBD) was first attempted in 1993 based on the observation that bone marrow transplantation improved Crohn’s disease [7]. Subsequent studies have demonstrated the utility of stem cells from bone marrow in regenerating damaged mucosa in the gastrointestinal tract [8-14]. Bone marrow-derived mesenchymal stem cells (MSCs) are generally less immunogenic [15] but does influence the activity of proinflammatory cytokines and chemokines [16]. Thus, MSCs transplantation may hold therapeutic potential for UC.
Oxidative stress has been implicated to be involved in the pathogenesis of UC [17]. Previous reports have demonstrated that local or systemic administration of MSCs is able to restore the damaged intestinal permeability in rats [18-20]. This may be a consequence of reducing the production of pro-inflammatory cytokines and ameliorating the clinical and pathological alterations in mice [21]. In the current study, the reduction of oxidative stress and recovery of the intestinal permeability/intestinal barrier regeneration by administration of MSCs in dextran sulfate sodium (DSS)-induced colitic mice are investigated.
Materials and methods
Mice
A total of 50 female Balb/c mice aged 8 weeks and 4 male mice aged 3 weeks were obtained from the Experimental Animal Research Center of the Chinese Military Medical Academy. All the animals were maintained and handled following the National Research Council Guide for the care and use of laboratory animals. And the experiment was approved by the Laboratory Animal Care and Use Committee at the Naval General Hospital, China.
Reagents
DMEM/F12 medium was purchased from Thermo (MA, USA). Fetal calf serum was from Invitrogen (CA, USA). PE-labelled mouse antibodies against human-CD34, -CD44, -CD45 and -CD90 were obtained from Biolegend (CA, USA). DSS was from MP Biomedicals (OH, USA). The ELISA kits for D-lactate (D-LAC) assay and diamine oxidase (DAO) assay, kits for malondialdehyde (MDA) assay and superoxide dismutase (SOD) assay were obtained from Sigma-Aldrich (MO, USA). Rabbit polyclonal antibodies against SRY protein and E-cadherin were from Santa Cruz (TX, USA). Dexamethasone, ascorbic acid-2-phosphate, β-glycerophosphate, alizarin red S, 3-isobutyl-1-methylxanthine, indomethacin, and oil red O were purchased from Sigma-Aldrich (MO, USA).
Isolation, characterization and culture of bone marrow-derived MSCs
Bone marrow-derived MSCs from male mice were isolated by the method described by Song et al [22]. Briefly, the tibia and femur of euthanized mice were removed and the cavum ossis flushed with culture media. The flushed media was then collected and filtered through multi-layer gauze and centrifuged at 1000 rpm for 5 minutes. The pellet was resuspended with DMEM/F12 medium and cultured at 37°C, 5% CO2. Cells were subjected to subculture, when the cells achieved 90% confluency. This subculture was analyzed for surface expression of CD34, CD44, CD45 and CD90 by flow cytometry. A total of 4 male mice were used for bone marrow-derived MSCs isolation.
The osteogenic and adipogenic differentiation potential of the cells were explored by the methods of Chen et al [23]. Briefly, 0.01 μmol/L dexamethasone, 50 mg/L ascorbic acid-2-phosphate, 10 mmol/L β-glycerophosphate, 10% fetal calf serum, 105 U/L penicillin, 100 mg/L streptomycin were added into DMEM/F-12 medium-an osteogenesis medium. Bone marrow-derived MSCs cultured at the 3rd passage were resuspended, and 1 × 104 cells were seeded onto a sterile cover slip. The cover slip was placed into a well of a 6-well culture plate and cultured with basic medium for 2 days and then changed to the osteogenesis medium. The medium was changed every 3 days. After 21 days of culture, the cover slip was recovered, washed twice with PBS, fixed with 95% ethanol, and stained with 0.1% alizarin red S at 37°C for 30 minutes to visualize the formation of bone nodules.
The adipogenic medium composed of DMEM/F-12 with 1 μmol/L dexamethasone, 0.5 mmol/L 3-isobutyl-1-methylxanthine, 0.01 mmol/L human insulin, 0.01 mmol/L indomethacin, 10% fetal calf serum, 105 U/L penicillin, and 100 mg/L streptomycin. The same procedure described for osteogenesis was also used for adipogenesis except that the cover slip was recovered in 14 days, fixed with 50% isopropanol for 1 minute, and stained with oil red O for 10 minutes.
