Abstract
Endometrial carcinoma is the most common gynecologic malignancy. Searching for a new molecule to more accurately predict survival of patients and act as therapy target is urgent. CHRM3 is a major player in many kinds of cancer. The expression level and prognostic value of CHRM3 in endometrial carcinoma remain unclear. In this study, we assayed the expression of CHRM3 in 257 endometrial carcinoma patients by immunohistochemistry. The results showed that CHRM3 expression level was closely correlated with the FIGO stage, vascular invasion and lymphatic metastasis. Although CHRM3 was highly expressed in advanced endometrial carcinoma, multivariate Cox proportional hazards regression analysis showed that CHRM3 expression was not an independent prognostic factor for endometrial carcinoma. Furthermore, to evaluate the prognostic efficiency of CHRM3 in endometrial carcinoma, we compared the sensitivity and specificity of CHRM3 in endometrial carcinoma prognosis by logistic regression. The result showed that CHRM3 combining with other clinicopathological risk factors was a stronger prognostic model than clinicopathological risk factor alone or combination of risk factors. Thus, CHRM3 potentially offers a clinical value in target therapy for endometrial carcinoma patients.
Keywords: CHRM3, endometrial carcinoma, immunohistochemistry, prognosis
Introduction
Endometrial carcinoma is the most common gynecologic malignancy. Incidence rates and death rates of this cancer are increasing year by year. In the United States, an estimated 52630 new cases are expected to be diagnosed in 2014, with 8590 women succumbing to their disease [1]. Early-stage patients have good prognosis and high cure rate. However, those with advanced stage symptom including metastasis may have poor prognosis. Therefore, there is an urgent need for finding new molecules with prognostic value to more accurately predict survival of endometrial carcinoma patients and to cure this disease efficiently.
Cholinergic nerves provide an important input to the uterus. Uterine activity is innervated by cholinergic system occurring in the uterine body and cervix from several species, and its contraction is regulated by released acetylcholine. This effect is mediated by the interaction of acetylcholine with muscarinic choline receptors (CHMRs). Muscarinic receptors belong to the superfamily of G protein-coupled receptors and are widely expressed in numerous organs and tissues, such as the central nervous system, salivary glands, heart, arteries, digestive organs, bladder, uterus, skeletal muscle, and smooth muscle. They are involved in signal transduction, secretion, peristalsis, and muscle contraction [2-5]. Molecular cloning studies have identified five CHMRs genes, each gene corresponding to the subtypes M1, M2, M3, M4 and M5 receptors (CHRM1-5) [6,7]. The muscarinic receptor subtypes responsible for mediating contraction of the myometrium have been investigated in a few animal species. In the guinea-pig uterus, the contractile response was triggered mainly by M3 receptors via the inositol phosphate pathway [8]. In the rat uterus, previous study had demonstrated the expression of both M2 and M3 muscarinic receptors and the physiological significance of M3 subtype in cholinergic uterine contractions [9]. In the porcine uterus, the existence of M3 subtype but not M2 muscarinic receptor have been indicated by functional and radio-ligand binding assays [10]. And in the mouse uterus, both M2 and M3 muscarinic receptors are expressed, but uterine contractions were predominantly mediated by the M3 receptor, because carbachol-induced contractions occurred in M2 knockout mice but did not occur in M3 knockout mice [11]. All these findings suggest that the muscarinic receptors expression exist species-related variations and M3 muscarinic receptor play a major role in mediating contractions of the uterus.
Although CHRM3 is expressed in uterus and mainly mediate uterine contractions, its expression level and prognostic value in endometrial carcinoma remain unclear. In this study, the CHRM3 expression level in endometrial carcinoma tissues was detected by immunohistochemistry, the correlation between variables and overall survival (OS) was analyzed by Cox proportional hazards regression, the prognostic accuracy of CHRM3 was compared with other clinicopathological risk factors by logistic regression, and the prognostic efficacy of CHRM3 in endometrial carcinoma patients was assessed.
