Abstract
Resistance to single or multiple chemotherapeutic drugs is a major obstacle in breast cancer therapy. Recent studies have suggested that GPR30 is implicated in mediating cancer cell proliferation. The aim of this study was to examine the anti-tumor effects of the GPR30 antagonist G15 in breast cancer. We found that low concentrations of G15 had little effect on breast cancer cell viability, but could enhance doxorubicin sensitivity in MDA-MB-231 and MCF-7 cells with epithelial phenotypes. In addition, G15 prevented epithelial breast cancer cells undergoing epithelial-mesenchymal transition (EMT) after doxorubicin induction. Moreover, downregulation of GPR30 suppressed the EMT in breast cancer cells. These results support that G15 enhanced doxorubicin sensitivity and prevented the EMT in epithelial breast cancer cells by inhibiting GPR30 expression.
Keywords: Breast cancer, GPR30, G15, epithelial-mesenchymal transition
Introduction
Breast cancer is currently one of the most common malignant tumors among women all over the world. It has become the leading cause of cancer death among females, accounting for 23% of the total cancer cases and 14% of the cancer deaths [1,2]. At present, combination chemotherapy serves as one of the important adjuvant therapies for breast cancer after surgery in the clinic [3]. Although chemotherapy improves survival rates in the adjuvant treatment, nearly 50% of treated patients will relapse due to the acquired resistance against the anticancer drugs [4,5]. Therefore, improving the chemotherapy sensitivity is imperative for optimized treatment for breast cancer.
It has been acknowledged that the pathogenesis of breast cancer is a multistep process regulated by aberrantly protein expression and alterations of morphological and molecular features during malignant progression [6-8]. Epithelial-mesenchymal transition (EMT) is a complex, reversible process which induces epithelial cells to transform to mesenchymal phenotype [9,10]. Although accumulating evidences suggest that EMT plays an important role in regulating the chemoresistance properties of breast cancer, but the molecular mechanism still remains elusive [11-13]. The G-protein coupled receptor 30 (GPR30), a seven-transmembrane domain protein, was recently identified as a novel estrogen receptor structurally distinguished from the classic ERα and ERβ [14,15]. In keratinocytes, GPR30 enhances cell proliferation by promoting cyclin D expression and inhibits oxidative stress-induced apoptosis by stimulating Bcl-2 expression [16,17]. Further-more, GPR30 has been shown to regulate the proliferative effects of tamoxifen and estrogen in endometrial cancer cells [18]. A recent study has demonstrated that GPR30 activates Mitogen-activated protein (MAP) kinases and contributes to tamoxifen resistance development in breast cancer [19]. G15 is a tetrahydro-3H-cyclopenta[c]quinoline analog that inhibits GPR30 with high affinity. It has been reported to be active against proliferation of uterine epithelial cells and induce apoptosis and autophagy in human oral squamous carcinoma cells [20,21]. In the present study, we aimed to evaluate the effects of G15 on the chemoresistance of breast cancer cells to doxorubicin.
Materials and methods
Cell culture and reagents
Human breast cancer cell lines MCF-7, MDA-MB-231 and Bcap-37 were purchase d from the ATCC (Manassas, VA, USA) and cultured in DMEM (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS and 1% penicillin/streptomycin. All cells were maintained at 37°C in 5% CO2 incubator. Doxorubicin and G15 were purchased from Sigma-Aldrich (St. Louis, MO, USA). The GPR30 siRNA was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
CCK-8 assay
Breast cancer cells were seeded onto 96-well plates at 3.0 × 103 cells/well. The medium was replaced with the corresponding serum-free medium for 24 h to synchronize the cell cycle, then serum-free medium was replaced with complete medium containing the drugs at the indicated concentrations for 48 h. Then 10 µL/well CCK8 solution (Dojindo, Kumamoto, Japan) was added, the plates incubated for 3 h, and absorbance was measured at 450 nm using an MRX II microplate reader (Dynex, Chantilly, VA, USA).