Induction of colitis in mice
Colitis was induced in the animals by consuming 5% DSS solution ad libitum for 7 days [24] in 50 female balb/c mice. During this period, examination of body weight, stool consistency and hematochezia were noted daily. Fecal occult blood was determined by tetramethyl benzidine testing. The disease activity index (DAI) was calculated based on above observations according to the method of Murano et al (Table 1) [25]. After 7 days, half of the mice (25 mice) received allogenic MSC transplantation with the rest (25 mice) serving as controls.
Table 1.
Scoring of disease activity index [25]
| Score | Weight loss | Stool consistency | Occult/gross hematochezia |
|---|---|---|---|
| 0 | None | Normal | Normal |
| 1 | 1-5% | ||
| 2 | 5-10% | Loose stools | Fecal occult blood test (+) |
| 3 | 10-15% | ||
| 4 | >15% | Watery stools | Gross hematochezia |
Note: DAI was scored by cumulating scores from the 3 categories.
Allogenic MSC transplantation and sample assay
From male mice, 0.4 ml of a concentration of cultured MSCs at 2.5 × 106 cells/ml was injected into the tail vein of 25 female mice. For controls, 0.4 ml of normal saline was used. Five animals in both the treatment and control groups were euthanized at 12 h, 24 h, 3 d, 7 d and 14 d. Immediately after this procedure, 1 ml of blood was collected through inferior vena cave vein of each mouse and centrifuged to obtain serum. The sera were stored at -80°C until D-LAC and DAO assays were performed.
Serum D-LAC and DAO levels, markers of intestinal permeability damage, were tested by ELISA according to the manufacturer’s instructions. MDA and SOD assays were also performed according to the manufacturer’s instructions. The specimen was a length of app-roximately 4 cm of the affected colon located approximately 1 cm above the anus as DSS induced colitis mainly affects large intestine and distal small intestine [26]. These samples were washed thoroughly with cold sterile saline (4°C). Part of the specimen was homogenized and centrifuged and the supernatant collected for MDA and SOD assays. MDA is an index of lipid oxidation whereas SOD works as an enzymatic antioxidant. SRY is a male sex determinant gene and its presence in female tissue represents the existence of allogenic cells. E-cadherin is considered as a transmembrane junctional adhesion molecule in intestinal epithelial cells. The rest of the colonic specimen was fixed with 4% buffered formaldehyde, embedded in paraffin, sectioned at 4 μm and stained with hematoxylin-eosin or immunohistochemically for SRY protein and E-cadherin.
Statistical analysis
SPSS17.0 software (IBM SPSS Statistics, NY, USA) was used for statistical analysis in this study. Data were expressed as mean ± standard deviation. A parametric method for the testing of continuous data was used and where the p value less than or equal to 0.05, this was considered statistically significant.
Results
Surface expression of CD44+CD90+CD34-CD45- characterized by MSCs
The surface expression ratio of CD34, CD45, CD44, and CD90 in MSCs cells were 0.61%, 0.28%, 99.42%, and 98.69% respectively [27].
MSC multipotent differentiation potential
In osteogenic medium, MSCs transformed from individual cells to congregated cell clusters within 3-4 days. After 3 weeks of culture in osteogenic medium, calcified bone nodules, stained by alizarin red S were noted in congregated cell clusters .
In adipogenic medium, tiny lipid droplets were present in the cytoplasm of some cells after 7 days. After 14 days, cytoplasmic lipid droplets fused into large lipid vacuoles which were observed with oil red O.
Inflammation activity progresses during colitis induction
During the induction period, DAI scores were recorded. The scores were increased over time of DSS administration, and were consistent with the manifestations of acute colitis, e.g. diarrhoea, bloody excrement and body weight loss. On days 3, 4, 5, 6, and 7, the scores were 1.06±0.96 (mean ± SD), 2.48±1.23, 4.32±1.43, 7.26±1.62, 8.92±1.01 respectively.
Male specific SRY protein expression in female murine bowels that receive male MSCs transplantation
SRY protein expression in murine bowel by immunohistochemistry is shown in Figure 1. Three days after transplantation of allogenic MSCs from male mice, SRY protein could be detected sparsely in the bowel of female mice. By day 7, the expression had increased and continued to do so until day 14. This enhanced expression was maintained to at least for 21 days. In control non-MSCs-transplanted colitic mice colon, there was no expression (data not shown).