Materials and methods
Patients and tumor samples
In this study, 257 formalin-fixed paraffin-embedded (FFPE) endometrial carcinoma tissue samples were obtained between April 2003 and March 2013 from Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, China, the Shanghai Changning District Central Hospital, China, and the First People’s Hospital of Huai’an City, Jiangsu, China. We excluded patients who had history of other solid tumors, underwent radical surgery treatment, radiotherapy, chemotherapy, or other anticancer therapies prior to surgery, had no complete clinicopathological and follow-up data, and without completed informed consent and approval of the ethics committee of each hospital for the use of samples. Two pathologists reassessed all these samples. The FFPE tissue samples comprised at least 80% tumor cells. OS was defined as the time from the date of surgery to the date of death or the last follow-up examination.
Tissue microarray construction
Tissue microarrays were constructed by Shang-hai Zuoli Biotechnology Co., Ltd (Zuoli Bio-technology Co, Shanghai, China). Pathologists stained tissue slice of EC samples with hematoxylin-eosin to confirm the diagnoses and marked at fixed points which displayed the most typical histological characteristics under a microscope. Cores with 1.1-mm diameter from per-donor block were diverted into a recipient block microarray. Four-micrometer-thick sections were cut from the recipient block and diverted to glass slides used with an adhesive tape transfer system in order to ultraviolet cross linkage.
Immunohistochemistry
The tissue microarray glass slides were deparaffinized through xylene, 50% xylene and hydrated via gradient concentrations of ethanol (in 100, 95, 85, and 75% ethanol for 10 min, respectively). Antigen retrieval was performed in 0.01 M citrate buffer (pH6.0) for 30 min at 100°C. Tissue sections were blocked for peroxidase activity with 0.3% Hydrogen peroxide at 37°C for 30 mins and then washed by Phosphate Buffered Saline (PBS) solution. Subsequently, the sections were blocked with 10% BSA for 1 h at room temperature. Then the tissues were incubated with CHRM3 antibody (1:100; Abcam Biotechnology, Cambridge, UK) overnight at 4°C. Next day, the tissues were washed with PBS for three times and incubated with goat anti-rabbit IgG-HRP (HUABIO) secondary antibody for one hour at room temperature. The slides were visualized using DAB substrate liquid (Thermo Scientific, USA) and chromogenic reaction was controlled under microscope. After immunostaining, tissues were immersed into hematoxylin for nuclear staining. The slides were then dehydrated through gradient concentrations of ethanol, cleared with xylene, and covered with neutral balsam.
Scoring was conducted according to the percentage of positive staining cells [12]; 0-5% scored 0, 6-35% scored 1, 36-70% scored 2, and more than 70% scored 3. The final score was designated as Low expression or High expression group as follows: score 0-1, Low expression, score 2-3, High expression. These scores were determined independently by two senior pathologists.
Statistical analysis
Statistical analysis were conducted using SPSS 19.0 software (Chicago, IL, USA). ROC curves were performed to compare the prognostic accuracy of CHRM3 with clinicopathological risk factors in all patients by logistic regression. For survival analysis, the Kaplan-Meier method was used to analyze the correlation between OS and variables, and the log-rank test was used to compare survival curves. Univariate analysis was based on the Cox proportional hazard regression model, and multivariate analysis was based on the Cox proportional hazard regression model with stepwise manner (forward: condition, entry α = 0.05, stay α = 0.1). Statistical significance was set at 0.05.
Results
CHRM3 expression in endometrial carcinoma patients
To determine the CHRM3 expression in endometrial carcinomas, we performed immunohistochemistry (IHC) study using tissue microarrays that contained 257 endometrial carcinoma samples. The clinicopathological characters of the samples were shown in Table 1. IHC scores of tissue samples were shown in Table 2. Representative stains of CHRM3 scored as 0, 1, 2 and 3 were shown in Figure 1. CHRM3 expression existed significant difference in endometrial carcinomas. The correlation be-tween CHRM3 expression and the clinicopathological features was shown in Table 3. Statistical analysis revealed that the CHRM3 protein expression level was closely correlated with the FIGO stage, vascular invasion and lymphatic metastasis but not with pregnancy, grade, or pathological type in the endometrial carcinomas. Moreover, the CHRM3-high expression rate was higher than CHRM3-low expression rate in stage III and IV patients, or in patients with vascular invasion, or in patients with lymphatic metastasis. These results suggest that CHRM3 might be associated with metastasis and lead to a poor prognosis in endometrial carcinoma. However, the prognostic value of CHRM3 in human endometrial carcinoma remains unclear.