EdU incorporation assay
Cell viability was calculated as a percentage of untreated control. Measurement of inhibitive rate of cell proliferation was carried out using a Click-iT EdU Imaging Kit (Invitrogen, Carlsbad, CA, USA) according to the procedure provided by the manufacture.
siRNA transfection
Breast cancer cells were transfected with GPR30 siRNA or negative control siRNA (Invitrogen) using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instruction. The transfection medium (Opti-MEM; Gibco, USA) was replaced with complete medium 12 h after transfection, and the cells were incubated for the indicated times.
Immunofluorescence
Breast cancer cells were seeded into 48-well plates at 6.0 × 103 cells/well. Cells were fixed with 4% formaldehyde for 15 min, washed with PBS, treated with 5% BSA for 30 min at room temperature, and incubated with mouse anti-human vimentin or anti-human E-cadherin primary antibodies (Cell Signaling Technology, USA) at 4°C overnight. The cells were incubated with goat anti-mouse FITC-conjugated secondary antibody (Abcam) at 4°C for 2 h, incubated with DAPI (Sigma-Aldrich) for 2 min at room temperature, washed twice with PBS, and observed using an inverted fluorescence microscope (Olympus, Tokyo, Japan).
Western blot analysis
Breast cancer cells were lysed in 50 μl cell lysis buffer (Cell Signaling, Danvers, MA, USA) containing protease inhibitors (Sigma, USA). Whole cell lysates were prepared and fractioned were separated by 10% SDS-PAGE and proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were then incubated with anti-GPR30 (Abcam, Cambridge, USA), anti-E-cadherin, or anti-Vimentin (Cell Signaling Technology, USA) antibodies at 4°C overnight. The membranes were washed three times with TBST and then incubated with the appropriate HRP-conjugated secondary antibodies for 1 h at room temperature. Protein expression was detected by chemiluminescence (GE Healthcare, Piscataway, NJ, USA).
Statistical analysis
Each experiment was performed in triplicate, and repeated at least three times. All the data were presented as means ± SD and treated for statistics analysis by SPSS program. Comparison between groups was made using ANOVA and statistically significant difference was defined as P < 0.05.
Results
Different doxorubicin sensitivity in breast cancer cells
Firstly, we performed CCK-8 assay to examine the sensitivity of different kinds of breast cancer cell types (MCF-7, MDA-MB-231 and Bcap-37) to doxorubicin. Compared with Bcap-37 cells, MCF-7 and MDA-MB-231 cells are more sensitive to doxorubicin (Figure 1A). Western blot was used to measure the expression of GPR30 in breast cancer cell lines. Interestingly, we observed the highest expression of GPR30 in Bcap-37 cells, and the lowest expression in MDA-MB-231 cells (Figure 1B), suggesting that GPR30 may be involved in the chemoresistance to doxorubicin.