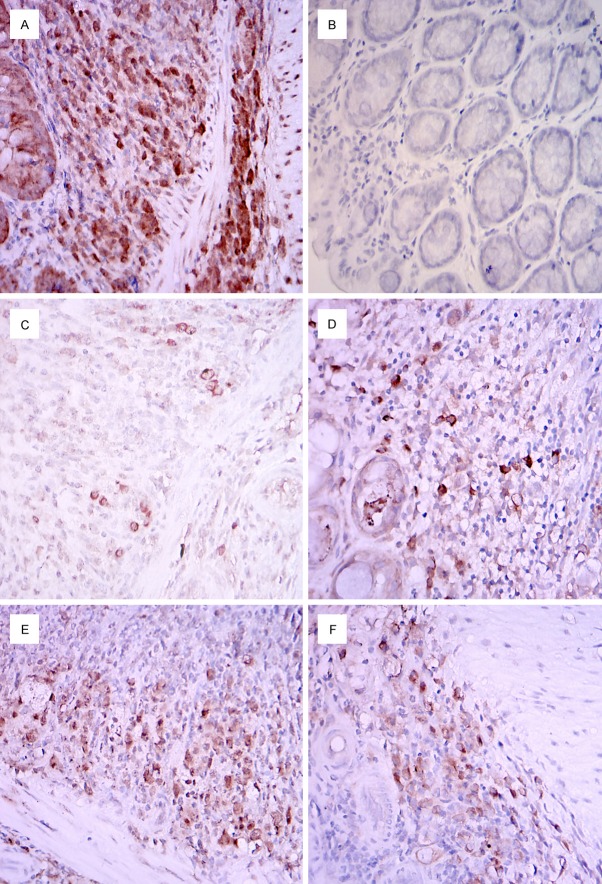
Figure 1.
Immunohistochemical detection of SRY protein expression in murine bowel. A. Positive control in male murine bowel. B. Negative control in female murine bowel. C. A dispersed expression was detected in the mucosal and submucosal layers of the bowel of female mice 3 days after receiving MSCs from male mice. D. Colonies were detected in the bowel of female mice 7 days after receiving homogeneous MSCs transplants from male mice. E. SRY protein expression in the female murine bowel after 14 days of male MSCs transplantation. F. Enhanced SRY expression was still noted at day 21 days post transplantation. Magnification is 100 ×.
MSCs transplantation decreases intestinal mucosal permeability and promotes tissue repair
Intestinal inflammation results in an increase in mucosal permeability and elevation of serum levels of D-LAC and DAO. When inflammation dissipated, serum levels of D-LAC and DAO returned to normal. MSCs transplantation accelerated this recovery process. Mice that were MSCs-transplanted normalized serum D-LAC and DAO levels within 72 hours while the control mice took 7 days (Figure 2).
Figure 2.
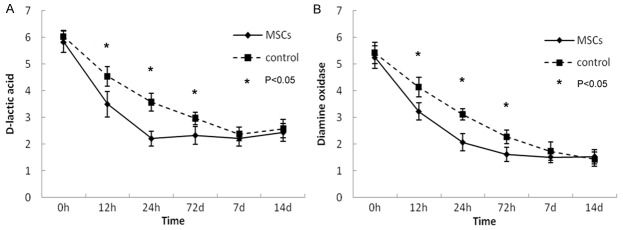
Serum levels of D-LAC and DAO in mice. MSCs transplantation normalizes serum D-LAC and DAO levels.
Murine colitic pathology of the distal colon consisted of multiple erosive, ulcerative or hemorrhagic lesions along with tissue edema and vascular congestion and inflammatory cell infiltration. In control mice, the resulting DSS injury of colonic crypts and infiltration of inflammatory cells into bowel tissue normalized within 7 days. By contrast in MSCs-transplanted mice, recovery was more prompt (Figure 3). Immunohistochemical staining of E-cadherin in mice bowel also showed similar findings. Colitic colon had decreased positive staining of E-cadherin. The expression of E-cadherin also normalized in 7 days after the cessation of DSS. Thus, MSCs transplantation is related to E-cadherin expression (Figure 4).
Figure 3.