Table 1.
Clinicopathological characters of endometrial carcinoma patients
| Number | % | |
|---|---|---|
| Age (years) | ||
| < 45 | 24 | 9.3 |
| ≥ 45 | 233 | 90.7 |
| Pregnancy | ||
| No | 13 | 5.1 |
| Yes | 244 | 94.9 |
| Pathological type | ||
| Adenocarcinoma | 233 | 90.7 |
| Squamous carcinoma, papillary serous carcinoma, and clear cell carcinoma | 24 | 9.3 |
| Stage | ||
| I | 216 | 84.0 |
| II | 19 | 7.4 |
| III and IV | 22 | 8.6 |
| Grade | ||
| G1 | 144 | 56.1 |
| G2 | 78 | 30.3 |
| G3 | 35 | 13.6 |
| Vascular invasion | ||
| No | 235 | 91.4 |
| Yes | 22 | 8.6 |
| Lymphatic metastasis | ||
| No | 243 | 94.6 |
| Yes | 14 | 5.4 |
Table 2.
IHC scores for CHRM3 expression in 257 endometrial carcinoma patients
| CHRM3 | % | |
|---|---|---|
| Score | ||
| 0 | 16 | 6.2 |
| 1 | 111 | 43.2 |
| 2 | 38 | 14.8 |
| 3 | 92 | 35.8 |
| Total | 257 | 100.0 |
Figure 1.
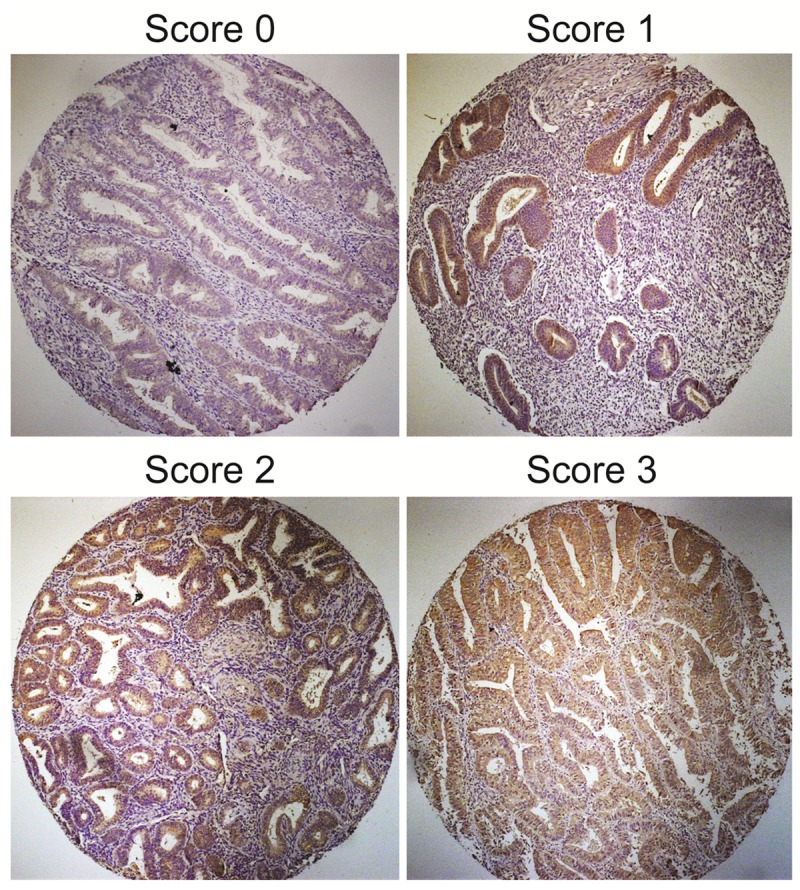
Representative immunohistochemical stains for CHRM3 in endometrial carcinoma patients.
Table 3.