Figure 1.
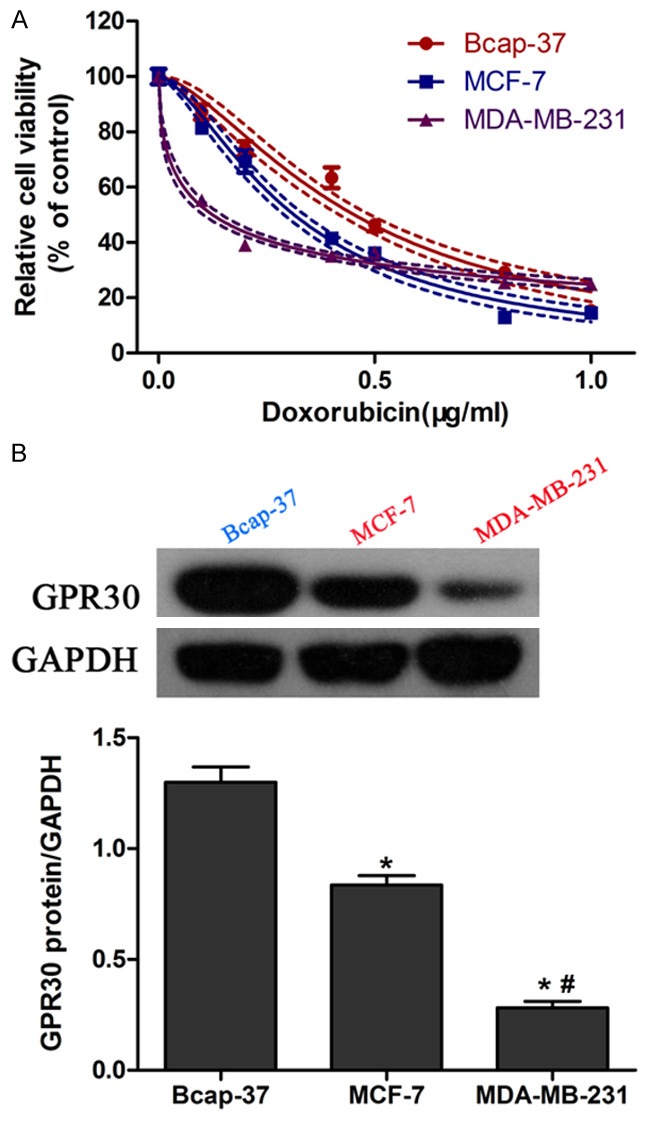
Different doxorubicin sensitivity in breast cancer cells. Three breast cancer cell lines including MCF-7, MDA-MB-231 and Bcap-37 were incubated with doxorubicin for 48 h. Cell viability was measured using CCK-8 method (A). Western blot was performed to determine GPR30 expression in MCF-7, MDA-MB-231 and Bcap-37 cells (B). *P < 0.05, compared with Bcap-37; #P < 0.05, compared with MCF-7.
Low concentrations of G15 had little cytotoxicity on breast cancer cells
A series of G15 concentrations ranging from 0~5.0 (µM) were incubated with three breast cells lines (MCF-7, MDA-MB-231, Bcap-37) and data from CCK-8 assay showed that G15 exerted little cytotoxicity in cancer cells between 0 and 0.625 µM. However, higher concentrations of G15 (1.25, 2.5, 5.0 µM) significantly inhibited the viability of the three cell lines (Figure 2A-C). Therefore, 0.625 µM G15 was used for further coadministrtion with doxorubicin.
Figure 2.
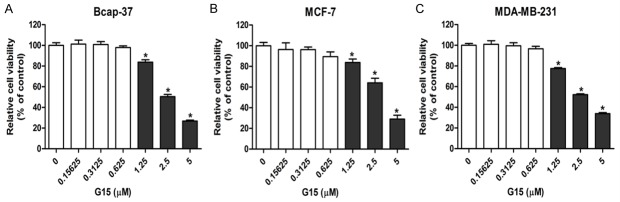
Low concentrations of G15 had little cytotoxicity on breast cancer cells. Three breast cancer cell lines including Bcap-37 (A), MCF-7 (B), and MDA-MB-231 (C) were incubated with different concentrations of G15 for 48 h. The CCK-8 values of the treated breast cancer cells were normalized to the control group with the absence of G15. *P < 0.05.
G15 enhanced doxorubicin sensitivity in epithelial breast cancer cells
To evaluate the synergistic cytotoxic effects of doxorubicin combined with G15, we used CCK-8 assay to measure cell viability treated with doxorubicin alone or doxorubicin plus G15 for 48 h. Surprisingly, the doxorubicin sensitivity was reduced in the mesenchymal Bcap-37 cells after co-administration with G15 (Figure 3A). By contrast, the breast cancer cells with epithelial features (MDA-MB-231 and MCF-7) showed a higher sensitivity to doxorubicin in combined treatment group (Figure 3B and 3C). In addition, EDU incorporation assay was used to measure cancer cell proliferation. As shown in Figure 3D, doxorubicin and G15 co-treatment resulted no significant differences in Bcap-37 cell growth. But the proliferation of MCF-7 (Figure 3E) and MDA-MB-231 (Figure 3F) cells was reduced after cotreatment with doxorubicin and G15. These results suggested that G15 could enhance the cytotoxicity of doxorubicin in epithelial breast cancer cells.
Figure 3.
