Abstract
Matrix metalloproteinase 14 (MMP14) has been shown to play a significant role in several types of cancers, but little is known about the function of MMP14 in nasopharyngeal carcinoma (NPC) carcinogenesis. The aim of this study was to investigate the role of MMP14 in NPC using NPC tumor samples or tissue microarray. We have shown that MMP14 was increased in NPC samples compared with normal nasopharynx (NP) tissues in microarray data (GSE13597). Both MMP14 mRNA and protein expression were markedly higher in NPC tissues than in NP tissues. High levels of MMP14 protein were found positively correlate with the status of late clinical stages of tumor and tumor with lymph node metastasis. Moreover, we have shown that MMP14 expression promoted the cell migration and invasion of NPC cells in vitro and regulated the expression of EMT-associated genes. Our data demonstrated that MMP14 plays an important role in regulation of migration and invasion of NPC cells, and constitutes a potential novel therapeutic target for NPC.
Keywords: Matrix metalloproteinase 14, nasopharyngeal carcinoma, biomarker, metastasis, epithelial-mesenchymal transition
Introduction
Nasopharyngeal carcinoma (NPC) is the most common malignant tumor of the nasopharynx. It is rare in most populations but is frequent in Southeast Asia, especially Southern China. It poses one of the serious health problems in southern China where an annual incidence of more than 20 cases per 100,000 is reported [1]. Most NPC patients tend to present with an advanced stage of disease at the time of diagnosis because of anatomical location and ambiguous symptoms. Thus, understanding the molecular events associated with NPC initiation, progression and prognosis may lead to earlier diagnosis, prognosis prediction, and potential therapeutic target.
The human matrix metalloproteinases (MMPs) family is comprised of twenty-four zinc-containing enzymes which share several functional domains. MMPs are often referred to by a descriptive name such as gelatinases (MMP2 and MMP9) and collagenases (MMP1, MMP8, MMP13, and MMP14) [2]. MMPs are involved in many phases of cancer progression, including tumor invasion, metastasis, and angiogenesis [2].
MMP14, the first member of membrane-type MMPs family, is distinguished from soluble MMPs by a C-terminal transmembrane domain and a cytoplasmic tail [3]. In the human genome, MMP14 is encoded by a single copy gene located on chromosome 14 [3]. MMP14 is generally considered pro-invasive and pro-tumorigenic because both the expression and the activity of MMP14 are increased in tumor cells, and because elevated expression of MMP14 directly correlate with enhanced cell invasion and migration [4]. The function of MMP14 is mainly responsible for degrading collagen type I to III and other extracellular matrix proteins [5]. At cell surface, MMP14 binds to tissue inhibitor of metalloprotease-2 and the secreted pro-MMP2, forming a trimolecular complex that results in the activation and release of active MMP2 [4,6]. Meanwhile, MMP14 activity is not restricted to extracellular matrix degradation and is necessary for induction of the epithelial-mesenchymal transition (EMT) [7]. Although MMP14 has been studied in several types of cancer, little is known about its significance in NPC.
The aim of this study was to investigate the roles of MMP14 in NPC. We have shown that MMP14 mRNA and protein were increased in NPC tissue compared with nasopharynx (NP) tissues and MMP14 expression was associated with clinical stages and lymph node metastasis of NPC. MMP14 expression was also increased in NPC cell lines compared with normal nasopharyngeal epithelial cell line. MMP14 was found to regulate NPC cell migration, invasion, and EMT-associated gene expression in vitro.
Materials and methods
Cell culture
NPC cell lines 5-8F, 6-10B, CNE-2, and CNE-1 were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; Gibco, USA). NP69, an immortalized human nasopharyngeal epithelial cell line, was grown in defined-KSFM medium supplemented with epidermal growth factor (EGF; Invitrogen, USA). All cell lines were incubated in a humidified chamber with 5% CO2 at 37°C.
Collection of primary NPC and noncancerous nasopharynx specimens
Fifty-eight fresh NPC tissues and twenty-seven fresh nasopharynx (NP) tissues were obtained at the time of diagnosis before any therapy from Second Hospital of Longyan City and Fujian Medical University. All fresh samples were immediately preserved in liquid nitrogen. The clinical processes were approved by the Ethics Committees of Second Hospital of Longyan City and Fujian Medical University. The patients provided informed consents. The clinical staging was based on the 7th AJCC Cancer Staging Manual.