Relationship between CHRM3 expression and clinicopathological features of endometrial carcinoma patients
| CHRM3 | P Value | ||||
|---|---|---|---|---|---|
|
|
|||||
| Low | % | High | % | ||
| Age (years) | |||||
| < 45 | 11 | 4.3 | 13 | 5.1 | 0.831 |
| ≥ 45 | 116 | 45.1 | 117 | 45.5 | |
| Pregnancy | |||||
| No | 5 | 1.9 | 8 | 3.1 | 0.571 |
| Yes | 122 | 47.5 | 122 | 47.5 | |
| Pathological type | |||||
| Adenocarcinoma | 119 | 46.3 | 114 | 44.4 | 0.133 |
| Squamous carcinoma, papillary serous carcinoma, and clear cell carcinoma | 8 | 3.1 | 16 | 6.2 | |
| Stage | |||||
| I | 113 | 44.0 | 103 | 40.1 | 0.009** |
| II | 10 | 3.9 | 9 | 3.5 | |
| III and IV | 4 | 1.5 | 18 | 7.0 | |
| Grade | |||||
| G1 | 71 | 27.6 | 73 | 28.4 | 0.796 |
| G2 | 37 | 14.4 | 41 | 16.0 | |
| G3 | 19 | 7.4 | 16 | 6.2 | |
| Vascular invasion | |||||
| No | 122 | 47.5 | 113 | 44.0 | 0.013* |
| Yes | 5 | 1.9 | 17 | 6.6 | |
| Lymphatic metastasis | |||||
| No | 125 | 48.6 | 118 | 45.9 | 0.011* |
| Yes | 2 | 0.8 | 12 | 4.7 | |
P < 0.05;
P < 0.01.
CHRM3 was a prognostic factor for the overall survival (OS) of endometrial carcinoma patients
The correlation between CHMR3 expression status and OS was evaluated by Kaplan-Meier method. The OS of the CHMR3-low expression group was significantly better than the CHRM3-high expression group (Figure 2F). A univariate analysis using Kaplan-Meier method showed that the pathological type, stage, grade, vascular invasion, lymphatic metastasis, and CHRM3 were predictors of OS in the endometrial carcinoma patients (Figure 2A-F). In addition, OS of CHRM3-high expression group was significantly worse than that of the CHRM3-low expression group for advanced