Abstract
Simple Summary
Beside their use to treat infections, antibiotics are used excessively as growth promoting factors in livestock industry. Animals discharge in their feces and urine between 70%–90% of the antibiotic administrated unchanged or in active metabolites. Because livestock manure is re-applied to land as a fertilizer, concerns are growing over spread of antibiotics in water and soil. Development of antibiotic resistant bacteria is a major risk. This paper reviewed the potential of anaerobic digestion to degrade antibiotics in livestock manure. Anaerobic digestion can degrade manure-laden antibiotic to various extents depending on the concentration and class of antibiotic, bioreactor operating conditions, type of feedstock and inoculum sources.
Abstract
Degrading antibiotics discharged in the livestock manure in a well-controlled bioprocess contributes to a more sustainable and environment-friendly livestock breeding. Although most antibiotics remain stable during manure storage, anaerobic digestion can degrade and remove them to various extents depending on the concentration and class of antibiotic, bioreactor operating conditions, type of feedstock and inoculum sources. Generally, antibiotics are degraded during composting > anaerobic digestion > manure storage > soil. Manure matrix variation influences extraction, quantification, and degradation of antibiotics, but it has not been well investigated. Fractioning of manure-laden antibiotics into liquid and solid phases and its effects on their anaerobic degradation and the contribution of abiotic (physical and chemical) versus biotic degradation mechanisms need to be quantified for various manures, antibiotics types, reactor designs and temperature of operations. More research is required to determine the kinetics of antibiotics’ metabolites degradation during anaerobic digestion. Further investigations are required to assess the degradation of antibiotics during psychrophilic anaerobic digestion.
Keywords: antibiotics, livestock, manure, degradation, fate, anaerobic digestion
1. Introduction
Feeding antimicrobials (antibiotics) as growth promoter at sub-therapeutic doses to swine, cattle, poultry, and fish [1,2] is an integral part of the farm animal/fish production. Antibiotics are relatively recalcitrant to degradation. At significant concentrations, they impose bactericidal or antimicrobial effects which inhibit bacterial activity or growth. Animals excrete a significant fraction of antibiotics in feces and urine; therefore, there is substantial risk that unaltered or still active metabolites would be found in the environment. Different pathways for antibiotics introduction into the environment within an agricultural context were suggested [3]. Land application of livestock manure spreads antibiotics into environment at large scale. The excretion of wastes by grazing animals, atmospheric dispersal of feed and manure dust containing antibiotics [4] and the incidental release of products from spills or discharge are also potential pathways introducing antibiotics into the environment. Antibiotics in food products from animals and plants [5], the development and spread of antibiotic resistant bacteria [6], and the aquatic environments contamination from manure land application are concerns about agricultural antibiotic usage.
1.1. Antibiotic Consumption in Livestock Industry
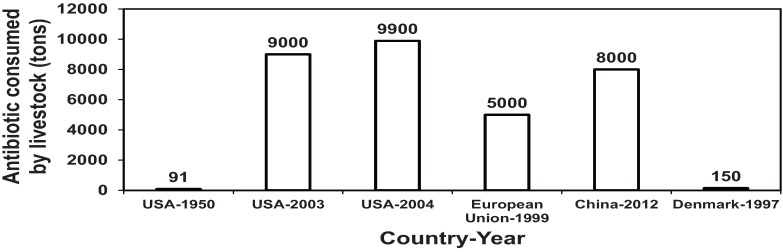
Antibiotic consumption in livestock industry in USA, European countries and China is given in Figure 1. Notice that in the USA, for example, the quantity of antibiotics used in 2004 is 108 times that used in 1950. This is partially because the recommended levels of growth-promoting antibiotics in poultry and pig diets increased from 4 ppm for the narrow spectrum and 10 ppm for the broad-spectrum antibiotics in 1950s to 200 ppm nowadays. About 91% of livestock operations in the USA use 11.2 million kg antibiotics sold over-the-counter as growth promoters annually [6,7,8,9,10]. Antibiotics fed to animals end up in manure and eventually in the environment.
Figure 1.
Quantities of antibiotics consumed by livestock in animal feed (Data from [7,8,9,11]).
1.2. Types of Antibiotics Used in Livestock
Major antibiotics used in livestock: Various antibiotics classes are used to various extents and frequencies therapeutically and sub-therapeutically in livestock industry [2] including:
b-Lactams: penicillins: amoxicillin, ampicillin, benzylpenicillin, cloxacilin, dicloxacilin, flucloxacillin, methicillin, mezlocillin, nafcillin, oxacillin, piperacillin, phenoxymethylcillin.
Macrolides: azithromycin, clarithromycin, clindamycin, erythromycin, roxithromycin, spiramycin, tylosin, vancomycin.
Sulphonamides: sulphadimidine, sulphamethoxazole.
Trimethoprim.
Fluorochinolones: ciprofloxacin, ofloxacin.
Tetracyclines: chlortetracycline, doxycycline, oxytetracycline, tetracycline.
Polyether antibiotic: monensin
The antibiotics in Italic font are the major antibiotics usually used in swine and cattle while other antibiotics are less frequent. Pan et al. [12] reported detection frequencies of 85%–97% (tetracyclines), 52% (sulphonamides), and 5% (macrolide) in 126 swine manure samples collected from 21 animal feeding operation in Shandong-China. Similar results were reported in China (Chen et al. [11] and Japan [13]). Tetracyclines (especially oxytetracycline (OTC) and chlortetracycline (CTC)) occur worldwide in lagoon samples or manures from livestock husbandry [14,15,16,17,18].
1.3. Excretion of Antibiotics in Livestock Manure
Animals excrete significant proportion of antibiotics (17%–90% for livestock) [7,19,20,21] directly into urine and feces, unchanged or as active metabolites (epimers or isomers) of the parent species [22]. Table 1 gives the percentage of antibiotics excreted by animals with their metabolic status (changed or unchanged from their administrated form). Some metabolites are more potent than their parent compounds, while others such as acetic conjugates of sulphonamides can revert back to their parent compounds during manure storage [23].
Table 1.
Level of excretion of antibiotics from animals.
| Antibiotic | Source of manure | Excretion level (%) | Status | Reference |
|---|---|---|---|---|
| Chlortetracycline | Steers feces | 75 | Not reported | [24] |
| Tetracycline | Animal feces | 25 | Not reported | [25] |
| Tylosin | Urine | 50–60 | Unchanged | [25] |
| Oxytetracycline | Castrate sheeps | 21 | Unchanged | [26] |
| Chlortetracycline | Young bulls | 17–75 | Unchanged | [26] |
| Tylosin | Pigs | 40 | Unaltered or as potent metabolites | [27] |
| Monensin | Beef cattle feces | 40% | Unchanged | [28] |
| Virginiamycin | Piggeries liquid manure | 20 | After several days of storage | [29] |
| Oxytetracycline | Calves manure (feces, urine, and bedding) | 23 | Not reported | [30] |
1.4. Concentration of Antibiotics in Livestock Manure
Typically, antibiotic concentrations in manure are between 1 to 10 mg·kg−1 or L−1 but may reach levels ≥ 200 mg·kg−1 or L−1 [20]. Concentrations of 100’s mg·kg−1 or mg·L−1 of veterinary antibiotics have been found in animal excreta in China [11,31,32]. It is not clear whether the high variation in the detected concentrations and the antibiotics excretion by animals were due to individual differences regarding antibiotics metabolism or to an inadequate extraction and quantification methods used during these studies. The concentration of the most commonly used antibiotics have been reported to be as high as 216 mg·L−1 of swine, beef, and poultry/turkey manures [21].