Analysis of microarray data
Microarray data set (GEO accession number: GSE13597) from 25 NPC samples, and 3 control samples was retrieved from the GEO database. Those differentially expressed genes were screened and identified by Real-time PCR for the following study.
Real-time PCR
To quantitate mRNA expression, total RNA was extracted from clinical samples with RNAiso Plus (Takara, Japan). The isolated total RNA was reverse transcribed using the PrimeScript RT Master Mix (Perfect Real Time) (Takara, Japan) for MMP14, according to manufacturer instructions. The sequence-specific forward and reverse primers sequences for MMP14 mRNA were 5’-GGATACCCAATGCCCATTGGCCA-3’ and 5’-CCTCGGTGCACCATGTTTGGC-3’ re-spectively. Forward and reverse primers sequences for GAPDH mRNA were 5’-GCACCGTCAAGGCTGAGAAC-3’ and 5’-TGGTGAAGACGCCAGTGGA-3’ respectively. qPCR was performed using SYBR Premix Ex TaqTM II (Takara, Japan) on a LightCycler (Roche Diagnostics, USA). Relative quantification of miRNA expression was calculated by using the 2-ΔΔCt method. The raw data were presented as the relative quantity of target mRNA, normalized with GAPDH, and relative to a calibrator sample. All qRT-PCR reactions were performed in triplicate.
Cell line transfection
Plasmid construct were examined according to previous study [8]. To produce a short-hairpin RNA (shRNA) targeting the MMP14 mRNA in position 436 to 456 (GenBank Acc.#NM_004995.2), two DNA sequences: 5’-GATC-CATGCAGAAGTTTTACGGCTTGTTCAAGAGACAA-GCCGTAAAACTTCTGCATTTTTTTGGAAA-3’ and 5’-AGCTTTTCCAAAAAATGCAGAAGTTTTACGGCTTGTCTCTTGAACAAGCCGTAAAACTTCTGCA-TG-3’ were annealed and cloned into the pSilencer 3.1-H1 hygro vector according to the manufacturer’s protocol. A scrambled sequence was provided by Ambion. It consists of the same pSilencer hygro plasmid with an shRNA sequence that is not found in the human, mouse or rat genome database. Full-length cDNA of MMP14 (GenBank Acc.#NM_004995) was amplified by PCR and cloned into the peak12 vector. This expression vector contains the EF-1 alpha promoter and the coding sequence of MT1-MMP was cloned between SpeI and HindIII restriction enzyme sites. 5-8F and 6-10B cells were transfected with the pSilencer or pSilencer-shMMP14 plasmid and pEAK12 or pEAK12-MMP14 plasmid, respectively, using the Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen, USA).
Cell proliferation assays
Cell proliferation was analyzed using MTT assay. Briefly, 1×103 cells were seeded into a 96-well plate with quadruplicate repeat for each condition. The cells were incubated for 1, 2, 3, and 4 days. Twenty microliters of MTT (5 mg/ml) (Sigma, USA) was added to each well and incubated for 4 h. At the end of incubation, the supernatants were removed and 150 μl of DMSO (Sigma, USA) was added to each well. The absorbance value (OD) of each well was measured at 490 nm. Experiments were performed three times.
Cell migration and invasion assays
In vitro cell migration and invasion assays were examined according to previous study [9]. Briefly, 1×105 cells were seeded on a fibronectin-coated polycarbonate membrane insert in a transwell apparatus (Corning, Corning, USA). After the cells were incubated for 12 h, Giemsa-stained cells adhering to the lower surface were counted under a microscope in five predetermined fields (100×). For the cell invasion assay, the procedure was similar to the cell migration assay, except that the transwell membranes were pre-coated with 24 mg/ml Matrigel (Corning, USA).
Western blot
Western blot was carried out according as described [8] with anti-MMP14, E-Cadherin, Vimentin, Snail, Slug, ZEB1 (1:1000; Cell signaling technology, USA), and anti-Twist (1:1000; Abcam, USA). HRP-conjugated anti-rabbit/mouse IgG antibody was used as the secondary antibody (1:2000; Cell signaling technology, USA). Signals were detected using enhanced chemiluminescence reagents (Pierce, USA).