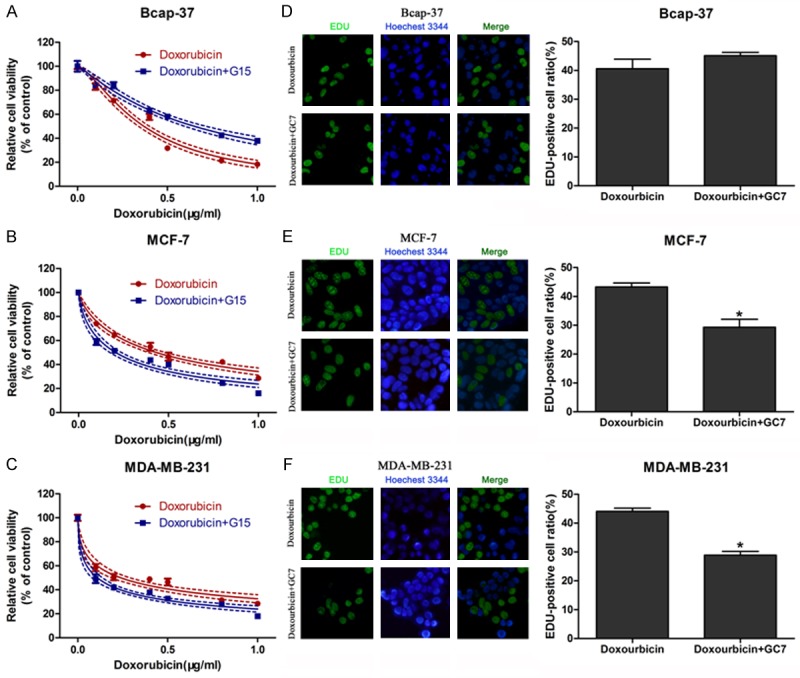
G15 enhanced doxorubicin sensitivity in epithelial breast cancer cells. G15 (0.625 µM) significantly reduced the cytotoxicity of doxorubicin in the mesenchymal Bcap-37 cells (A), but increased the doxorubicin sensitivity in epithelial MCF-7 (B) MDA-MB-231 (C) cells. Photomicrographs and bar charts depict the EdU staining and relative EdU-positive ratio, respectively, of Bcap-37 (D), MCF-7 (E) and MDA-MB-231 (F) cells after treatment with doxorubicin or doxorubicin plus G15 for 48 h. *P < 0.05.
G15 regulated doxorubicin-induced EMT in epithelial breast cancer cells
In order to investigate whether doxorubicin induced EMT in breast cancer cells, the expression levels of epithelial/mesenchymal markers in breast cancer cells were examined. Western blot analysis showed that doxorubicin treatment led to significant down-regulation of E-cadherin and up-regulation of Vimentin in MCF-7, MDA-MB-231, Bcap-37 cells (Figure 4A). These results suggested that doxorubicin could induce EMT in breast cancer cells. In addition, combined treatment with G15 reversed such changes in MDA-MB-231 and MCF-7 cells (epithelial phenotype), but not in Bcap-37 cells (mesenchymal phenotype). Immunofluorescent staining also showed similar results consistent with the western blotting analysis (Figure 4B). These data suggested that G15 could reverse the doxorubicin-induced EMT in breast cancer cells with epithelial features.
Figure 4.
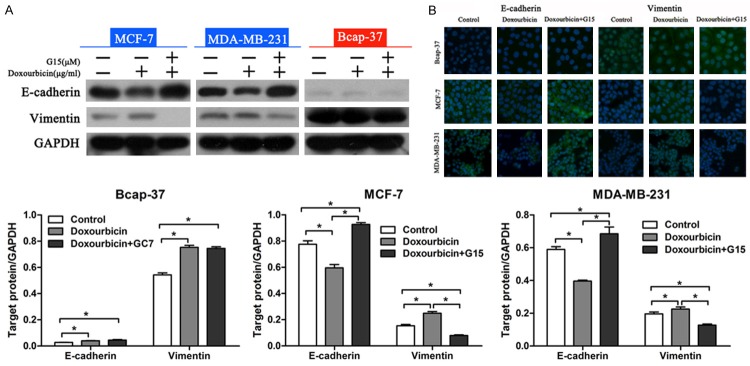
G15 prevented EMT in epithelial breast cancer cells. A. Western blot analysis of E-cadherin and Vimentin expression in breast cancer cells. Relative protein expression in Bcap-37, MCF-7, and MDA-MB-231 cells was quantified by band density with GAPDH served as control. *P < 0.05. B. EdU staining of Bcap-37, MCF-7, and MDA-MB-231 cells after treatment with doxorubicin or doxorubicin plus G15 for 48 h.