endometrial carcinoma patients who in stage II, III and IV or in grad 2 and 3 (Figure 3A, 3B). In no lymphatic metastasis EC patients, however, the OS of CHRM3-low expression group was also remarkably better than that of CHRM3-high expression group (Figure 3C). Furthermore, univariate Cox proportional hazards regression analysis indicated that pathological type, stage, grade, vascular invasion, lymphatic metastasis and CHRM3 were hazardous prognostic factors for the OS of endometrial carcinoma patients. However, multivariate Cox proportional hazards regression analysis showed that CHRM3 expression was not an independent prognostic factor for endometrial carcinoma (Table 4). These results suggested that CHRM3 was a prognostic factor for the OS of endometrial carcinoma patients, but was not an independent prognostic factor.
Figure 2.
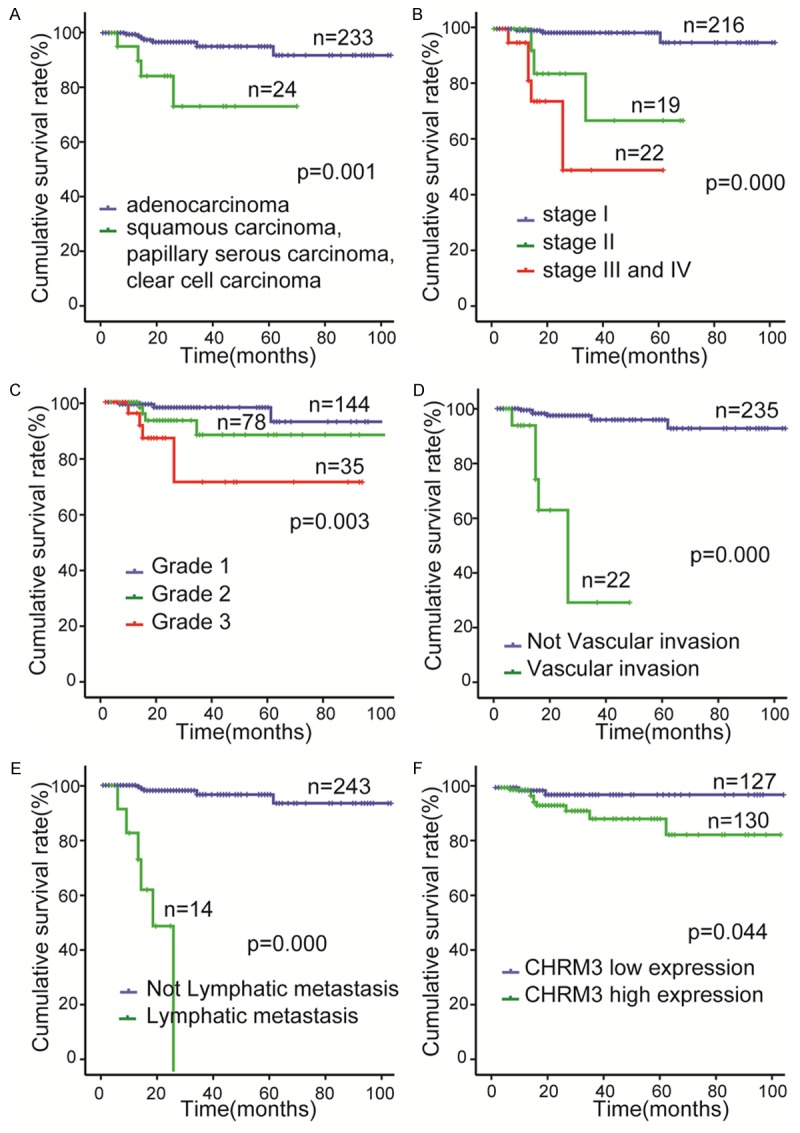
Univariate analyses of factors associated with overall survival (OS) in endometrial carcinoma patients by Kaplan-Meier method and log-rank test. OS according to (A) Pathological type; (B) stage; (C) grade; (D) vascular invasion; (E) lymphatic metastasis; (F) CHRM3 expression.
Figure 3.
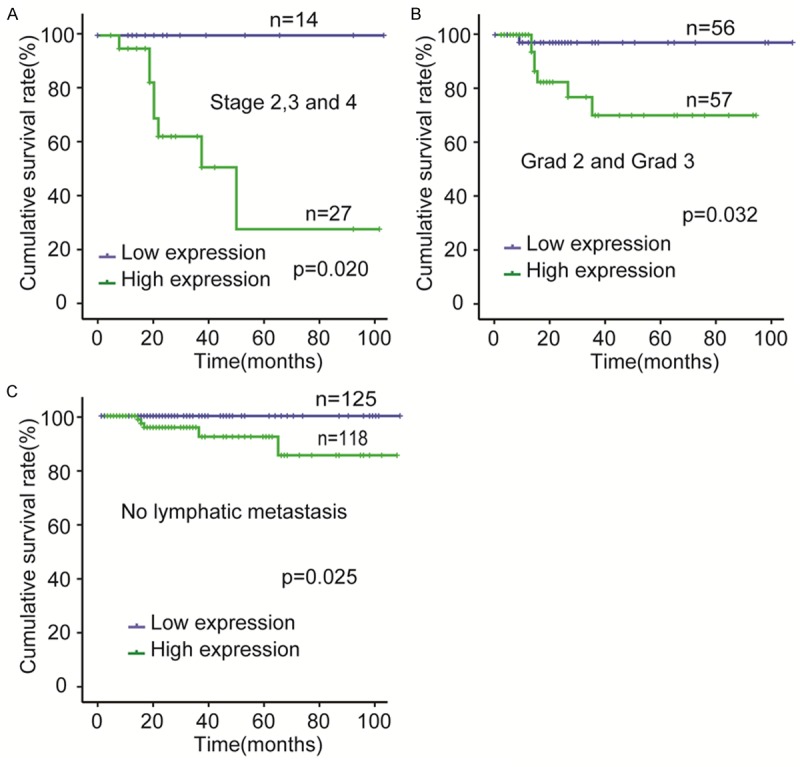
Prognostic significance of CHRM3 expression was assessed for all 257 samples by Kaplan-Meier method and log-rank test. A. Comparisons of OS between CHRM3 low expression and CHRM3 high expression groups in G2 and G3 cohort. B. Comparisons of OS between CHRM3 low expression and CHRM3 high expression groups in stage II and stage III and IV cohort. C. Comparisons of OS between CHRM3 low expression and CHRM3 high expression groups in no lymphatic metastasis cohort.