Statistical analysis
All data were analyzed for statistical significance using SPSS 13.0 software. Two-tailed Student’s t test was used for comparisons of two independent groups including examinations of cell migration and invasion assays, relationships between MMP14 expression levels and clinicopathologic characteristics, and differences of MMP14 expression between NPC and NP tissues). One-way ANOVA was used to determine cell growth in vitro. A P-value of less than 0.05 was considered statistically significant.
Results
MMP14 is increased in NPC tissue
From our microarray data, MMP14 was highly expressed in NPC tissues compared with NP tissues with an average of 2.40 folds (Figure 1A). Furthermore, Using real-time PCR to measure the expression of MMP14 transcripts, we found that the MMP14 expression level was significantly increased with an average increase of 3.58-fold in NPC tissue in comparison to NP tissue (P<0.001, Figure 1B). Meanwhile, The MMP14 protein expression was detected by western blot, and increased expression of MMP14 was observed in NPC tissue compared to NP tissue (Figure 1F).
Figure 1.
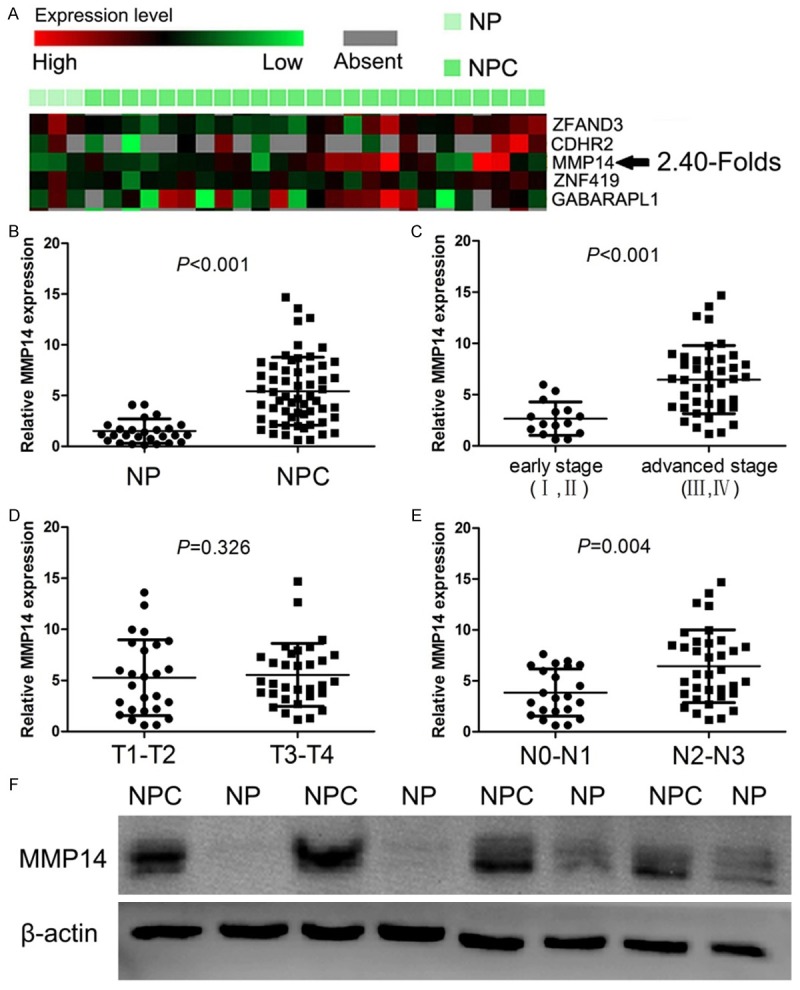
Expression of MMP14 in NPC tissues. A. Increased MMP14 expression was shown in NPC and NP samples by microarray data analysis of GSE13597 data set retrieved from the GEO database. B. The mRNA expression of MMP14 was increased in NPC. C-E. Relationship between clinicopathological characteristics and expression of MMP14 in NPC patients. F. The protein expression of MMP14 was increased in NPC than those in NP.