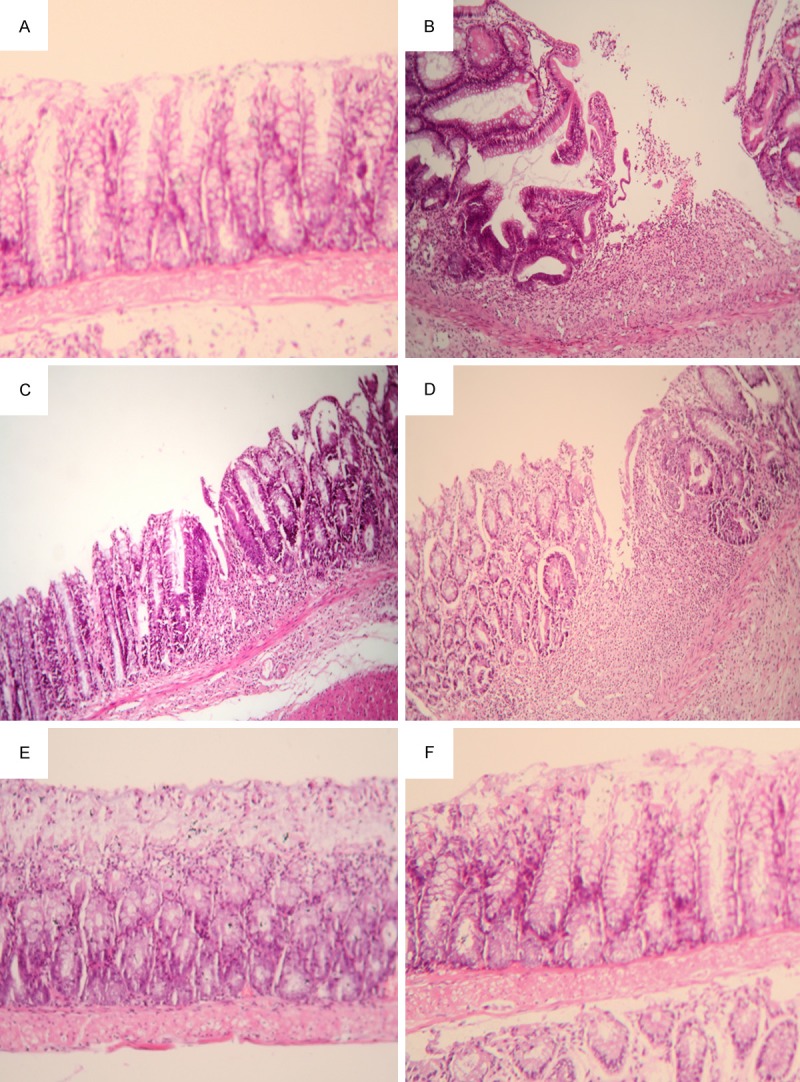
MSCs transplantation promotes the recovery of bowel tissue injury. Bowel tissue from normal control mice showed intact intestinal crypts without inflammatory cell infiltration (A). After induction of colitis, epithelial barrier disruption, crypt and gland loss with condensed inflammatory cell infiltration were detected (B). Three days after the withdrawal of DSS, partial recovery of collapsed intestinal crypts were present (C), and the mice that were MSCs-transplanted showed a recovery in a shorter time frame compared to controls (D). Both control (E) and the transplanted mice (F) had similar profiles 7 days after the cessation of the toxin. The sections were stained with H & E. Magnification is 100 ×.
Figure 4.