G15 enhanced doxorubicin sensitivity via inhibition of GPR30
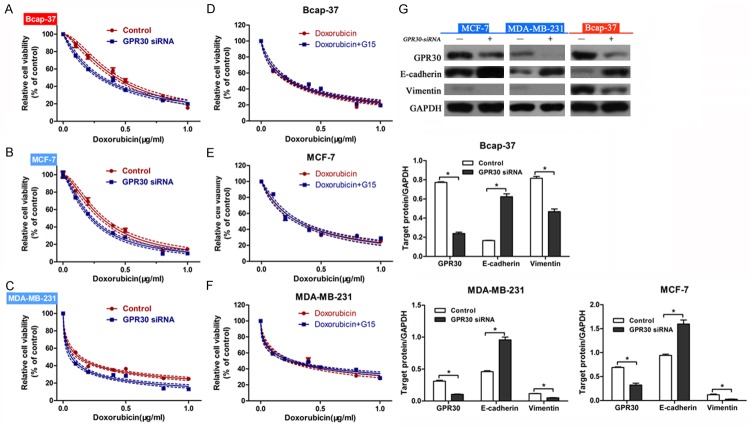
In order to investigate the mechanism by which G15 enhanced doxorubicin sensitivity, RNAi was applied to knockdown the expression of GPR30 in breast cancer cells. We found that downregulation of GPR30 increased the sensitivity to doxorubicin in Bcap-37 (Figure 5A), MCF-7 (Figure 5B) and MDA-MB-231 (Figure 5C) cells. We then treated these transfected cells with doxorubicin alone, or doxorubicin combined with G15, and carried out CCK-8 cell viability assays. Results showed that there were little changes in the doxorubicin sensitivity between two groups (Figure 5D-F), suggesting that GPR30 was involved in the chemosensitivity. In order to verify whether GPR30 was a key factor in G15 regulation of EMT, we transfected GPR30 siRNA into breast cancer cells without G15 treatment. Western blot analysis indicated that GPR30 knockdown led to upregulation of E-cadherin and downregulation of vimentin in cancer cells (Figure 5G). Taken together, these data demonstrated that downregulation of GPR30 could suppress EMT in breast cancer cells.
Figure 5.
G15 enhanced doxorubicin sensitivity through inhibition of GPR30. Knockdown of GPR30 enhanced the doxorubicin sensitivity of Bcap-37 (A), MCF-7 (B), and MDA-MB-231 (C) cells. After transfection with GPR30 siRNA, CCK-8 cell viability assays were performed to determine the chemosensitivity of Bcap-37 (D), MCF-7 (E), and MDA-MB-231 (F) cells treated with doxorubicin alone or doxorubicin plus G15. (G) Western blot analysis of E-cadherin and Vimentin expression in GPR30 siRNA transfected cells. Relative protein expression in Bcap-37, MCF-7, and MDA-MB-231 cells was quantified by band density with GAPDH served as control. *P < 0.05.
Discussion
Accumulating evidences suggest that the acquired drug resistance to traditional chemotherapeutics has become a major obstacle to the triumph of chemotherapy [22,23]. In this study, we examined whether G15, a GPR30 antagonist, could enhance the cytotoxicity of doxorubicin in human breast cancer cells. Interestingly, our data showed that G15 increased the doxorubicin sensitivity in epithelial breast cancer cells (MDA-MB-231 and MCF-7) but not in mesenchymal cells (Bcap-37).