Relationship between clinicopathological characteristics and expression of MMP14 in NPC patients
The relationship between clinicopathological characteristics and MMP14 expression levels in patients with NPC were analyzed (Figure 1C-E). We found that MMP14 was positively associated with clinical stage (I-II vs. III-IV, P<0.001) and N classification (N0-N1 vs. N2-N3, P=0.004) in NPC. However, we did not find any significant association of MMP14 expression levels with patient’s gender, age, and T classification.
Expression of MMP14 in NPC cancer cell lines
We first analyzed the expression level of MMP14 in NP cell line and NPC cell lines with different degrees of differentiation and metastatic ability including CNE-1 (high differentiation), CNE-2 (low differentiation), 5-8F (high metastatic ability), 6-10B (low metastatic ability) (Figure 2A). We observed that MMP14 expression was markedly higher in NPC cells compared with NP cells, and relatively higher in the cell with high metastatic ability compared with the cell with low metastatic ability, suggesting that MMP14 expression may be associated with the metastatic ability of NPC cells. Based on this expression pattern, we therefore chose 5-8F cell for the following loss-of-function studies and 6-10B for the following gain-of-function studies.
Figure 2.
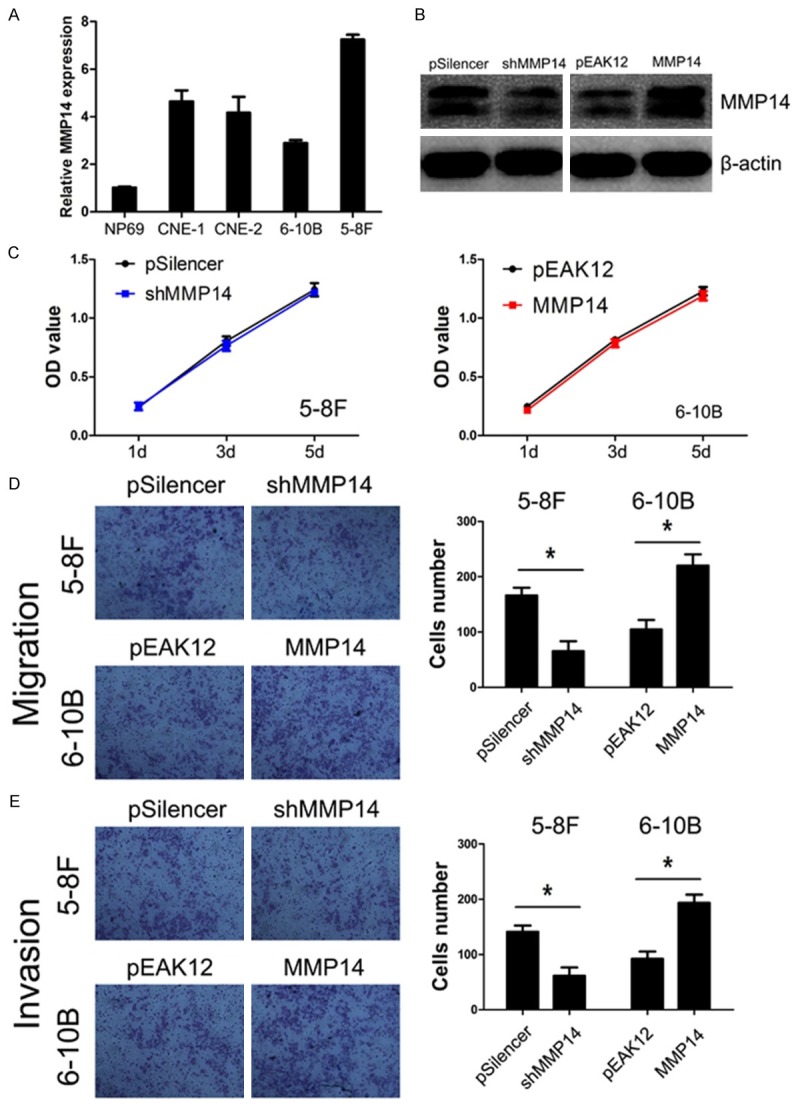
The effect of MMP14 expression on proliferation, migration, and invasion capacities of NPC cell in vitro. A. MMP14 expression is elevated in NPC cell lines. B. Efficiencies of plasmids were distinguished by western blot in NPC cells. C. MMP14 expression had no effect on the proliferation of NPC cells. D, E. MMP14 expression regulated cells migration and invasion in NPC.