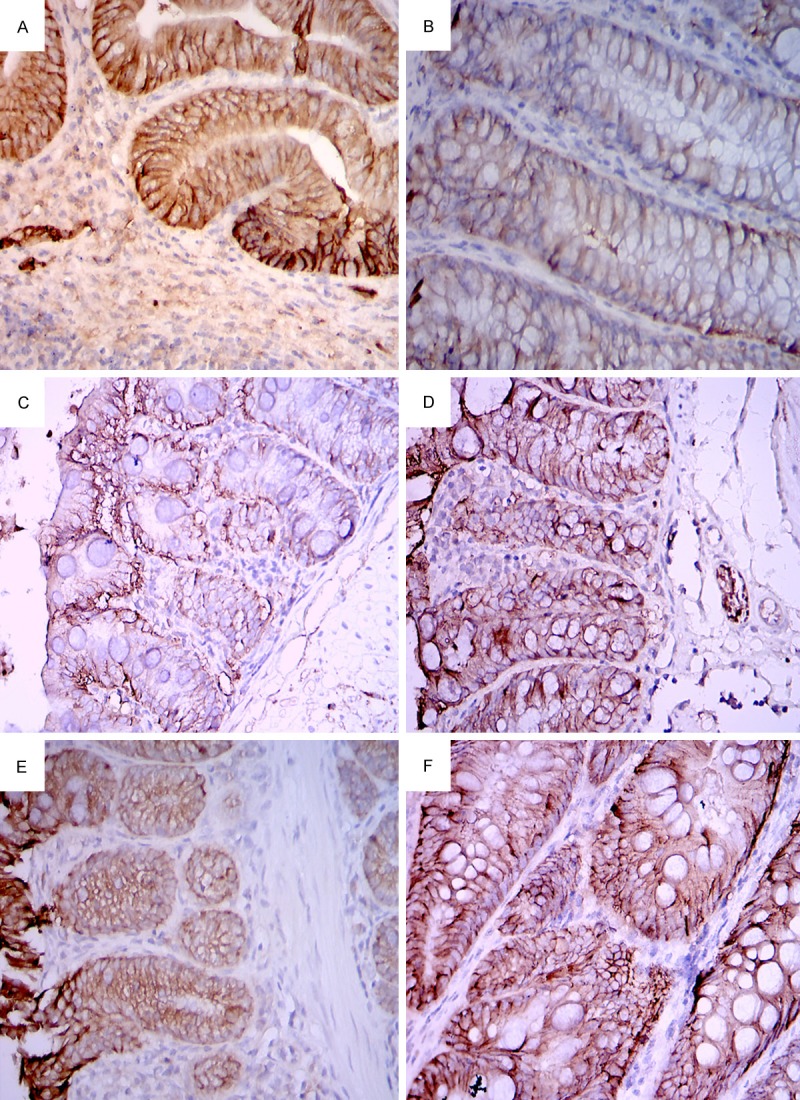
Immunohistochemistry of E-cadherin in murine intestine. Strong positive staining in the intestine of normal control mice (A). Induction of colitis significantly reduced the E-cadherin expression (B). Three days after MSCs transplantation, the E-cadherin expression in murine intestine was enhanced (D) in comparison with the control mice (C). Seven days after transplantation, the intestinal E-cadherin expression normalized in both the transplanted (E) and the control mice (F). Magnification is 100 ×.
MSCs transplantation reduces markers of oxidative stress in colitic tissue
Induction of colitis by DSS resulted in increased amounts of MDA and decreased amounts of SOD in intestinal tissue. Twelve hours after transplantation, MDA levels in intestinal tissue reduced and declined steadily until normal levels were reached in 14 days. SOD increased in MSCs-transplanted mice and normalized in 14 days (Figure 5). Differences of between transplanted mice and controls in levels of MDA and SOD were noted at 12 h, 24 h, 72 h, and 7 d.
Figure 5.
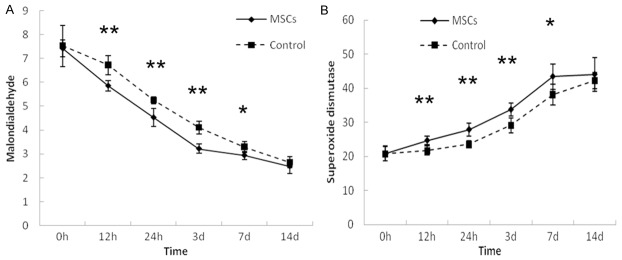
Alteration of MDA and SOD levels in intestinal tissue of mice after MSCs transplantation. A. MDA level in the mice that were MSCs-transplanted reduced in a shorter time period compared to controls. B. SOD level in mice that received MSCs transplantation compared to controls were statistically significant. **P<0.01, *P<0.05.
Discussion
MSCs have the potential to differentiate into a variety of cell types [28]. Previous studies have focused on the use of MSCs in the repair of tissue that are injured by various mechanisms [29]. The colonization of MSCs in injured sites is critical for this repair and thus there is interest in its application in the treatment of IBD [30].