Table 4.
Univariate & multivariate cox proportional hazards model to predict factors associated with OS of endometrial carcinoma patients
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| Hazard Ratio (95% Cl) | P value | Hazard Ratio (95% Cl) | P value | |
| Age | 0.405 (0.087-1.891) | 0.250 | 0.362 (0.033-3.956) | 0.405 |
| Pregnancy | 0.509 (0.065-3.982) | 0.520 | 8.789 (0.219-353.313) | 0.249 |
| Pathological type | 6.180 (1.787-21.372) | 0.004** | 0.110 (0.007-1.672) | 0.112 |
| Stage | 4.703 (2.435-9.081) | < 0.001** | 1.536 (0.421-5.602) | 0.515 |
| Grade | 2.595 (1.254-5.370) | 0.010* | 1.623 (0.543-4.852) | 0.386 |
| Vascular invasion | 18.851 (5.389-65.940) | < 0.001** | 1.373 (0.189-9.986) | 0.754 |
| Lymphatic metastasis | 47.233 (11.65-191.492) | < 0.001** | 63.766 (4.493-904.945) | 0.002** |
| CHRM3 | 4.249 (0.918-19.673) | 0.046* | 2.792 (0.458-17.026) | 0.266 |
P < 0.05;
P < 0.01.
The sensitivity and specificity of CHRM3 for endometrial carcinoma prognosis
To further confirm the prognostic accuracy of CHRM3 in endometrial carcinoma, the sensitivity and specificity of CHRM3 in endometrial carcinoma prognosis were compared by logistic regression. For receiver operating characteristic curve (ROC) analysis, eight models were constructed including CHRM3, a single clinicopathological risk factor, combinations of clinicopathological risk factors, and CHRM3 combined with clinicopathological risk factors. Then, an ROC curve comparing the prognostic accuracy of CHRM3 with that of other clinicopathological risk factors was performed (Figure 4). The area under the curve (AUC) was 0.641 for pathological type, 0.802 for stage, 0.682 for grade, 0.693 for vascular invasion, 0.756 for lymphatic metastasis, 0.663 for CHRM3, 0.894 for combined clinical prognostic factors, and 0.930 for CHMR3 and combined clinical prognostic factors (Table 5). Statistical analyses showed that AUC for CHRM3 combined with other clinicopathological prognostic factors was higher than any individual factor or other clinicopathological prognostic factors’ combination. These results indicated that CHRM3 combined with other clinicopathological prognostic factors had more sensitivity and specificity and was a stronger prognostic model than the single risk factor or their combination.
Figure 4.
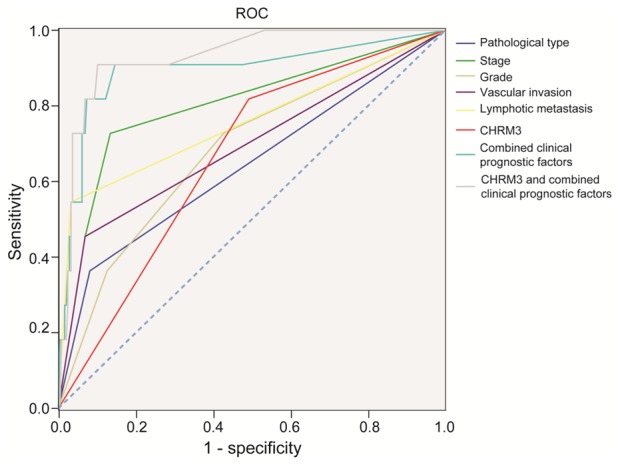
ROC curve compares the prognostic accuracy of CHRM3 with clinicopathological risk factors in all 257 endometrial carcinoma patients by logistic regression.
Table 5.