Knockdown of MMP14 inhibits the NPC cells migration and invasion
We used shRNA to generate a stable MMP14 knockdown in the 5-8F cell line, which is the highest MMP14 expression cell line in the four NPC cell lines (Figure 2A). The transfection efficiency was confirmed using western blot. As shown in Figure 2B, the 5-8F cells that had been transfected with the MMP14 shRNA plasmid displayed significantly decreased MMP14 expression at protein levels compared with the control cells.
The effects of MMP14 knockdown on cell growth was assayed by MTT. As shown in Figure 2C, MMP14 knockdown had no effect on the growth of 5-8F cells. Furthermore, we examined whether MMP14 knockdown could inhibit the migratory and invasive capacities of 5-8F cells. The effect of MMP14 knockdown on cell migration and invasion were measured by transwell migration and invasion assays, we found knocking down MMP14 expression significantly reduced the abilities of migration and invasion in 5-8F cells (both P<0.001, Figure 2D, 2E).
Overexpression of MMP14 promotes the NPC cells migration and invasion
The 6-10B cell line, which was the lowest MMP14 expression cell line in the four NPC cells (Figure 2A), had been transfected with the MMP14 expression plasmid displayed significantly increased MMP14 expression at protein levels compared with the vector cell lines (Figure 2B).
Increased MMP14 expression had no effect on NPC cell growth (Figure 2C), but significantly promoted the migration and invasion of 6-10B cells (both P<0.001, Figure 2D, 2E), which was contrary to the phenotype of migration and invasion induced by knocking down MMP14 expression.
MMP14 regulates the expression of EMT-associated genes
To further study the mechanism by which MMP14 regulates cell migration and invasion, we examined protein levels EMT-associated genes in NPC cells with suppressed and increased MMP14 expression respectively (Figure 3). We found that knockdown of MMP14 decreased the expression of Vimentin, Twist, and ZEB1 and increased E-cadherin expression. On the contrary, overexpression of MMP14 promoted the activation of Vimentin, Twist, and ZEB1 and suppressed the expression of E-cadherin. However, the expression of Snail and Slug were not affected by MMP14.
Figure 3.
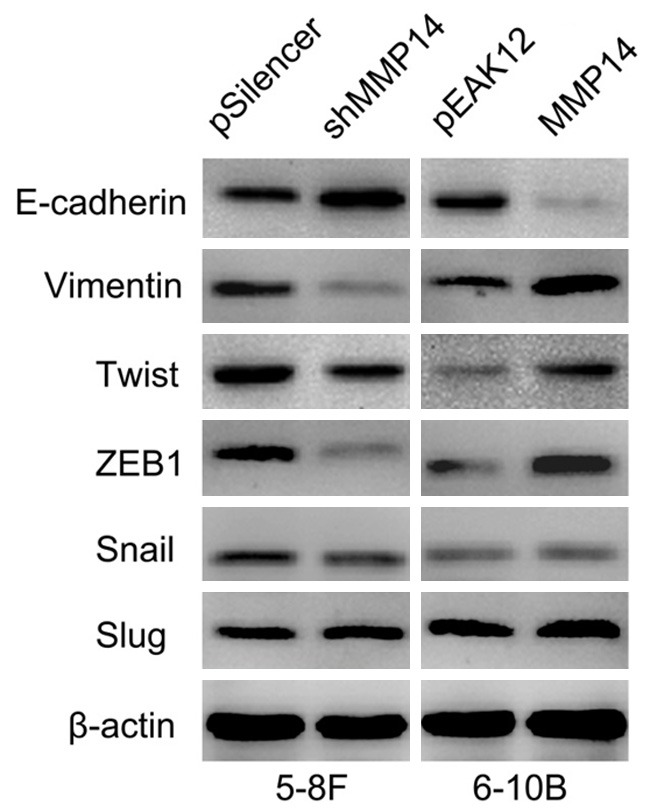
MMP14 regulates the expression of EMT-associated genes. Knockdown of MMP14 decreased the expression of Vimentin, Twist, and ZEB1 and increased E-cadherin expression. On the contrary, overexpression of MMP14 promoted the activation of Vimentin, Twist, and ZEB1 and suppressed the expression of E-cadherin. However, the expression of Snail and Slug were not affected by MMP14.