This integrity of intestinal mucosal barrier is functionally reflected by the permeability of the mucosal barrier [31]. E-cadherin is one of the major transmembrane junctional adhesion molecules in intestinal epithelial cells and plays an important role in maintaining the cellular structure of the epithelium as well as the intercellular conjunction [32]. It has been reported that the expression of E-cadherin is down-regulated in patients with Crohn’s disease or regional intestinal ulcer [33]. The current study revealed that although the intestinal expression of E-cadherin reduced upon the induction of colitis by DSS, normalization within 7 days upon cessation of the toxin occurred. Transplantation of MSCs accelerated the restoration of E-cadherin expression in the injured intestine, i.e. promoted repair of the intestinal barrier.
D-LAC is a bacterial metabolite produced by intestinal flora. The abnormal increase of intestinal permeability allows D-LAC passage through the intestinal barrier. As it is not degraded by the liver, the elevation of D-LAC level in blood represents damage to the intestinal barrier [34]. DAO is an intracellular enzyme in the intestinal epithelium of mammals. It is released into intestinal lumen as enterocytes’ detachment and the dissolution of such cells. When intestinal permeability is abnormally increased as in damage to the intestinal barrier, luminal DAO enters the blood resulting in elevated serum DAO level [35,36]. MSCs transplantation reduced the elevated serum D-LAC and DAO levels and restored to normal in 72 h. In contrast, it took 7 days to recover these levels to normal in control mice. The results demonstrated that MSCs transplantation does promote the repair of intestinal barrier.
A recent study reported that bone marrow transplantation was able to ameliorate the post-radiation increase in intestinal permeability [37]. However, the major component of bone marrow is hemopoietic stem cells with the non-hemopoietic MSCs accounting for a small quantity [38]. MSCs transplantation in DSS-induced colitis and busulphan-induced hypoplastic marrow rats resulted in restoration of epithelial barrier integrity [20]. The question of whether MSCs are the sources of the regenerated intestinal cells is not known.
Oxidative stress has been implicated as an etiological and triggering factor for IBD [39]. MDA has been recognized as a biomarkers of lipid oxidation [40]. An increased level of MDA in vivo represents excessive oxidative damage in the body [41]. SOD is one of the main enzymatic antioxidants in vivo. The quantity of this enzyme would decrease in response to a severe oxidative stress and its recovery represent recession of oxidative stress [42,43]. These results are consonant with previous published studies demonstrating that an increased level of MDA and a decreased level of SOD were present in DSS induced colitis. MSCs transplantation significantly reduced MDA and promoted SOD levels suggestive of an antioxidant property of MSCs.
Using the allosome tracing method [44], it was found that MSCs can colonize in the injured intestine. Putatively, MSCs directly differentiate into mucosal epithelium cells. It was demonstrated that SRY protein, a sex determinant gene located in Y chromosome can be detected in the bowel of female mice 3, 7 and 14 days after receipt of MSCs from male donors confirming the ability of MSCs homing in damaged intestinal tissue. The possible role of transplanted MSCs in non-inflamed intestine, if any, is undefined.
Conclusion
This study demonstrated that MSCs is capable of migrating to and homing in the damaged bowel tissue in the restoration of the injured tissue. This results in restoring permeability of intestinal mucosa and easing oxidative stress.
Acknowledgements
This study was supported by the 11th Five-Year Programs of Chinese People’s Liberation Army (06MA017) and the Beijing Medicine Research and Development Fund Project (2009-3083).
Disclosure of conflict of interest
None.