Several studies have confirmed that antibiotics used in animal production are present in fresh manure, manure storage tanks, soil, surface and underground water [33,34]. Jacobsen and Halling-Sørensen [18] detected tetracycline and sulphonamides in swine manure, but no tylosin was detected because of poor recoveries of tylosin from manure. De Liguoro et al. [35] found 0.11 mg·kg−1 of tylosin and 10 mg·kg−1 of OTC in fresh calf manure, but found negligible concentrations of these compounds in soil and water. Dolliver and Gupta [36] found that 1.2% to 1.8% chlortetracycline, monensin and tylosin were lost from manure stockpile by runoff water. Campagnolo et al. [37] found significant quantity of macrolides, sulphonamides and fluoroquinolones in the nearby surface water.
While the measured concentrations shown in Table 2 and elsewhere assess for the presence of antibiotics in various environmental samples and collectively provided strong evidences of their widespread in manure, each study reports its own quantification technique with particular recovery efficiencies, sensibility and reliability.
Table 2.
Concentration of some antibiotics in manures.
| Antibiotic | Matrix | Concentration | Reference |
|---|---|---|---|
| Oxytetracycline | Manure | 136 mg·L−1 | [14] |
| Chlortetracycline | 46 mg·L−1 | ||
| Tetracycline | Swine manure | 98 mg·L−1 | [11] |
| Oxytetracycline | 354 mg·L−1 | ||
| Chlortetracycline | 139 mg·L−1 | ||
| Doxycycline | 37 mg·L−1 | ||
| Sulfadiazine | 7.1 mg·L−1 | ||
| Tetracycline | Swine manure | 30 mg·kg−1 DM | [18] |
| Sulphonamides | 2 mg·kg−1 DM | ||
| Tylosin | Fresh calf manure | 0.11 mg·kg−1 | [35] |
| Oxytetracycline | 10 mg·kg−1 | ||
| Chlortetracycline, | Beef manure stockpile | 6.6 mg·kg−1 | [36] |
| Monensin | 120 mg·kg−1 | ||
| Tylosin | 8.1 mg·kg−1 | ||
| Oxytetracycline | Cow manure | 0.5–200 mg·L−1 | [38] |
| Chlortetracycline | Swine manure | 764.4 mg·L−1 | [12] |
| Chlortetracycline | Swine manure storage lagoon | 1 mg·L−1 | [37] |
| Oxytetracycline | 0.41 mg·L−1 |
There is no standardized and reliable method for antibiotics quantification in complex matrices, such as soil and biological sludge, making inter- and even intra-study comparisons difficult. Most studies report results without sufficiently describing the condition of the manure handling and management before sampling. Partitioning of antibiotics into the liquid and solid phases affects the results. For example, sampling a leaching manure pile would indicate the solid phase fraction of the antibiotics rather than the total concentration.
Most of the antibiotic residues in manure form complexes with soluble organics and remain stable during manure storage. When manure is applied to agriculture fields, a fraction of the antibiotics becomes mobile with the flow of water in the soil and contaminate the surrounding environment including surface and groundwater. The extent of fractioning of an antibiotic between solid and aqueous phases, and hence its mobility, depends on the properties of the antibiotic, soil, and the hydrological effects. More research is required to understand kinetics of biodegradation and potencies of degraded products of various antibiotics in different environments (soils, manures and waste water).
1.5. Environmental Transport of Antibiotics from Livestock Manure
Antibiotics behavior and transport in the environment are related to their physicochemical properties [33]. Numerous antibiotics comprise a non-polar core associated with polar functional moieties; many antibiotics are amphiphilic or amphoteric and ionized. However, physicochemical properties vary widely among compounds from the various structural classes and antibiotics of the same class do not necessarily exhibit identical behaviors. Adsorption of antibiotics to the organic and mineral exchange sites in soil is mostly due to charge transfer and ion interaction and not only to hydrophobic partitioning [34].
Wu et al. [39] demonstrated that ciprofloxacin, tetracycline, doxycycline, and clindamycin were strongly sorbed on aerobically digested biosolids, while sulfamethazine and sulfamethoxazole were only weakly sorbed to particles. Davis et al. [40] investigated the transport of seven different antibiotics used in animal production during a simulated rainfall event and determined their association with the sediment or the aqueous phase. They reported that the percentage of partitioning of the antibiotics into (aqueous, solid) phases for sulfathiazole (77,23), sulfamethazine (95,5), monensin (91,9), erythromycin (26,74), and tylosin (23,77). Therefore, sulfathiazole, sulfamethazine, and monensin mostly associated with aqueous phase while tylosin and erythromycin associated with the solid phase. The tendency of some antibiotics to adsorb on particles reduces their bioavailability [39,41] and results in low degradation rates. Chen et al. [11] found tetracycline, oxytetracycline, chlortetracycline, and doxycycline (0.1–205 μg·kg−1) in manure-amended soils near swine farms. Dolliver et al. [5] found that corn, lettuce, and potato took sulfamethazine from a manure-amended soil and accumulated 0.1 mg to 1.2 mg sulfamethazine kg−1 of dry plant tissue after 45 days of growth; although the accumulated concentration is relatively low it might still pose a health concern. Therefore, surface waters, agricultural soils, and groundwaters may become reservoirs to antibiotics because of the current manure management practices [42,43]. Many antibiotics have short half-lives (days to weeks) [41,44], but at high concentration some persist for months to years within agricultural-related matrices [27,45]. For example, manure storage does not affect tetracyclines and sulfadiazine [11]. Lamshöft et al. [46] observed that metabolites of sulfadiazine have been reversibly converted to sulfadiazine; therefore, they suggested that frequent fertilization of soil by manure contaminated with sulfadiazine and its metabolites may cause them to accumulate in soil and results in environmental contamination. The physicochemical properties, the structure of antibiotics and their degradation by-products determine whether they degrade during biological treatment [47], some compounds and their metabolites may persist for days to months [11].
Evaluating the data available on degradation and fate of antibiotics during anaerobic digestion of livestock manure is essential to the development of this technology as an integral part of the strategy to control the spread of antibiotic resistant bacteria. This paper summarizes what is currently known about behavior, of the major antibiotic used in livestock therapeutically or as growth promoters, during anaerobic digestion, their metabolic by-products, and fractioning into aqueous and solid fractions. There is little information regarding the effect and fate (removal) of antibiotics during the anaerobic digestion of manure [30,48].
2. Persistence and Biodegradation of Antibiotics during Biological Processes of Manure Treatment
2.1. Persistence of Antibiotics in Manure
A summary of the reported half-life (t1/2) of some antibiotics during manure storage or in soil environment is given in Table 3. Generally, a wide range of half-lives has been reported in the scientific literature regarding antibiotic degradation from different environmental conditions.
Table 3.