Discussion
Matrix metalloproteinase-14 (MMP14) was the first membrane type matrix metalloproteinase discovered, and hence is also referred to as membrane type 1-matrix metalloproteinase (MT1-MMP). MMP14 has been suggested to involve in many biological processes, such as proliferation, invasion, angiogenesis, and basement membrane remodeling [10]. Generally, MMPs are produced by tissues as inactive zymogens and require further activation. However, MMP14 does not require additional activation, due to its capacity to be presented on the cell membrane in its active form [4].
The high expression of MMP14 was observed in human lung cancer [11], breast cancer [12], colon cancer [12], prostate cancer [13], and glioblastomas [14] in comparison to benign tumor or normal tissue, but little is known about the role of MMP14 in NPC. In a recent microarray analysis, we found significantly high levels of MMP14 in squamous cell lung cancer compared to normal lung tissues [15]. Similar to a microarray analysis performed by Bose et al. (GSE13597) [16], we found MMP14 was higher level in NPC samples compared with normal nasopharyngeal samples. In addition, we further presented evidence to verify the mRNA and protein expression levels of MMP14 were higher in NPC tissues than those in normal nasopharyngeal epithelial tissues through Real-time PCR and western blot. This expression pattern was similar to the microarray data.
Recent study indicated that MMP14 overexpression was significantly correlated with N classification (lymph node metastasis), M classification (distance metastasis), and clinical stages in patients with non-small cell lung cancer [11]. In small cell lung cancer, Michael et al. also demonstrated that increased MMP14 expression significantly correlated with clinical stages (limited and extensive) [17]. Similar to reports in gastric cancer, He et al. and Peng et al. demonstrated that the MMP14 expression related to serosa invasion and clinical stages in gastric cancer patients [18,19]. In our results, we found that MMP14 expression was significantly increased in advanced clinical stages compared with early clinical stages, and in high N classification compared with low N classification. Our results implicated that MMP14 plays significant roles in NPC progression through promoting NPC cells invasion and metastasis.
In order to explore the role of MMP14 in NPC cells proliferation, migration, and invasion, the experiments in vitro were performed. Our results indicated that the MMP14 expression had no effect on NPC cell proliferation, but knocking down MMP14 expression significantly reduced the abilities of migration and invasion and increased MMP14 expression significantly promoted the migration and invasion in NPC cells. Similarly, present study indicated that a selective MMP-14 inhibitor reduced cancer cell motility and tumor growth in human melanoma, fibrosarcoma, tongue squamous cell carcinoma, oral carcinoma, and breast carcinoma cell line [20]. Moreover, increased plasma membrane localization of MMP14 can facilitate prostate cancer cell invasion and metastasis [21].
MMP14 was originally known as a tumor specific activator of MMP2 [6,22] and is now identified to induce the epithelial-mesenchymal transition (EMT). EMT is a critical process by which epithelial cells lose their epithelial morphology and acquire a mesenchymal phenotype, characterized by the decrease of epithelial proteins such as E-cadherin, and the increase of mesenchymal proteins such as vimentin [23,24]. Several transcription factors have been identified to induce the process of EMT by inhibiting E-cadherin, such as Twist, ZEB1, Snail, and Slug [25-28]. It is widely accepted that EMT plays a significant role during tumor invasion and metastasis, and aggressive cancer cells often present with a loss of epithelial characteristics and acquire a mesenchymal phenotype [29]. In Oral squamous cell carcinoma, Yang et al. indicated that overexpression of MMP14 induces EMT and results in the acquisition of cancer stem cell-like properties [7]. Similar to our study in NPC, we found MMP14 markedly regulated the expression of EMT-associated genes including E-cadherin, vimentin, Twist, and ZEB1, but had no effect on Snail and Slug expression. These results consistently suggested that MMP14 play an important role in regulating the process of EMT.
In conclusion, our studies suggested that the expression of MMP14 is significantly increased in NPC cell lines and clinical sample and correlated with clinical stage and lymph node metastasis of NPC patients. Furthermore, The MMP14 expression significantly regulated the cell migration and invasion in vitro and the expression of EMT-associated genes.