Abbreviations
- DAI
disease activity index
- DAO
diamine oxidase
- D-LAC
D-lactate
- DSS
dextran sulfate sodium
- IBD
inflammatory bowel diseases
- MDA
malondialdehyde
- MSC
mesenchymal stem cell
- SOD
superoxide dismutase
- UC
ulcerative colitis
References
- 1.Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010;11:134–147. doi: 10.1111/j.1751-2980.2010.00429.x. [DOI] [PubMed] [Google Scholar]
- 2.Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 3.Abreu MT, Sparrow MP. Translational research in inflammatory bowel disease. Mt Sinai J Med. 2006;73:1067–1073. [PubMed] [Google Scholar]
- 4.Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79–94. doi: 10.1128/CMR.15.1.79-94.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg LN, Peppercorn MA. Efficacy and safety of drugs for ulcerative colitis. Expert Opin Drug Saf. 2010;9:573–592. doi: 10.1517/14740331003639412. [DOI] [PubMed] [Google Scholar]
- 7.Drakos PE, Nagler A, Or R. Case of Crohn’s disease in bone marrow transplantation. Am J Hematol. 1993;43:157–158. doi: 10.1002/ajh.2830430223. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto T, Okamoto R, Yajima T, Mori T, Okamoto S, Ikeda Y, Mukai M, Yamazaki M, Oshima S, Tsuchiya K, Nakamura T, Kanai T, Okano H, Inazawa J, Hibi T, Watanabe M. Increase of bone marrow-derived secretory lineage epithelial cells during regeneration in the human intestine. Gastroenterology. 2005;128:1851–1867. doi: 10.1053/j.gastro.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 9.Clerici M, Cassinotti A, Onida F, Trabattoni D, Annaloro C, Della Volpe A, Rainone V, Lissoni F, Duca P, Sampietro G, Fociani P, Vago G, Foschi D, Ardizzone S, Deliliers GL, Porro GB. Immunomodulatory effects of unselected haematopoietic stem cells autotransplantation in refractory Crohn’s disease. Dig Liver Dis. 2011;43:946–952. doi: 10.1016/j.dld.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Komori M, Tsuji S, Tsujii M, Murata H, Iijima H, Yasumaru M, Nishida T, Irie T, Kawano S, Hori M. Involvement of bone marrow-derived cells in healing of experimental colitis in rats. Wound Repair Regen. 2005;13:109–118. doi: 10.1111/j.1067-1927.2005.130114.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Gong JF, Zhang W, Zhu WM, Li JS. Effects of transplanted bone marrow mesenchymal stem cells on the irradiated intestine of mice. J Biomed Sci. 2008;15:585–594. doi: 10.1007/s11373-008-9256-9. [DOI] [PubMed] [Google Scholar]
- 12.Khalil PN, Weiler V, Nelson PJ, Khalil MN, Moosmann S, Mutschler WE, Siebeck M, Huss R. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007;132:944–954. doi: 10.1053/j.gastro.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N, Kawano S. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther. 2008;326:523–531. doi: 10.1124/jpet.108.137083. [DOI] [PubMed] [Google Scholar]
- 15.Nasef A, Ashammakhi N, Fouillard L. Immunomodulatory effect of mesenchymal stromal cells: possible mechanisms. Regen Med. 2008;3:531–546. doi: 10.2217/17460751.3.4.531. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka F, Tominaga K, Ochi M, Tanigawa T, Watanabe T, Fujiwara Y, Ohta K, Oshitani N, Higuchi K, Arakawa T. Exogenous administration of mesenchymal stem cells ameliorates dextran sulfate sodium-induced colitis via anti-inflammatory action in damaged tissue in rats. Life Sci. 2008;83:771–779. doi: 10.1016/j.lfs.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Jena G, Trivedi PP, Sandala B. Oxidative stress in ulcerative colitis: an old concept but a new concern. Free Radic Res. 2012;46:1339–1345. doi: 10.3109/10715762.2012.717692. [DOI] [PubMed] [Google Scholar]
- 18.Shen ZY, Zhang J, Song HL, Zheng WP. Bone-marrow mesenchymal stem cells reduce rat intestinal ischemia-reperfusion injury, ZO-1 downregulation and tight junction disruption via a TNF-alpha-regulated mechanism. World J Gastroenterol. 2013;19:3583–3595. doi: 10.3748/wjg.v19.i23.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang H, Qu L, Li Y, Gu L, Shi Y, Zhang J, Zhu W, Li J. Bone marrow mesenchymal stem cells reduce intestinal ischemia/reperfusion injuries in rats. J Surg Res. 2011;168:127–134. doi: 10.1016/j.jss.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Yabana T, Arimura Y, Tanaka H, Goto A, Hosokawa M, Nagaishi K, Yamashita K, Yamamoto H, Adachi Y, Sasaki Y, Isobe M, Fujimiya M, Imai K, Shinomura Y. Enhancing epithelial engraftment of rat mesenchymal stem cells restores epithelial barrier integrity. J Pathol. 2009;218:350–359. doi: 10.1002/path.2535. [DOI] [PubMed] [Google Scholar]