Half life of antibiotics under storage and natural environment conditions.
| Antibiotic | Medium matrix | Half-life (days unlessindicated otherwise) | Reference |
|---|---|---|---|
| Tetracycline | Biosolids storage | 37 to >77 | [49] |
| Tetracycline | Stored feedlot manure | 17.2 | [50] |
| Chlortetracycline | Composted manure | 3 | [51] |
| Chlortetracycline | Dairy manure | 6.8 | [50] |
| Chlortetracycline | Stored feedlot manure | 13.5 | [50] |
| Oxytetracycline | Stockpiled fresh manure (low-intensity composting) | 21 | [35] |
| Oxytetracycline | Dairy manure | 17.7 | [50] |
| Oxytetracycline | Stored feedlot manure | 31.1 | [50] |
| Oxytetracycline | Horse manure | 8.4 | [50] |
| Tylosin | Aerobic soil-manure slurry | 3.3–8.1 | [41] |
| Olaquindox | Aerobic soil-manure slurry | 5.8–8.8 | [41] |
| Metronidazole | Aerobic soil-manure slurry | 13.1–26.9 | [41] |
| Erythromycin | Storage of pig manure | 41 | [52] |
| Erythromycin | Biosolids storage | 7.0-17 | [49] |
| Roxithromycin | Storage of pig manure | 130 | [52] |
| Salinomycin | Storage of pig manure | 6 | [52] |
| Doxycycline | Biosolids storage | 53 to >77 | [49] |
| Clindamycin | Biosolids storage | 1.0–1.6 | [49] |
| Clarithromycin | Biosolids storage | 1.1–1.9 | [49] |
Storteboom et al. [50] found that OTC persists in dairy manure (t1/2 = 17.7 d) longer than in horse manure (t1/2 = 8.4 d). However, matrix differences could have influenced antibiotic recoveries during extraction and quantification methods used and thus biased half-lives.
In addition, different types of biological processes affect antibiotic persistence differently; for example, chlortetracycline half-life has been found to increase in the order: composting > manure storage > soil. Half-lives for the primary degradation in aerobic soil-manure slurries ranging from 3.3 to 8.1 days for tylosin, 5.8 to 8.8 days for olaquindox, and 13.1 to 26.9 days for metronidazole were observed [41]. Schlüsener et al. [53] indicated that erythromycin, roxithromycin and salinomycin, tetracycline, doxycycline, clindamycin, and clarithromycin were more persistent under anaerobic conditions than aerobic condition with a longer t1/2 by a factor of 1.5 to 2, suggesting that aerobic degradation might be a more important mechanism to eliminate these compounds from the environment [49]. However, the results were not sufficiently strong to support this assumption. The authors emphasized on the need to obtain more data for compounds from different classes to support this assumption and to perform further research to clarify the degradation pathways and identify the metabolites. Also, the poor antibiotic recoveries related to the extraction techniques used (31%–83% recoveries) and high matrix effects (50%–90%) can account in part for the variation in results. Table 4 gives the half-lives of major antibiotic classes in manure environment; notice that tetracyclines and quinolones are very persistent with an average half-live of around 100 days. Recently, improved extraction, cleaning, and quantification methods have been developed. Hence, better recoveries are expected from recent studies on antibiotics degradation during the anaerobic digestion of manure.
Table 4.
Persistence of major classes of veterinary antibiotics in manure (adapted from Boxall et al. [3]).
| Chemical group | Half-life (d) | Persistence class |
|---|---|---|
| Aminoglycosides | 30 | Moderately persistent |
| β-lactams | 5 | Slightly persistent |
| Macrolides | <2 to 21 | Impersistent to slightly persistent |
| Quinolones | 100 | Very persistent |
| Sulphonamides | <8 to 30 | Slightly to moderately persistent |
| Tetracyclines | 100 | Very persistent |
2.2. Biodegradation Level of Antibiotics in Manure Biological Treatment
Bioavailability of antibiotics determines their degradation rate, however, bioavailability depends on the compound's hydrophobicity [54]. Therefore, antibiotic's chemical properties and manure-related matrix characteristics modify the antibiotics' reluctance to biodegradation and play a significant role in antibiotic removal, respectively [50]. Motoyama et al. [13] related differences in the measured concentrations of the same antibiotic in different types of manures (swine, cattle, and horses) to the specific adsorption characteristics of the different manures’ matrices. The physicochemical characteristics of various antibiotics correlated with their degradation profiles and support these assumptions [34].
Degradation of antibiotics in compost, soil, manure, and sediments follows the same metabolic mechanisms [55] though differences among different media matrices affect the fractioning of antibiotics between liquid and solid phases. Results of antibiotics’ degradation studies should be considered cautiously depending on how the bioassay has been conducted, the extraction recovery efficiency, and the resolution of the quantification protocol. Studies reporting on degradation of antibiotics as sole substrate using a standardized bacterial consortium in closed bottles incubated in the dark at 20 °C and assessing the oxygen consumed on theoretical oxygen demand (ThOD) [56] provide a limited information. More reliable results should be obtained from studies conducted with antibiotic-containing manure simulating real situations using mixed anaerobic cultures and monitored by gas production, COD removal, and VFAs consumption. Table 5 presents a summary of antibiotics degradation in livestock manure biological treatment. Notice that the removal of oxytetracycline varied from as low as 55%–70% (soil) to 55%–75% (anaerobic digestion) to 85%–99% (composting). Except for the 99% removal during composting, all other removals efficiencies have been achieved using the same initial oxytetracycline concentration (20 mg·L−1).
Table 5.
Biodegradation of antibiotics in manure.
| Treatment | Antibiotic | Concentration | Observed reduction | Reference |
|---|---|---|---|---|
| I. Anaerobic digestion | ||||
| Anaerobic digestion of swine manure 21 days | Chlortetracycline | [62] | ||
| 5.9 mg·L−1 | 98% (55 °C) | |||
| Anaerobic digestion of cattle manure (28 days) | Monensin | [62] | ||
| 0.30 mg·L−1 | 27% (55 °C) | |||
| Batch anaerobic digestion | Oxytetracycline | 20 mg·L−1 | 55%–73% at 37 °C | [63] |
| Anaerobic sequence batch reactor (ASBR) | Tylosin A | [1] | ||
| 5.8 mg·kg−1 | Decreased to 0.01 mg·L−1 in 48 h | |||
| Swine manure from lagoons | Tylosin | 0–400 mg·kg−1 | 95%–75% | [64] |
| II. Composting | ||||
| Composting (22–35 days) | Chlortetracycline | 1.5 mg·kg−1 | 99% | [65] |
| [65] | ||||
| Tylosin | 3.7 mg·kg−1 | 54% | ||
| Sulfamethazine | 10.8 mg·kg−1 | –76% | [65] | |
| Composting beef manure (35 days) abiotic removal | Oxytetracycline | 115 μg·g−1 DM | 99% (laboratory)25% (22 °C) | [51] |
| Composting | 20 mg·L−1 | [13] | ||
| Carbamazepine | 37% | |||
| III. Manure amended soil | ||||
| Soil | [66] | |||
| Chlortetracycline | 4.7 µg·kg−1 | 0%0% | ||
| Sulphanilamide | 0.25–1.0 mg·L−1 | 0% | [67] | |
| Tylosin | 5.6 µg·L−1 | 0% | [68] | |
| Erythromecin | 5.6 µg·L−1 | 25% | [68] | |
| Storage | [46] | |||
| Difloxacin | 17.6 mg·L−1 | 7% (10 °C and 20 °C) | ||
Careful examination of Table 5 reveals several trends. The high removals during composting are likely due to the effects of the additional aerobic bioactivity compared to anaerobic digestion alone. Although both soil and composting share the same aerobic-anoxic conditions composting showed higher removals likely because of the presence of good inoculum compared to soil condition. No sound conclusion could be drawn regarding the effect of the biological action temperature on antibiotic removal. For example, mesophilic and thermophilic anaerobic digestion operation showed higher removals of chlortetracycline than psychrophilic operation, however, for monensin both psychrophilic and mesophilic showed low removals compared to thermophilic. Interestingly, oxytetracycline was removed in soil by almost the same efficiency at 5 °C and 15 °C, while at 25 °C a 20% increase in the removal efficiency was observed.