Acknowledgements
This research was supported by the grants from Foundation of Longyan Science and Technology Program (2015LY, 2013LY57) and The Foundation of Ningde Science and Technology Program (No. 20130157).
Disclosure of conflict of interest
None.
References
- 1.Cho WC. Nasopharyngeal carcinoma: molecular biomarker discovery and progress. Mol Cancer. 2007;6:1. doi: 10.1186/1476-4598-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strongin AY. Proteolytic and non-proteolytic roles of membrane type-1 matrix metalloproteinase in malignancy. Biochim Biophys Acta. 2010;1803:133–141. doi: 10.1016/j.bbamcr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 4.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 5.Ulasov I, Yi R, Guo D, Sarvaiya P, Cobbs C. The emerging role of MMP14 in brain tumorigenesis and future therapeutics. Biochim Biophys Acta. 2014;1846:113–120. doi: 10.1016/j.bbcan.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Nishida Y, Miyamori H, Thompson EW, Takino T, Endo Y, Sato H. Activation of matrix metalloproteinase-2 (MMP-2) by membrane type 1 matrix metalloproteinase through an artificial receptor for proMMP-2 generates active MMP-2. Cancer Res. 2008;68:9096–9104. doi: 10.1158/0008-5472.CAN-08-2522. [DOI] [PubMed] [Google Scholar]
- 7.Yang CC, Zhu LF, Xu XH, Ning TY, Ye JH, Liu LK. Membrane Type 1 Matrix Metalloproteinase induces an epithelial to mesenchymal transition and cancer stem cell-like properties in SCC9 cells. BMC Cancer. 2013;13:171. doi: 10.1186/1471-2407-13-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perentes JY, Kirkpatrick ND, Nagano S, Smith EY, Shaver CM, Sgroi D, Garkavtsev I, Munn LL, Jain RK, Boucher Y. Cancer cell-associated MT1-MMP promotes blood vessel invasion and distant metastasis in triple-negative mammary tumors. Cancer Res. 2011;71:4527–4538. doi: 10.1158/0008-5472.CAN-10-4376. [DOI] [PubMed] [Google Scholar]
- 9.Zhao B, Han H, Chen J, Zhang Z, Li S, Fang F, Zheng Q, Ma Y, Zhang J, Wu N, Yang Y. MicroRNA let-7c inhibits migration and invasion of human non-small cell lung cancer by targeting ITGB3 and MAP4K3. Cancer Lett. 2014;342:43–51. doi: 10.1016/j.canlet.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Pahwa S, Stawikowski MJ, Fields GB. Monitoring and Inhibiting MT1-MMP during Cancer Initiation and Progression. Cancers (Basel) 2014;6:416–435. doi: 10.3390/cancers6010416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YZ, Wu KP, Wu AB, Yang ZC, Li JM, Mo YL, Xu M, Wu B, Yang ZX. MMP-14 overexpression correlates with poor prognosis in non-small cell lung cancer. Tumour Biol. 2014;35:9815–9821. doi: 10.1007/s13277-014-2237-x. [DOI] [PubMed] [Google Scholar]
- 12.Okada A, Bellocq JP, Rouyer N, Chenard MP, Rio MC, Chambon P, Basset P. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci U S A. 1995;92:2730–2734. doi: 10.1073/pnas.92.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Udayakumar TS, Chen ML, Bair EL, Von Bredow DC, Cress AE, Nagle RB, Bowden GT. Membrane type-1-matrix metalloproteinase expressed by prostate carcinoma cells cleaves human laminin-5 beta3 chain and induces cell migration. Cancer Res. 2003;63:2292–2299. [PubMed] [Google Scholar]
- 14.Munaut C, Noel A, Hougrand O, Foidart JM, Boniver J, Deprez M. Vascular endothelial growth factor expression correlates with matrix metalloproteinases MT1-MMP, MMP-2 and MMP-9 in human glioblastomas. Int J Cancer. 2003;106:848–855. doi: 10.1002/ijc.11313. [DOI] [PubMed] [Google Scholar]