- 21.He XW, He XS, Lian L, Wu XJ, Lan P. Systemic infusion of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis in mice. Dig Dis Sci. 2012;57:3136–3144. doi: 10.1007/s10620-012-2290-5. [DOI] [PubMed] [Google Scholar]
- 22.Song C, Li G. CXCR4 and matrix metalloproteinase-2 are involved in mesenchymal stromal cell homing and engraftment to tumors. Cytotherapy. 2011;13:549–561. doi: 10.3109/14653249.2010.542457. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, McClurg A, Zhou GQ, McCaigue M, Armstrong MA, Li G. Chondrogenic differentiation alters the immunosuppressive property of bone marrow-derived mesenchymal stem cells, and the effect is partially due to the upregulated expression of B7 molecules. Stem Cells. 2007;25:364–370. doi: 10.1634/stemcells.2006-0268. [DOI] [PubMed] [Google Scholar]
- 24.Kihara N, de la Fuente SG, Fujino K, Takahashi T, Pappas TN, Mantyh CR. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut. 2003;52:713–719. doi: 10.1136/gut.52.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, Sasaki S, Saitoh O, Katsu K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51–58. doi: 10.1046/j.1365-2249.2000.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18:279–288. doi: 10.4196/kjpp.2014.18.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 28.Ren G, Chen X, Dong F, Li W, Ren X, Zhang Y, Shi Y. Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med. 2012;1:51–58. doi: 10.5966/sctm.2011-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson WM, Nesti LJ, Tuan RS. Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med. 2012;1:44–50. doi: 10.5966/sctm.2011-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricart E. Current status of mesenchymal stem cell therapy and bone marrow transplantation in IBD. Dig Dis. 2012;30:387–391. doi: 10.1159/000338134. [DOI] [PubMed] [Google Scholar]
- 31.Scaldaferri F, Pizzoferrato M, Gerardi V, Lopetuso L, Gasbarrini A. The gut barrier: new acquisitions and therapeutic approaches. J Clin Gastroenterol. 2012;46 (Suppl):S12–17. doi: 10.1097/MCG.0b013e31826ae849. [DOI] [PubMed] [Google Scholar]
- 32.Hermiston ML, Wong MH, Gordon JI. Forced expression of E-cadherin in the mouse intestinal epithelium slows cell migration and provides evidence for nonautonomous regulation of cell fate in a self-renewing system. Genes Dev. 1996;10:985–996. doi: 10.1101/gad.10.8.985. [DOI] [PubMed] [Google Scholar]
- 33.Dogan A, Wang ZD, Spencer J. E-cadherin expression in intestinal epithelium. J Clin Pathol. 1995;48:143–146. doi: 10.1136/jcp.48.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray MJ, Barbose JJ, Cobb CF. Serum D(-)-lactate levels as a predictor of acute intestinal ischemia in a rat model. J Surg Res. 1993;54:507–509. doi: 10.1006/jsre.1993.1078. [DOI] [PubMed] [Google Scholar]
- 35.Luk GD, Bayless TM, Baylin SB. Diamine oxidase (histaminase). A circulating marker for rat intestinal mucosal maturation and integrity. J Clin Invest. 1980;66:66–70. doi: 10.1172/JCI109836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song WB, Lv YH, Zhang ZS, Li YN, Xiao LP, Yu XP, Wang YY, Ji HL, Ma L. Soluble intercellular adhesion molecule-1, D-lactate and diamine oxidase in patients with inflammatory bowel disease. World J Gastroenterol. 2009;15:3916–3919. doi: 10.3748/wjg.15.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garg S, Wang W, Prabath BG, Boerma M, Wang J, Zhou D, Hauer-Jensen M. Bone marrow transplantation helps restore the intestinal mucosal barrier after total body irradiation in mice. Radiat Res. 2014;181:229–239. doi: 10.1667/RR13548.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baghaban Eslaminejad M, Malakooty Poor E. Mesenchymal stem cells as a potent cell source for articular cartilage regeneration. World J Stem Cells. 2014;6:344–354. doi: 10.4252/wjsc.v6.i3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci. 2007;52:2015–2021. doi: 10.1007/s10620-006-9622-2. [DOI] [PubMed] [Google Scholar]
- 40.Lykkesfeldt J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin Chim Acta. 2007;380:50–58. doi: 10.1016/j.cca.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 41.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ, Fisher SE, Mullin GE. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–2086. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 43.Miller AF. Superoxide dismutases: ancient enzymes and new insights. FEBS Lett. 2012;586:585–595. doi: 10.1016/j.febslet.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, Seguro AC, Pacheco-Silva A, Saraiva Camara NO. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27:3063–3073. doi: 10.1002/stem.214. [DOI] [PubMed] [Google Scholar]