Although the degradation half-lives were reported for antibiotics in stored solids or in soil where there is a “passive” biodegradation, these values do not reflect degradation rates in the presence of an active biomass such as in waste treatment processes. Aerobic and anaerobic waste treatment processes have shown their efficiency to remove many xenobiotics and pharmaceuticals from effluents. Some studies have evaluated the biodegradability, mostly in aerobic conditions, of various antibiotics used for human health and animal production. Al-Ahmad et al. [57] have shown, when testing the biodegradability of cefotiam, ciprofloxacin, meropenem, penicillin G, and sulfamethoxazole using closed bottle test [56], that only pencillin G was biodegradable to some degree (27%), prolonging the test from 28 to 40 days increased the removal to 35%. Using the same test to evaluate the biodegradability of ciproflaxin, ofloxacin and metronidazole, Kümmerer et al. [58] observed no biodegradation of those antibiotics, without loss of their genotoxicity.
Wang et al. [59] found that degradation kinetics of sulfadimethoxine was affected by its initial concentration because microorganisms are inhibited at high antibiotic concentrations; this result could presumably be extrapolated to any antimicrobials degradation kinetic. Shi et al. [60] found that tetracycline and sulfamethoxydiazine initial concentrations of up to 50 mg·L−1 decreased by 50% within 12 h of continuous anaerobic digestion (OLR 1.88 kg COD m−3·d−1) and only traces of antibiotics were detected after 2–3 days. These researchers did not provide evidence whether the reduction of the antibiotics concentration was due to sorption or biodegradation. Loke et al. [61] found a half-life value lower than 2 days for tylosin A in manure spiked with 25 mg·L−1 during anaerobic digestion of swine waste at 20 °C. Moreover, in aerobic conditions the disappearance rates of tylosin A increased with increasing concentrations of solids, but it was not clear if removal was due to bacterial or abiotic degradation, or that sorption on manure particles was responsible for low aqueous antibiotic concentrations. Loke et al. [61] did not observe instant sorption of tylosin on manure particles, with 102% to 108% recoveries during method validation. However, recovery efficiencies were not assessed for long term contact between the antibiotic and manure particles. Hence, it is plausible that the more concentrated solids adsorb more of the antibiotic over time and less is recovered, which gives the impression that the half-life is shorter in this condition.
The effects of individual and mixtures of antimicrobials on manure biological treatment depend on inhibition and resistance mechanisms, the manure matrix, the composition of the microbial community, biotic and abiotic degradation of antimicrobials, and sorption of antimicrobials [69].
2.2.1. Tetracyclines
Abiotic mechanisms were responsible for a removal of 98% of CTC (initial concentration (Ci) = 113 µg·g−1) during 30 days of beef manure composting (TS = 30%) [9]. However, there was an increased loss of extractable CTC residues with increased time, probably due to sorption to organic matter, rendering its quantification difficult. Approximately 60% removal of OTC (Ci = 9.8 mg·L−1) was achieved in 64 days by anaerobic digestion at 35 °C (TS 4.0% to 4.7%) yielding a calculated half-life of 56 days for OTC [30,48]. Also, approximately 75% removal of buffer extractable CTC (Ci = 5.9 mg·L−1) was achieved in 33 days by anaerobic digestion at 35 °C yielding a calculated value half-life of about 18 days. However, these removals during anaerobic digestion cannot be directly related to biological activity or abiotic mechanisms since no sorption analysis was performed. Anaerobic digestion decreased concentrations of OTC from 13.5, 56.9 and 95.0 mg·L−1 to 5.7, 26.6 and 30.7 mg·L−1 in 21 days, respectively, while CTC was decreased from 9.8, 46.1 and 74.0 mg·L−1 to 0.9, 4.0 and 7.5 mg·L−1, respectively [70]. CTC was transformed and epimerized at faster rates than that for OTC. CTC decreased in the solid fraction at a slower rate than that observed in the aqueous phase likely because water-extractable antibiotics are most “available” for degradation by microorganisms and that 100% of CTC concentration has been found water-extractable [65]. Finally, the degradation product and epimer of CTC, 4-epi-chlortetracycline (ECTC), was completely removed at high rate [70]. Arikan et al. [48] and [30] reported a significant removal of the parent compounds of CTC and OTC during the first 10 days of incubation, then, OTC was degraded in 60–70 days whereas CTC was removed at a slower rate. These finding agrees with the half-life of OTC (22–27 days) determined during batch anaerobic digestion of manure [63].
The adsorption of OTC and CTC was limited by the available superficial area of the inoculum and pig manure [70]. Furthermore, OTC and CTC form strong complexes with divalent cations, which are abundant in pig manure, adsorb onto proteins, particles and organic matter [71]. At 35 °C and pH 7, 40% and 60% of OTC and CTC, respectively, were removed in the first hour. 4-epi-oxytetracycline (EOTC), an epimer of OTC and ECTC degraded quickly. After 7 days, 6% of the initial amount of OTC remained in the assay [70].
Álvarez et al. [70] determined also the first-order degradation constants for OTC (0.045 to 0.058 d−1) and CTC (0.169 to 0.216 d−1) while Arikan et al. [30] reported lower first-order degradation constants (0.012 and 0.039 d−1 for OTC and CTC, respectively). This inconsistency might have been caused by the higher organic matter content in the assays (50 g·L−1 of cattle manure), which could have increased the stability of both compounds due to their strong adsorption onto the solid fraction [70].
The half-life of oxytracycline in manure was 30 days and it was detectable (820 ug·kg−1) after 5 months of maturation [35]. Søeborg et al. [72] suggested that some portion of chlortetracycline degradation during composting may be due to abiotic processes.
2.2.2. Tylosin
De Liguoro et al. [35] found that tylosin degraded rapidly and it was undetectable in manure after 45 days; no trace (>10 ug·L−1) of the compound was detected in soil or surrounding water. Chelliapan et al. [64] reported that 95% tylosin reduction with a COD reduction of 93% were achieved in an up-flow anaerobic stage reactor (UASR) treating pharmaceutical wastewater (contains tylosin 0 to 400 mg·L−1) at a HRT of 4 d and OLR of 1.86 kg COD m−3·d−1. However, at concentrations of 600 and 800 mg·L−1 the COD reduction was 85% and the tylosin removal was 75% [64]. They concluded that tylosin concentrations ≤ 400 mg·L−1 had a minimal effect on reactor performance. Methanogens were active in the reactor even at 800 mg·L−1 tylosin which did not affect the CH4 yield. Similar findings that such as high concentrations of tylosin are unlikely to create problems in the treatment of wastewater by anaerobic digestion have been reported in other studies [73,74].