GPR30, a novel estrogen receptor at the plasma membrane, has been proposed as a candidate for triggering a broad range biological activities, including mobilization of intracellular calcium stores, stimulation of adenylyl cyclase, and activation of mitogen-activated protein kinase and phosphoinositide 3-kinase signaling pathways [24,25]. GPR30 is recognized as a promising target for cancer treatment because it is expressed in many types of cancer, such as breast, endometrial and ovarian cancer [26-28]. In our study, results showed that the doxorubicin sensitivity and GPR-30 expression varied in different cancer cells. Specifically, Bcap-37 cells with highest expression of GPR30 exhibited lowest sensitivity to doxorubicin, while MDA-MB-231 cells with lowest expression of GPR30 showed greatest sensitivity. These results implied that GPR30 may be involved in the drug resistance in breast cancer cells.
G15, a GPR30-specific antagonist, has been investigated in several cancers. Incubation with G15 promotes cell death, G2/M arrest as well as autophagy in human oral squamous carcinoma cells [21]. Combination treatment with the G15 and tamoxifen induces cytocidal action in vitro and anti-tumor progression in vivo, suggesting that GPR30 might be a useful target in developing better treatments for breast cancer patients [19]. In the present research, we found that G15 co-treatment enhanced the cytotoxicity of doxorubicin in epithelial breast cancer cells (MDA-MB-231 and MCF-7) but not in mesenchymal cells (Bcap-37).
Many studies have shown that EMT plays a key role in carcinogenicity, metastasis, progression and acquired chemoresistance in many kinds of cancers, including breast cancer [29,30]. During EMT, epithelial markers such as Ecadherin decrease, while mesenchymal markers such as vimentin increase [31]. In our study, we found that doxorubicin treatment induced EMT in breast cancer cells. However, combined administration with G15 reversed EMT in MDA-MB-231 and MCF-7 cells (epithelial phenotype), but not in Bcap-37 cells (mesenchymal phenotype). These results demonstrated that G15 regulated EMT in breast cancer cells with epithelial phenotype.
The etiology of breast cancer involves a complex interplay of various factors, of which the accumulation of oncogenes and loss of tumor repressors are crucial events in the initiation and progression of breast cancer [32,33]. EMT has been recognized as a critical procedure regulating the pathogenesis of breast cancer [34]. In order to explore the molecular mechanism by which G15 modulated EMT, GPR30 was downregulated in breast cancer cells. We found that depletion of GPR30 increased the doxorubicin sensitivity. However, there were no significant differences in the doxorubicin sensitivity with or without G15, demonstrating that G15 enhanced doxorubicin sensitivity via inhibition of GPR30. In addition, our results indicated that GPR30 knockdown suppressed EMT in tumor cells. These data suggested that GPR30 might be an upstream factor regulating EMT and thereby plays an important role in EMT phenotype changes.
In conclusion, the present study demonstrated that G15 prevented epithelial breast cancer cells from undergoing EMT changes via inhibition of GPR30. Furthermore, these results suggested that GPR30 plays a key role in EMT and drug resistance in breast cancer. Therefore, inhibition of GPR30 could enhance the anti-tumor drugs sensitivity and prevent EMT of breast cancer cells. These findings may provide a novel therapeutic strategy for breast cancer therapy.