The area under the ROC curve
| Variable | AUC area (95% Cl) | P value |
|---|---|---|
| Pathological type | 0.641 (0.449-0.833) | 0.113 |
| Stage | 0.802 (0.644-0.959) | 0.001** |
| Grade | 0.682 (0.514-0.850) | 0.041* |
| Vascular invasion | 0.693 (0.502-0.883) | 0.031* |
| Lymphatic metastasis | 0.756 (0.571-0.942) | 0.004** |
| CHRM3 | 0.663 (0.516-0.810) | 0.067 |
| Combined clinical prognostic factors | 0.894 (0.769-1.000) | 0.000** |
| CHMR3 and combined clinical prognostic factors | 0.930 (0.861-0.999) | 0.000** |
P < 0.05;
P < 0.01.
Discussion
Endometrial carcinoma, which is a tumor within the female genital system, remains a leading cause of women death. Thus, searching for new prognostic markers to assess prognosis or act as pharmacological target are urgent for this disease.
CHRM3 is one of the five muscarinic receptors which plays an important role in many pathological processes. Recent studies have shown that CHRM3 is a major player in many kinds of cancer, like colon cancer, gastric cancer, breast cancer, and lung cancer [13-17]. It has been shown that acetylcholine (Ach) promotes proliferation and cell migration of several cancers through CHRM3, and that such promotion can be inhibited by CHRM3 antagonists [18,19]. This suggests that inhibition of CHRM3 may be a novel therapeutic approach. We have known uterine contraction is induced via activation of CHRM3. However, the correlation between the CHRM3 expression and prognostic value in endometrial carcinoma remains unclear.
We investigated the status of CHRM3 expression in a large number of endometrial carcinoma tissues, and we showed that CHRM3 was highly expressed in patients with advanced endometrial carcinoma. CHRM3 expression was significantly correlated with FIGO stage, vascular invasion and lymphatic metastasis in endometrial carcinoma samples. These results suggested that M3 receptor high expression may be involved in the cancer metastasis and poor prognosis in endometrial carcinoma. Overexpression of CHRM3 may represent an prognostic factor for endometrial carcinoma patients influencing OS. Patients with higher CHRM3 expression had a shorter overall survival time, whereas patients with lower CHRM3 expression had better survival. These results are consistent with the previous reports concerning the roles of muscarinic receptors in the carcinogenesis and progression of cancer. From these results, we can see that CHRM3 may become a valuable predictor for prognosis among endometrial carcinoma patients. We compared the prognostic accuracy of CHRM3 with other clinicopathological risk factors. The results showed that CHRM3 combined with other clinicopathological risk factors was a stronger prognostic model.
The limitations of our study are mainly in follow-up time and sample size for advanced endometrial carcinoma patients. In addition, as an initial report of the potential role of CHRM3 in endometrial carcinoma, further work, such as prospective study and molecular mechanism of CHRM3, are still needed.
In conclusion, our study shows that CHRM3 expression was associated with FIGO stage, vascular invasion, and lymphatic metastasis. Moreover, CHRM3 combined with other clinicopathological risk factors is a better prognostic tool. M3 receptor may be a novel antineoplastic therapy in the advanced endometrial carcinoma.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Shew RL, Papka RE, McNeill DL. Calcitonin gene-related peptide in the rat uterus: presence in nerves and effects on uterine contraction. Peptides. 1990;11:583–589. doi: 10.1016/0196-9781(90)90062-a. [DOI] [PubMed] [Google Scholar]
- 3.Houdeau E, Rossano B, Prud’homme MJ. Regional and muscle layer variations in cholinergic nerve control of the rat myometrium during the oestrous cycle. Auton Neurosci. 2003;104:1–9. doi: 10.1016/s1566-0702(02)00233-3. [DOI] [PubMed] [Google Scholar]