The tylosin A half-life of (2.5 h) in high-rate anaerobic digester is shorter than its half-lives (2–8 days) in soils or passively stored manure [1,41,61,75]. Tylosin was degraded in soil columns (half-life 3.3–8.1 days) [41]. Kolz et al. [27] found that 90% of tylosin A in anaerobic sludge was sorbed and degraded (abiotic or biotic) within 5 days in anaerobic digestion. Angenent et al. [1] concluded that tylosin was removed by degradation rather than sorption in anaerobic batch experiment and ASBR; with dehydroxy-tylonolide as a by-product. Such conclusion could be explained by the fact that water-extractable antibiotics are most “available” for degradation by microorganisms given that 85% of the total-extractable concentration of tylosin was water-extractable [65].
Chelliapan et al. [76] reported tylosin (initial concentration 10–220 mg·L−1) removal of 70–88% in upflow anaerobic stage reactor operating at OLR 1.86 kg COD m−3·d−1 with a COD removal of 70%–75%. At higher OLR (2.84–3.73 kg COD m−3·d−1), tylosin removal increased and was stable between 93%–99% despite that the COD removal declined to about 45%. Obviously, there is no agreement on which mechanism is responsible for the removal of tylosin in anaerobic digestion.
2.2.3. Other Antibiotics
Sulfamethazole was utilized as carbon and nitrogen source by the microorganisms in absence of those nutrients, but remained intact in the presence of acetate and ammonium [77]. Antibiotics like ciprofloxacin, ofloxacin, and virginiamycin degrade very slowly and may persist in soil in its original form up to 30–80 days while bambermycin and erythromycin completely degrade in a period of one month at temperatures ranging from 20–30 °C [21].
Carballa et al. [78] found that mesophilic anaerobic digestion (STR of 30 days) degraded 99 and 94% of sulfamethoxazole, and roxithromycin, respectively. Water-extractable antibiotics are most “available” for degradation by microorganisms. The percentage of initial water-extractable antibiotic concentration out of the total-extractable was 40% for monensinand 85% for sulfamethazine [65].
Kim et al. [79] observed decrease in tetracyclines, sulfamethazine, and tylosin concentrations from 20 mg·kg−1 to less than 0.8, 0.2, and 1.0 mg·kg−1, respectively, during composting of pig manure with saw dust. Presence of saw dust correlated with the decline in tetracyclines and sulfamethazine concentrations, but not with tylosin. Again, it is debatable to compare results from different studies because of the utilization of various antibiotic quantification techniques having different reliabilities and precision. Moreover, most of these studies did not discuss the possibility that antibiotics would be adsorbed on particles and thus not quantified, biasing the degradation rates obtained.
2.4. Metabolites
Fedler and Day [80] suggested that the antibiotics themselves may not inhibit bacteria but their metabolites produced in the gastrointestinal tract of the animal may. 4-Epi-oxytetracycline (EOTC), a-apo-oxytetracycline (a-Apo-OTC) and b-apo-oxytetracycline (b-Apo-OTC) are degradation products of oxytetracycline (OTC) [48] whereas 4-epi-chlortetracycline (ECTC) is a degradation product and epimer of chlortetracycline (CTC). These metabolites are similar to their parent in creating complexes with metal ions, humic acids, proteins, particles and organic matter in the manure matrix [71] thus they are strongly adsorbed in manure. Unfortunately, almost all of the studies on antibiotics in manure focused on the parent compounds except several studies on OTC and CTC where antibiotic degradation progenies were monitored. It has been concluded that antibiotic metabolites produced in the gastrointestinal tract of the animal may inhibit bacterial activity more than the original molecule [73]. On the contrary, Halling-Sørensen et al. [81] found that the degradation products of OTC have less biological activity on sludge and soil bacteria than OTC. These authors also found a similar trend of biotransformation between the parent and the intermediate compounds (EOTC and ECTC), as well as the removal of these intermediates [70].
3. Required Future Research
Developing standard protocols to assess the impact, degradation, and fate of various antibiotics and their metabolites during anaerobic digestion is essential to enable a reasonable comparison among results generated from different studies. Assessing the effect of culture matrices, solid content, and nature of manure organic fraction on the degradation dynamic of various antibiotic during anaerobic digestion is required with a focus on kinetic and metabolic modeling and simulation of inhibition, recovery, and adaptation mechanisms. Particularly, better understanding and prediction of the contribution of abiotic (physical and chemical) versus biotic degradation mechanisms of the different antibiotic classes is required. Fractioning of manure-laden antibiotics into liquid and solid phases and its effects on their anaerobic degradation needs to be understood and quantified under various manure classes, antibiotics types, reactor design and operation. For design purposes, kinetic data is required for the degradation of the antibiotic parent compounds and their metabolites in different anaerobic reactor designs and operation. Effects of process staging and modification need to be explored to avoid operational problems due to the effects of high antibiotics’ concentrations on the anaerobic digestion. Potential of psychrophilic anaerobic digestion of livestock manure to eliminate antibiotics and antibiotic resistant bacteria has not been investigated yet.
4. Conclusions
Most antibiotics form complexes with metals and soluble organics in manure and remain stable during storage, however; anaerobic digestion can degrade them to various extents depending on the concentration and class of antibiotic, operation condition, and type of culture.
Antibiotic’s chemical properties and manure-related matrix characteristics interact to modify their reluctance to biodegradation and play a significant role in antibiotics' removal. The physicochemical characteristics of various antibiotics correlate with their degradation profile. Antibiotics' degradation during anaerobic digestion depends on their water-extractability which affects their bioavailability to microorganisms. Therefore, fractioning of antibiotics into liquid and solid phases of manure, its effects on their anaerobic degradation, and contribution of abiotic (physical and chemical) versus biotic degradation mechanisms need to be determined and quantified for various manures, antibiotics types, reactor designs and operation conditions. Different types of biological processes affect antibiotic persistence differently; composting > anaerobic digestion > soil.
Antibiotics and their metabolites are strongly adsorbed in manure because of chemical combination with metals and organics. More research is required to evaluate kinetics and fate of antibiotic degradation progenies. It is strongly suggested that standard analytical protocols be developed for the detection, extraction, and quantification antibiotics from manure. Such standard methods will enable sound comparison of the results generated from different studies and making better conclusion regarding the impact, degradation, and fate of various antibiotics and their metabolites during anaerobic digestion. Assessing the effect of culture matrices, solid content, and nature of manure organic fraction on the degradation kinetics of various antibiotics during anaerobic digestion is required with a focus on kinetic, metabolic modeling, simulation of inhibition, recovery, and adaptation mechanisms. Further investigations are required to assess the degradation of antibiotics during psychrophilic anaerobic digestion.
Acknowledgments
This project has been financially supported through contributions from Agriculture and Agri-Food Canada, the Canadian Dairy Commission and Dairy Farmers of Canada under the Dairy Research Cluster Program.