- 15.Kettunen E, Anttila S, Seppanen JK, Karjalainen A, Edgren H, Lindstrom I, Salovaara R, Nissen AM, Salo J, Mattson K, Hollmen J, Knuutila S, Wikman H. Differentially expressed genes in nonsmall cell lung cancer: expression profiling of cancer-related genes in squamous cell lung cancer. Cancer Genet Cytogenet. 2004;149:98–106. doi: 10.1016/S0165-4608(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 16.Bose S, Yap LF, Fung M, Starzcynski J, Saleh A, Morgan S, Dawson C, Chukwuma MB, Maina E, Buettner M, Wei W, Arrand J, Lim PV, Young LS, Teo SH, Stankovic T, Woodman CB, Murray PG. The ATM tumour suppressor gene is down-regulated in EBV-associated nasopharyngeal carcinoma. J Pathol. 2009;217:345–352. doi: 10.1002/path.2487. [DOI] [PubMed] [Google Scholar]
- 17.Michael M, Babic B, Khokha R, Tsao M, Ho J, Pintilie M, Leco K, Chamberlain D, Shepherd FA. Expression and prognostic significance of metalloproteinases and their tissue inhibitors in patients with small-cell lung cancer. J. Clin. Oncol. 1999;17:1802–1808. doi: 10.1200/JCO.1999.17.6.1802. [DOI] [PubMed] [Google Scholar]
- 18.He L, Chu D, Li X, Zheng J, Liu S, Li J, Zhao Q, Ji G. Matrix metalloproteinase-14 is a negative prognostic marker for patients with gastric cancer. Dig Dis Sci. 2013;58:1264–1270. doi: 10.1007/s10620-012-2513-9. [DOI] [PubMed] [Google Scholar]
- 19.Peng CW, Wang LW, Fang M, Yang GF, Li Y, Pang DW. Combined features based on MT1-MMP expression, CD11b+ immunocytes density and LNR predict clinical outcomes of gastric cancer. J Transl Med. 2013;11:153. doi: 10.1186/1479-5876-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suojanen J, Salo T, Koivunen E, Sorsa T, Pirila E. A novel and selective membrane type-1 matrix metalloproteinase (MT1-MMP) inhibitor reduces cancer cell motility and tumor growth. Cancer Biol Ther. 2009;8:2362–2370. doi: 10.4161/cbt.8.24.10139. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Wilson MJ, Slaton JW, Sinha AA, Ewing SL, Pei D. Increased aggressiveness of human prostate PC-3 tumor cells expressing cell surface localized membrane type-1 matrix metalloproteinase (MT1-MMP) J Androl. 2009;30:259–274. doi: 10.2164/jandrol.108.006494. [DOI] [PubMed] [Google Scholar]
- 22.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metallopro-teinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 23.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno-Bueno G, Cubillo E, Sarrio D, Peinado H, Rodriguez-Pinilla SM, Villa S, Bolos V, Jorda M, Fabra A, Portillo F, Palacios J, Cano A. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial-mesenchymal transition. Cancer Res. 2006;66:9543–9556. doi: 10.1158/0008-5472.CAN-06-0479. [DOI] [PubMed] [Google Scholar]
- 26.Castro Alves C, Rosivatz E, Schott C, Hollweck R, Becker I, Sarbia M, Carneiro F, Becker KF. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol. 2007;211:507–515. doi: 10.1002/path.2138. [DOI] [PubMed] [Google Scholar]
- 27.Lee KW, Kim JH, Han S, Sung CO, Do IG, Ko YH, Um SH, Kim SH. Twist1 is an independent prognostic factor of esophageal squamous cell carcinoma and associated with its epithelial-mesenchymal transition. Ann Surg Oncol. 2012;19:326–335. doi: 10.1245/s10434-011-1867-0. [DOI] [PubMed] [Google Scholar]
- 28.Huang MT, Wei PL, Liu JJ, Liu DZ, Huey-Chun H, An J, Wu CC, Wu CH, Ho YS, Yang YY, Chang YJ. Knockdown of thrombomodulin enhances HCC cell migration through increase of ZEB1 and decrease of E-cadherin gene expression. Ann Surg Oncol. 2010;17:3379–3385. doi: 10.1245/s10434-010-1163-4. [DOI] [PubMed] [Google Scholar]
- 29.Scheel C, Weinberg RA. Phenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells? Int J Cancer. 2011;129:2310–2314. doi: 10.1002/ijc.26311. [DOI] [PMC free article] [PubMed] [Google Scholar]