Acknowledgements
This study was supported by Zhejiang province natural science fund (No. LY13H160006); co-constructed plan by Ministry of Health and Zhejiang Province (No. 201343278); Traditional Chinese medicine scientific research fund project of Zhejiang province (No. 2013ZA076; No. 2012ZA079).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Engelhardt EG, Garvelink MM, de Haes JH, van der Hoeven JJ, Smets EM, Pieterse AH, Stiggelbout AM. Predicting and communicating the risk of recurrence and death in women with early-stage breast cancer: a systematic review of risk prediction models. J. Clin. Oncol. 2014;32:238–250. doi: 10.1200/JCO.2013.50.3417. [DOI] [PubMed] [Google Scholar]
- 3.Miles DW, Dieras V, Cortes J, Duenne AA, Yi J, O’Shaughnessy J. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann Oncol. 2013;24:2773–2780. doi: 10.1093/annonc/mdt276. [DOI] [PubMed] [Google Scholar]
- 4.de Ronde JJ, Lips EH, Mulder L, Vincent AD, Wesseling J, Nieuwland M, Kerkhoven R, Vrancken PM, Sonke GS, Rodenhuis S, Wessels LF. SERPINA6, BEX1, AGTR1, SLC26A3, and LAPTM4B are markers of resistance to neoadjuvant chemotherapy in HER2-negative breast cancer. Breast Cancer Res Treat. 2013;137:213–223. doi: 10.1007/s10549-012-2340-x. [DOI] [PubMed] [Google Scholar]
- 5.Croker AK, Allan AL. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44(+) human breast cancer cells. Breast Cancer Res Treat. 2012;133:75–87. doi: 10.1007/s10549-011-1692-y. [DOI] [PubMed] [Google Scholar]
- 6.Hergovich A. YAP-Hippo signalling downstream of leukemia inhibitory factor receptor: implications for breast cancer. Breast Cancer Res. 2012;14:326. doi: 10.1186/bcr3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cellurale C, Girnius N, Jiang F, Cavanagh-Kyros J, Lu S, Garlick DS, Mercurio AM, Davis RJ. Role of JNK in mammary gland development and breast cancer. Cancer Res. 2012;72:472–481. doi: 10.1158/0008-5472.CAN-11-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartman AR, Kaldate RR, Sailer LM, Painter L, Grier CE, Endsley RR, Griffin M, Hamilton SA, Frye CA, Silberman MA, Wenstrup RJ, Sandbach JF. Prevalence of BRCA mutations in an unselected population of triple-negative breast cancer. Cancer. 2012;118:2787–2795. doi: 10.1002/cncr.26576. [DOI] [PubMed] [Google Scholar]
- 9.Lee EK, Jeon WK, Chae MY, Hong HY, Lee YS, Kim JH, Kwon JY, Kim BC, Park SH. Decreased expression of glutaredoxin 1 is required for transforming growth factor-beta1-mediated epithelial-mesenchymal transition of EpRas mammary epithelial cells. Biochem Biophys Res Commun. 2010;391:1021–1027. doi: 10.1016/j.bbrc.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Masui T, Ota I, Yook JI, Mikami S, Yane K, Yamanaka T, Hosoi H. Snail-induced epithelial-mesenchymal transition promotes cancer stem cell-like phenotype in head and neck cancer cells. Int J Oncol. 2014;44:693–699. doi: 10.3892/ijo.2013.2225. [DOI] [PubMed] [Google Scholar]
- 11.Son H, Moon A. Epithelial-mesenchymal Transition and Cell Invasion. Toxicol Res. 2010;26:245–252. doi: 10.5487/TR.2010.26.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon JE, Jung WH, Koo JS. Molecules involved in epithelial-mesenchymal transition and epithelial-stromal interaction in phyllodes tumors: implications for histologic grade and prognosis. Tumour Biol. 2012;33:787–798. doi: 10.1007/s13277-011-0296-9. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Zhang G, Zhang H, Zhang F, Zhou B, Ning F, Wang HS, Cai SH, Du J. Acquisition of epithelial-mesenchymal transition phenotype and cancer stem cell-like properties in cisplatin-resistant lung cancer cells through AKT/beta-catenin/Snail signaling pathway. Eur J Pharmacol. 2014;723:156–166. doi: 10.1016/j.ejphar.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 15.Pang Y, Thomas P. Involvement of estradiol-17beta and its membrane receptor, G protein coupled receptor 30 (GPR30) in regulation of oocyte maturation in zebrafish, Danio rario. Gen Comp Endocrinol. 2009;161:58–61. doi: 10.1016/j.ygcen.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanda N, Watanabe S. 17beta-estradiol inhibits oxidative stress-induced apoptosis in keratinocytes by promoting Bcl-2 expression. J Invest Dermatol. 2003;121:1500–1509. doi: 10.1111/j.1523-1747.2003.12617.x. [DOI] [PubMed] [Google Scholar]