Author Contributions
Conceived the project on antibiotic degradation: DIM; obtained the financial support: DIM; performed the critical literature review: IG, DIM and NMCS; wrote the paper: IG, DIM and NMCS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Angenent L.T., Mau M., George U., Zahn J.A., Raskin L. Effect of the presence of the antimicrobial tylosin in swine waste on anaerobic treatment. Water Res. 2008;42:2377–2384. doi: 10.1016/j.watres.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Kemper N., Färber H., Skutlarek D., Krieter J. Analysis of antibiotic residues in liquid manure and leachate of dairy farms in northern germany. Agr. Water Manag. 2008;95:1288–1292. doi: 10.1016/j.agwat.2008.05.008. [DOI] [Google Scholar]
- 3.Boxall A.B.A., Fogg L.A., Blackwell P.A., Blackwell P., Kay P., Pemberton E.J., Croxford A. Veterinary medicines in the environment. Rev. Environ. Contam. Toxicol. 2004;180:1–91. doi: 10.1007/0-387-21729-0_1. [DOI] [PubMed] [Google Scholar]
- 4.Hamscher G., Pawelzick H., Sczesny S., Nau H., Hartung J. Antibiotics in dust originating from a pig-fattening farm: A new source of health hazard for farmers? Environ. Health Perspect. 2003;111:1590–1594. doi: 10.1289/ehp.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolliver H., Kumar K., Gupta S. Sulfamethazine uptake by plants from manure-amended soil. J. Environ. Qual. 2007;36:1224–1230. doi: 10.2134/jeq2006.0266. [DOI] [PubMed] [Google Scholar]
- 6.Mellon M., Benbrook C., Benbrook K. Hogging it: Estimates of Antimicrobial Abuse in Livestock. Union of Concerned Scientists; Cambridge, MA, USA: 2001. [Google Scholar]
- 7.Sarmah A.K., Meyer M.T., Boxall A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere. 2006;65:725–759. doi: 10.1016/j.chemosphere.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Shea K.M. Antibiotic resistance: What is the impact of agricultural uses of antibiotics on children's health? Pediatrics. 2003;112:253–258. [PubMed] [Google Scholar]
- 9.Arikan O.A., Mulbry W., Rice C. Management of antibiotic residues from agricultural sources: Use of composting to reduce chlortetracycline residues in beef manure from treated animals. J. Hazard. Mater. 2009;164:483–489. doi: 10.1016/j.jhazmat.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 10.National Research Council . The Use of Drugs in Food Animals: Benefits and Risks. National Academy Press; Washington, DC, USA: 1999. p. 25. [PubMed] [Google Scholar]
- 11.Chen Y., Zhang H., Luo Y., Song J. Occurrence and assessment of veterinary antibiotics in swine manures: A case study in east china. Chin. Sci. Bull. 2012;57:606–614. doi: 10.1007/s11434-011-4830-3. [DOI] [Google Scholar]
- 12.Pan X., Qiang Z., Ben W., Chen M. Residual veterinary antibiotics in swine manure from concentrated animal feeding operations in shandong province, china. Chemosphere. 2011;84:695–700. doi: 10.1016/j.chemosphere.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Motoyama M., Nakagawa S., Tanoue R., Sato Y., Nomiyama K., Shinohara R. Residues of pharmaceutical products in recycled organic manure produced from sewage sludge and solid waste from livestock and relationship to their fermentation level. Chemosphere. 2011;84:432–438. doi: 10.1016/j.chemosphere.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 14.Martínez-Carballo E., González-Barreiro C., Scharf S., Gans O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in austria. Environ. Pollut. 2007;148:570–579. doi: 10.1016/j.envpol.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Aust M.O., Godlinski F., Travis G.R., Hao X., McAllister T.A., Leinweber P., Thiele-Bruhn S. Distribution of sulfamethazine, chlortetracycline and tylosin in manure and soil of canadian feedlots after subtherapeutic use in cattle. Environ. Pollut. 2008;156:1243–1251. doi: 10.1016/j.envpol.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Christian T., Schneider R.J., Färber H.A., Skutlarek D., Meyer M.T., Goldbach H.E. Determination of antibiotic residues in manure, soil, and surface waters. Acta Hydrochim. Hydrobiol. 2003;31:36–44. doi: 10.1002/aheh.200390014. [DOI] [Google Scholar]
- 17.Karci A., Balcioǧlu I.A. Investigation of the tetracycline, sulfonamide, and fluoroquinolone antimicrobial compounds in animal manure and agricultural soils in turkey. Sci. Total Environ. 2009;407:4652–4664. doi: 10.1016/j.scitotenv.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen A.M., Halling-Sørensen B. Multi-component analysis of tetracyclines, sulfonamides and tylosin in swine manure by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2006;384:1164–1174. doi: 10.1007/s00216-005-0261-9. [DOI] [PubMed] [Google Scholar]
- 19.Bound J.P., Voulvoulis N. Pharmaceuticals in the aquatic environment—A comparison of risk assessment strategies. Chemosphere. 2004;56:1143–1155. doi: 10.1016/j.chemosphere.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Kumar K., Gupta S.C., Chander Y., Singh A.K. Antibiotic use in agriculture and its impact on the terrestrial environment. Adv. Agron. 2005;87:1–54. doi: 10.1016/S0065-2113(05)87001-4. [DOI] [Google Scholar]
- 21.Antibiotics in Manure and Soil—A Grave Threat to Human and Animal Health. National Academy of Agriculture Science; New Delhi, India: 2010. p. 20. Policy Paper 43. [Google Scholar]
- 22.Mackie R.I., Koike S., Krapac I., Chee-Sanford J., Maxwell S., Aminov R.I. Tetracycline residues and tetracycline resistance genes in groundwater impacted by swine production facilities. Anim. Biotech. 2006;17:157–176. doi: 10.1080/10495390600956953. [DOI] [PubMed] [Google Scholar]
- 23.Boxall A.B., Blackwell P., Cavallo R., Kay P., Tolls J. The sorption and transport of a sulphonamide antibiotic in soil systems. Toxicol. Lett. 2002;131:19–28. doi: 10.1016/S0378-4274(02)00063-2. [DOI] [PubMed] [Google Scholar]
- 24.Elmund G.K., Morrison S.M., Grant D.W., Nevins M.P. Role of excreted chlortetracycline in modifying the decomposition process in feedlot waste. Bull. Environ. Contam. Toxicol. 1971;6:129–132. doi: 10.1007/BF01540093. [DOI] [PubMed] [Google Scholar]
- 25.Feinman S.E., Matheson J.C. Draft Environmental Impact Statement Subtherapeutic Antibacterial Agents in Animal Feeds. FDA; Rockville, MD, USA: 1978. [Google Scholar]
- 26.Montforts M.H.M.M., Kalf D.F., Van Vlaardingen P.L.A., Linders J.B.H.J. The exposure assessment for veterinary medicinal products. Sci. Total Environ. 1999;225:119–133. doi: 10.1016/S0048-9697(98)00338-6. [DOI] [PubMed] [Google Scholar]
- 27.Kolz A.C., Moorman T.B., Ong S.K., Scoggin K.D., Douglass E.A. Degradation and metabolite production of tylosin in anaerobic and aerobic swine-manure lagoons. Water Environ. Res. 2005;77:49–56. doi: 10.2175/106143005X41618. [DOI] [PubMed] [Google Scholar]