- 4.Boyle FC, Digges KG. Responses to catecholamines of the rat isolated uterus throughout the natural oestrous cycle. Naunyn Schmiedebergs Arch Pharmacol. 1982;321:56–62. doi: 10.1007/BF00586350. [DOI] [PubMed] [Google Scholar]
- 5.Sato Y, Hotta H, Nakayama H, Suzuki H. Sympathetic and parasympathetic regulation of the uterine blood flow and contraction in the rat. J Auton Nerv Syst. 1996;59:151–158. doi: 10.1016/0165-1838(96)00019-7. [DOI] [PubMed] [Google Scholar]
- 6.Kubo T, Fukuda K, Mikami A, Maeda A, Takahashi H, Mishina M, Haga T, Haga K, Ichiyama A, Kangawa K, et al. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986;323:411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- 7.Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- 8.Leiber D, Marc S, Harbon S. Pharmacological evidence for distinct muscarinic receptor subtypes coupled to the inhibition of adenylate cyclase and to the increased generation of inositol phosphates in the guinea pig myometrium. J Pharmacol Exp Ther. 1990;252:800–809. [PubMed] [Google Scholar]
- 9.Abdalla FM, Marostica E, Picarelli ZP, Abreu LC, Avellar MC, Porto CS. Effect of estrogen on muscarinic acetylcholine receptor expression in rat myometrium. Mol Cell Endocrinol. 2004;213:139–148. doi: 10.1016/j.mce.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 10.Kitazawa T, Uchiyama F, Hirose K, Taneike T. Characterization of the muscarinic receptor subtype that mediates the contractile response of acetylcholine in the swine myometrium. Eur J Pharmacol. 1999;367:325–334. doi: 10.1016/s0014-2999(98)00946-7. [DOI] [PubMed] [Google Scholar]
- 11.Kitazawa T, Hirama R, Masunaga K, Nakamura T, Asakawa K, Cao J, Teraoka H, Unno T, Komori S, Yamada M, Wess J, Taneike T. Muscarinic receptor subtypes involved in carbachol-induced contraction of mouse uterine smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:503–513. doi: 10.1007/s00210-007-0223-1. [DOI] [PubMed] [Google Scholar]
- 12.Jiang SH, He P, Ma MZ, Wang Y, Li RK, Fang F, Fu Y, Tian GA, Qin WX, Zhang ZG. PNMA1 promotes cell growth in human pancreatic ductal adenocarcinoma. Int J Clin Exp Pathol. 2014;7:3827–3835. [PMC free article] [PubMed] [Google Scholar]
- 13.Xie G, Cheng K, Shant J, Raufman JP. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am J Physiol Gastrointest Liver Physiol. 2009;296:G755–763. doi: 10.1152/ajpgi.90519.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiszman GL, Middonno MC, de la Torre E, Farina M, Espanol AJ, Sales ME. Activation of muscarinic cholinergic receptors induces MCF-7 cells proliferation and angiogenesis by stimulating nitric oxide synthase activity. Cancer Biol Ther. 2007;6:1106–1113. doi: 10.4161/cbt.6.7.4330. [DOI] [PubMed] [Google Scholar]
- 15.Kodaira M, Kajimura M, Takeuchi K, Lin S, Hanai H, Kaneko E. Functional muscarinic m3 receptor expressed in gastric cancer cells stimulates tyrosine phosphorylation and MAP kinase. J Gastroenterol. 1999;34:163–171. doi: 10.1007/s005350050238. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Zhou J, Yao L, Lang Y, Liang Y, Chen L, Zhang J, Wang F, Wang Y, Chen H, Ma J. High expression of M3 muscarinic acetylcholine receptor is a novel biomarker of poor prognostic in patients with non-small cell lung cancer. Tumour Biol. 2013;34:3939–3944. doi: 10.1007/s13277-013-0982-x. [DOI] [PubMed] [Google Scholar]
- 17.Shah N, Khurana S, Cheng K, Raufman JP. Muscarinic receptors and ligands in cancer. Am J Physiol Cell Physiol. 2009;296:C221–232. doi: 10.1152/ajpcell.00514.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song P, Sekhon HS, Lu A, Arredondo J, Sauer D, Gravett C, Mark GP, Grando SA, Spindel ER. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res. 2007;67:3936–3944. doi: 10.1158/0008-5472.CAN-06-2484. [DOI] [PubMed] [Google Scholar]
- 19.Lin G, Sun L, Wang R, Guo Y, Xie C. Overexpression of muscarinic receptor 3 promotes metastasis and predicts poor prognosis in non-small-cell lung cancer. J Thorac Oncol. 2014;9:170–178. doi: 10.1097/JTO.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]