- 17.Kanda N, Watanabe S. 17beta-estradiol stimulates the growth of human keratinocytes by inducing cyclin D2 expression. J Invest Dermatol. 2004;123:319–328. doi: 10.1111/j.0022-202X.2004.12645.x. [DOI] [PubMed] [Google Scholar]
- 18.Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2006;20:631–646. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- 19.Mo Z, Liu M, Yang F, Luo H, Li Z, Tu G, Yang G. GPR30 as an initiator of tamoxifen resistance in hormone-dependent breast cancer. Breast Cancer Res. 2013;15:R114. doi: 10.1186/bcr3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai LY, Weng JR, Hu JL, Wang D, Sargeant AM, Chiu CF. G15, a GPR30 antagonist, induces apoptosis and autophagy in human oral squamous carcinoma cells. Chem Biol Interact. 2013;206:375–384. doi: 10.1016/j.cbi.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Feng M, Zheng G, Chen Y, Wang X, Pen B, Yin J, Yu Y, He Z. Chemoresistance to 5-fluorouracil induces epithelial-mesenchymal transition via up-regulation of Snail in MCF7 human breast cancer cells. Biochem Biophys Res Commun. 2012;417:679–685. doi: 10.1016/j.bbrc.2011.11.142. [DOI] [PubMed] [Google Scholar]
- 23.Fatemian T, Chowdhury EH. Targeting Oncogenes and Tumor Suppressors genes to Mitigate Chemoresistance. Curr Cancer Drug Targets. 2014;14:599–609. doi: 10.2174/156800961407140926104458. [DOI] [PubMed] [Google Scholar]
- 24.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 25.Prossnitz ER, Barton M. Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat. 2009;89:89–97. doi: 10.1016/j.prostaglandins.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, Steinhoff MM, Sabo E. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res. 2006;12:6359–6366. doi: 10.1158/1078-0432.CCR-06-0860. [DOI] [PubMed] [Google Scholar]
- 27.Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, Prossnitz ER. GPR30: a novel indicator of poor survival for endometrial carcinoma. Am J Obstet Gynecol. 2007;196:381–389. doi: 10.1016/j.ajog.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Smith HO, Arias-Pulido H, Kuo DY, Howard T, Qualls CR, Lee SJ, Verschraegen CF, Hathaway HJ, Joste NE, Prossnitz ER. GPR30 predicts poor survival for ovarian cancer. Gynecol Oncol. 2009;114:465–471. doi: 10.1016/j.ygyno.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Feng M, Zheng G, Chen Y, Wang X, Pen B, Yin J, Yu Y, He Z. Chemoresistance to 5-fluorouracil induces epithelial-mesenchymal transition via up-regulation of Snail in MCF7 human breast cancer cells. Biochem Biophys Res Commun. 2012;417:679–685. doi: 10.1016/j.bbrc.2011.11.142. [DOI] [PubMed] [Google Scholar]
- 30.Fang X, Cai Y, Liu J, Wang Z, Wu Q, Zhang Z, Yang CJ, Yuan L, Ouyang G. Twist2 contributes to breast cancer progression by promoting an epithelial-mesenchymal transition and cancer stem-like cell self-renewal. Oncogene. 2011;30:4707–4720. doi: 10.1038/onc.2011.181. [DOI] [PubMed] [Google Scholar]
- 31.Thomson S, Petti F, Sujka-Kwok I, Mercado P, Bean J, Monaghan M, Seymour SL, Argast GM, Epstein DM, Haley JD. A systems view of epithelial-mesenchymal transition signaling states. Clin Exp Metastasis. 2011;28:137–155. doi: 10.1007/s10585-010-9367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azhar M. Non-redundant tumour supressor functions of transforming growth factor beta in breast cancer. J Biosci. 2001;26:9–12. doi: 10.1007/BF02708975. [DOI] [PubMed] [Google Scholar]
- 33.Zhao H, Lo YH, Yu L, Wang SC. Overcoming resistance to fulvestrant (ICI182,780) by downregulating the c-ABL proto-oncogene in breast cancer. Mol Carcinog. 2011;50:383–389. doi: 10.1002/mc.20721. [DOI] [PubMed] [Google Scholar]
- 34.Al SS, Sharaf LH, Luqmani YA. Signalling pathways involved in endocrine resistance in breast cancer and associations with epithelial to mesenchymal transition (Review) Int J Oncol. 2011;38:1197–1217. doi: 10.3892/ijo.2011.942. [DOI] [PubMed] [Google Scholar]