- 28.Donoho A., Manthey J., Occolowitz J., Zornes L. Metabolism of monensin in the steer and rat. J. Agr. Food Chem. 1978;26:1090–1095. doi: 10.1021/jf60219a005. [DOI] [PubMed] [Google Scholar]
- 29.Cocito C. Antibiotics of the virginiamycin family, inhibitors which contain synergistic components. Microbiol. Rev. 1979;43:145–198. doi: 10.1128/mr.43.2.145-192.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arikan O.A. Degradation and metabolization of chlortetracycline during the anaerobic digestion of manure from medicated calves. J. Hazard. Mater. 2008;158:485–490. doi: 10.1016/j.jhazmat.2008.01.096. [DOI] [PubMed] [Google Scholar]
- 31.Hu X.G., Luo Y., Zhou Q.X., Xu L. Determination of thirteen antibiotics residues in manure by solid phase extraction and high performance liquid chromatography. Chin. J. Anal. Chem. 2008;36:1162–1166. doi: 10.1016/S1872-2040(08)60063-8. [DOI] [Google Scholar]
- 32.Xian Q., Hu L., Chen H., Chang Z., Zou H. Removal of nutrients and veterinary antibiotics from swine wastewater by a constructed macrophyte floating bed system. J. Environ. Manag. 2010;91:2657–2661. doi: 10.1016/j.jenvman.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 33.Pruden A. Hormones and Pharmaceuticals Generated by Concentrated Animal Feeding Operations. Springer-Verlag; New York, NY, USA: 2009. Production and transport of antibiotics from cafos; pp. 63–70. [Google Scholar]
- 34.Thiele-Bruhn S. Pharmaceutical antibiotic compounds in soils–A review. J. Plant Nutr. Soil Sci. 2003;166:145–167. doi: 10.1002/jpln.200390023. [DOI] [Google Scholar]
- 35.De Liguoro M., Cibin V., Capolongo F., Halling-Sørensen B., Montesissa C. Use of oxytetracycline and tylosin in intensive calf farming: Evaluation of transfer to manure and soil. Chemosphere. 2003;52:203–212. doi: 10.1016/S0045-6535(03)00284-4. [DOI] [PubMed] [Google Scholar]
- 36.Dolliver H.A.S., Gupta S.C. Antibiotic losses from unprotected manure stockpiles. J. Environ. Qual. 2008;37:1238–1244. doi: 10.2134/jeq2007.0391. [DOI] [PubMed] [Google Scholar]
- 37.Campagnolo E.R., Johnson K.R., Karpati A., Rubin C.S., Kolpin D.W., Meyer M.T., Esteban J.E., Currier R.W., Smith K., Thu K.M., et al. Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations. Sci. Total Environ. 2002;299:89–95. doi: 10.1016/S0048-9697(02)00233-4. [DOI] [PubMed] [Google Scholar]
- 38.Ince B., Coban H., Turker G., Ertekin E., Ince O. Effect of oxytetracycline on biogas production and active microbial populations during batch anaerobic digestion of cow manure. Bioprocess Biosyst. Eng. 2013;36:541–546. doi: 10.1007/s00449-012-0809-y. [DOI] [PubMed] [Google Scholar]
- 39.Wu C., Spongberg A.L., Witter J.D. Sorption and biodegradation of selected antibiotics in biosolids. J. Environ. Sci. Health A. 2009;44:454–461. doi: 10.1080/10934520902719779. [DOI] [PubMed] [Google Scholar]
- 40.Davis J.G., Truman C.C., Kim S.C., Ascough J.C., II, Carlson K. Antibiotic transport via runoff and soil loss. J. Environ. Qual. 2006;35:2250–2260. doi: 10.2134/jeq2005.0348. [DOI] [PubMed] [Google Scholar]
- 41.Ingerslev F., Halling-Sørensen B. Biodegradability of metronidazole, olaquindox, and tylosin and formation of tylosin degradation products in aerobic soil-manure slurries. Ecotoxicol. Environ. Safety. 2001;48:311–320. doi: 10.1006/eesa.2000.2026. [DOI] [PubMed] [Google Scholar]
- 42.Hu X., Zhou Q., Luo Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern china. Environ. Pollut. 2010;158:2992–2998. doi: 10.1016/j.envpol.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Chang X., Meyer M.T., Liu X., Zhao Q., Chen H., Chen J.A., Qiu Z., Yang L., Cao J., Shu W. Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in chongqing region of three gorge reservoir in china. Environ. Pollut. 2010;158:1444–1450. doi: 10.1016/j.envpol.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 44.Teeter J.S., Meyerhoff R.D. Aerobic degradation of tylosin in cattle, chicken, and swine excreta. Environ. Res. 2003;93:45–51. doi: 10.1016/S0013-9351(02)00086-5. [DOI] [PubMed] [Google Scholar]
- 45.Winckler C., Grafe A. Use of veterinary drugs in intensive animal production: Evidence for persistence of tetracycline in pig slurry. J. Soils Sediments. 2001;1:66–70. doi: 10.1007/BF02987711. [DOI] [Google Scholar]
- 46.Lamshöft M., Sukul P., Zuhlke S., Spiteller M. Behaviour of (14)c-sulfadiazine and (14)c-difloxacin during manure storage. Sci. Total Environ. 2010;408:1563–1568. doi: 10.1016/j.scitotenv.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Ben W., Qiang Z., Adams C., Zhang H., Chen L. Simultaneous determination of sulfonamides, tetracyclines and tiamulin in swine wastewater by solid-phase extraction and liquid chromatography-mass spectrometry. J. Chromatogr. A. 2008;1202:173–180. doi: 10.1016/j.chroma.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 48.Arikan O.A., Sikora L.J., Mulbry W., Khan S.U., Rice C., Foster G.D. The fate and effect of oxytetracycline during the anaerobic digestion of manure from therapeutically treated calves. Process Biochem. 2006;41:1637–1643. doi: 10.1016/j.procbio.2006.03.010. [DOI] [Google Scholar]
- 49.Wu C., Spongberg A.L., Witter J.D. Determination of the persistence of pharmaceuticals in biosolids using liquid-chromatography tandem mass spectrometry. Chemosphere. 2008;73:511–518. doi: 10.1016/j.chemosphere.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 50.Storteboom H.N., Kim S.C., Doesken K.C., Carlson K.H., Davis J.G., Pruden A. Response of antibiotics and resistance genes to high-intensity and low-intensity manure management. J. Environ. Qual. 2007;36:1695–1703. doi: 10.2134/jeq2007.0006. [DOI] [PubMed] [Google Scholar]
- 51.Arikan O.A., Sikora L.J., Mulbry W., Khan S.U., Foster G.D. Composting rapidly reduces levels of extractable oxytetracycline in manure from therapeutically treated beef calves. Bioresour. Technol. 2007;98:169–176. doi: 10.1016/j.biortech.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 52.Schlusener M.P., Bester K. Persistence of antibiotics such as macrolides, tiamulin and salinomycin in soil. Environ. Pollut. 2006;143:565–571. doi: 10.1016/j.envpol.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 53.Schlüsener M., von Arb M., Bester K. Elimination of macrolides, tiamulin, and salinomycin during manure storage. Arch. Environ. Contam. Toxicol. 2006;51:21–28. doi: 10.1007/s00244-004-0240-8. [DOI] [PubMed] [Google Scholar]
- 54.Ingerslev F., Halling-Sorensen B. Biodegradability properties of sulfonamides in activated sludge. Environ. Toxicol. Chem. 2000;19:2467–2473. doi: 10.1002/etc.5620191011. [DOI] [Google Scholar]
- 55.Büyüksönmez F., Rynk R., Hess T.F., Bechinski E. Occurrence, degradation and fate of pesticides during composting: Part II: Occurrence and fate of pesticides in compost and composting systems. Compost Sci. Utilization. 2000;8:61–81. doi: 10.1080/1065657X.2000.10701751. [DOI] [Google Scholar]
- 56.Guidelines for Testing of Chemicals. OECD; Paris, France: 1992. 301D Closed Bottle Test. Adopted by the Council on 17th July 1992. [Google Scholar]
- 57.Al-Ahmad A., Daschner F.D., Kümmerer K. Biodegradability of cefotiam, ciprofloxacin, meropenem, penicillin g, and sulfamethoxazole and inhibition of waste water bacteria. Arch. Environ. Contam. Toxicol. 1999;37:158–163. doi: 10.1007/s002449900501. [DOI] [PubMed] [Google Scholar]
- 58.Kümmerer K., Al-Ahmad A., Mersch-Sundermann V. Biodegradability of some antibiotics, elimination of the genotoxicity and affection of wastewater bacteria in a simple test. Chemosphere. 2000;40:701–710. doi: 10.1016/S0045-6535(99)00439-7. [DOI] [PubMed] [Google Scholar]
- 59.Wang Q.-Q., Bradford S.A., Zheng W., Yates S.R. Sulfadimethoxine degradation kinetics in manure as affected by initial concentration, moisture, and temperature. J. Environ. Qual. 2006;35:2162–2169. doi: 10.2134/jeq2006.0178. [DOI] [PubMed] [Google Scholar]
- 60.Shi J.C., Liao X.D., Wu Y.B., Liang J.B. Effect of antibiotics on methane arising from anaerobic digestion of pig manure. Anim. Feed Sci. Technol. 2011;166–167:457–463. doi: 10.1016/j.anifeedsci.2011.04.033. [DOI] [Google Scholar]
- 61.Loke M.-L., Ingerslev F., Halling-Sørensen B., Tjørnelund J. Stability of tylosin a in manure containing test systems determined by high performance liquid chromatography. Chemosphere. 2000;40:759–765. doi: 10.1016/S0045-6535(99)00450-6. [DOI] [PubMed] [Google Scholar]
- 62.Varel V.H., Wells J.E., Shelver W.L., Rice C.P., Armstrong D.L., Parker D.B. Effect of anaerobic digestion temperature on odour, coliforms and chlortetracycline in swine manure or monensin in cattle manure. J. Appl. Microbiol. 2012;112:705–715. doi: 10.1111/j.1365-2672.2012.05250.x. [DOI] [PubMed] [Google Scholar]
- 63.Turker G., Ince O., Ertekin E., Akyol C., Ince B. Changes in performance and active microbial communities due to single and multiple effects of mixing and solid content in anaerobic digestion process of otc medicated cattle manure. Int. J. Renew. Energ. Res. 2013;3:144–148. [Google Scholar]
- 64.Chelliapan S., Wilby T., Sallis P.J. Performance of an up-flow anaerobic stage reactor (UASR) in the treatment of pharmaceutical wastewater containing macrolide antibiotics. Water Res. 2006;40:507–516. doi: 10.1016/j.watres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 65.Dolliver H., Gupta S., Noll S. Antibiotic degradation during manure composting. J. Environ. Qual. 2008;37:1245–1253. doi: 10.2134/jeq2007.0399. [DOI] [PubMed] [Google Scholar]
- 66.Hamscher G., Sczesny S., Hoper H., Nau H. Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Anal. Chem. 2002;74:1509–1518. doi: 10.1021/ac015588m. [DOI] [PubMed] [Google Scholar]
- 67.Frankenberger W.T., Tabatabai M.A. Transformations of amide nitrogen in soils1. Soil Sci. Soc. Am. J. 1982;46:280–284. doi: 10.2136/sssaj1982.03615995004600020013x. [DOI] [Google Scholar]
- 68.Gavalchin J., Katz S.E. The persistence of fecal-borne antibiotics in soil. J. AOAC Int. 1994;77:481–485. [Google Scholar]
- 69.Amin M.M., Zilles J.L., Greiner J., Charbonneau S., Raskin L., Morgenroth E. Influence of the antibiotic erythromycin on anaerobic treatment of a pharmaceutical wastewater. Environ. Sci. Technol. 2006;40:3971–3977. doi: 10.1021/es060428j. [DOI] [PubMed] [Google Scholar]
- 70.lvarez J.A., Otero L., Lema J.M., Omil F. The effect and fate of antibiotics during the anaerobic digestion of pig manure. Bioresource Technol. 2010;101:8581–8586. doi: 10.1016/j.biortech.2010.06.075. [DOI] [PubMed] [Google Scholar]
- 71.Loke M.L., Jespersen S., Vreeken R., Halling-Sørensen B., Tjørnelund J. Determination of oxytetracycline and its degradation products by high-performance liquid chromatography-tandem mass spectrometry in manure-containing anaerobic test systems. J. Chromatogr. B. 2003;783:11–23. doi: 10.1016/S1570-0232(02)00468-3. [DOI] [PubMed] [Google Scholar]
- 72.Søeborg T., Ingerslev F., Halling-Sørensen B. Chemical stability of chlortetracycline and chlortetracycline degradation products and epimers in soil interstitial water. Chemosphere. 2004;57:1515–1524. doi: 10.1016/j.chemosphere.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 73.Massé D.I., Lu D., Masse L., Droste R.L. Effect of antibiotics on psychrophilic anaerobic digestion of swine manure slurry in sequencing batch reactors. Bioresource Technol. 2000;75:205–211. doi: 10.1016/S0960-8524(00)00046-8. [DOI] [Google Scholar]
- 74.Poels J., Van Assche P., Verstraete W. Effects of disinfectants and antibiotics on the anaerobic digestion of piggery waste. Agr. Wastes. 1984;9:239–247. doi: 10.1016/0141-4607(84)90083-0. [DOI] [Google Scholar]
- 75.Rabølle M., Spliid N.H. Sorption and mobility of metronidazole, olaquindox, oxytetracycline and tylosin in soil. Chemosphere. 2000;40:715–722. doi: 10.1016/S0045-6535(99)00442-7. [DOI] [PubMed] [Google Scholar]
- 76.Chelliapan S., Wilby T., Sallis P.J., Yuzir A. Tolerance of the antibiotic tylosin on treatment performance of an up-flow anaerobic stage reactor (UASR) Water Sci. Technol. 2011;63:1599–1606. doi: 10.2166/wst.2011.222. [DOI] [PubMed] [Google Scholar]
- 77.Drillia P., Dokianakis S.N., Fountoulakis M.S., Kornaros M., Stamatelatou K., Lyberatos G. On the occasional biodegradation of pharmaceuticals in the activated sludge process: The example of the antibiotic sulfamethoxazole. J. Hazard. Mater. 2005;122:259–265. doi: 10.1016/j.jhazmat.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 78.Carballa M., Omil F., Ternes T., Lema J.M. Fate of pharmaceutical and personal care products (ppcps) during anaerobic digestion of sewage sludge. Water Res. 2007;41:2139–2150. doi: 10.1016/j.watres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 79.Kim K.R., Owens G., Ok Y.S., Park W.K., Lee D.B., Kwon S.I. Decline in extractable antibiotics in manure-based composts during composting. Waste Manag. 2012;32:110–116. doi: 10.1016/j.wasman.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 80.Fedler C.B., Day D.L. Anaerobic Digestion of Swine Manure Containing an Antibiotic Inhibitor. Trans. ASAE. 1985;28:523–530. [Google Scholar]
- 81.Halling-Sørensen B., Sengeløv G., Tjørnelund J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch. Environ. Contam. Toxicol. 2002;42:263–271. doi: 10.1007/s00244-001-0017-2. [DOI] [PubMed] [Google Scholar]