Abstract
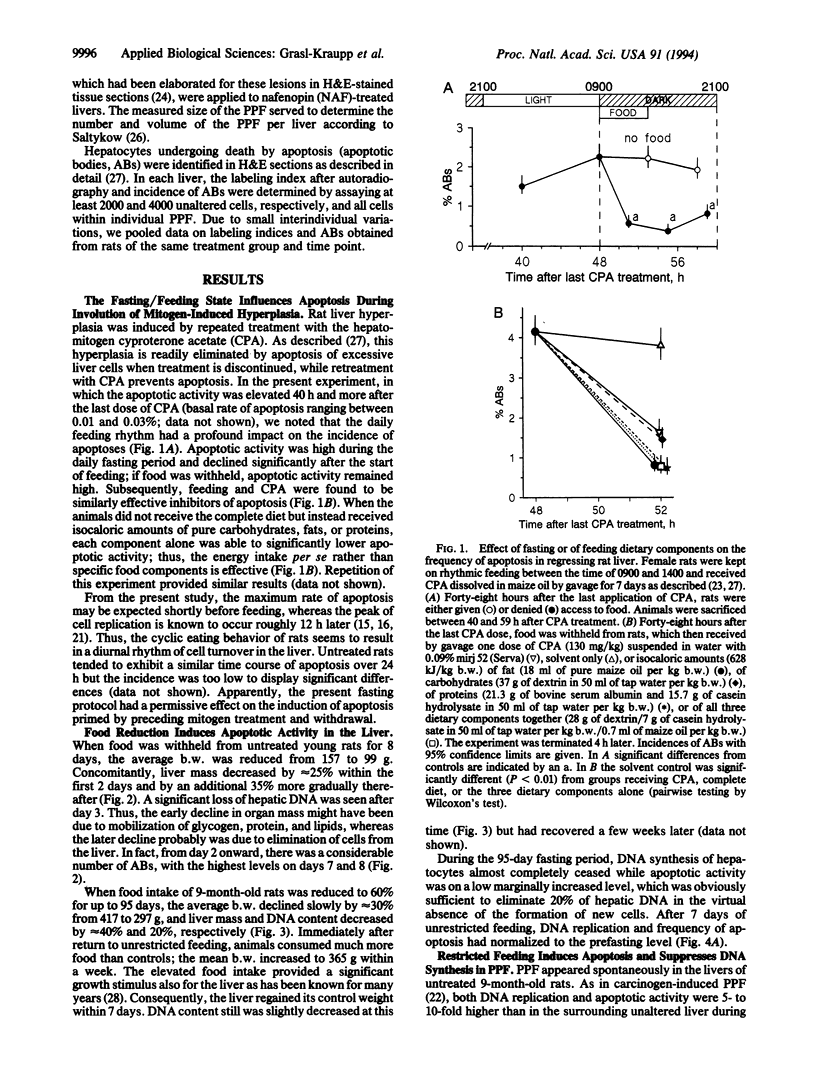
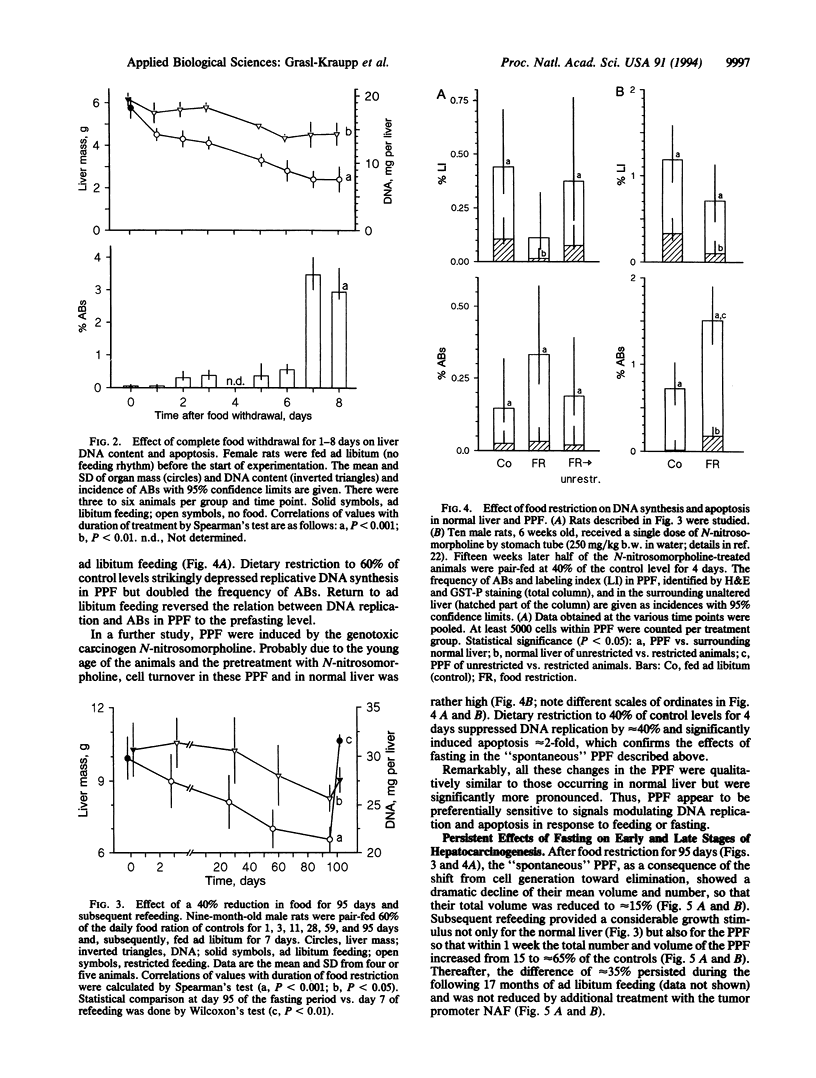
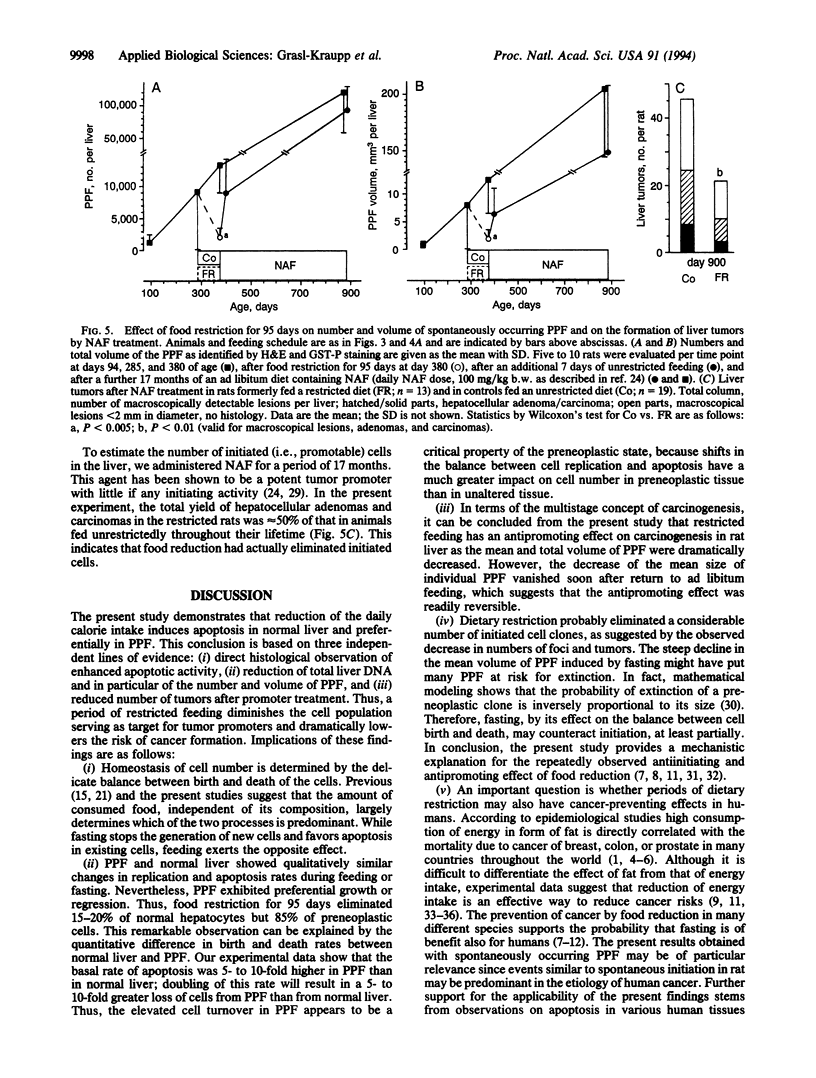
Restriction of dietary calories reduces cancer formation in experimental animals and probably also in humans. This effect is generally attributed to the inhibitory effect of fasting on cell proliferation. Here we studied the effect of fasting on physiological cell death through apoptosis by using rat liver as a model. (i) In normal liver, involution of hyperplasia by apoptosis was reinforced by food withdrawal and suppressed by feeding. Complete food withdrawal for 8 days or food reduction by 40% for 3 months eliminated 20-30% of normal liver cells through apoptosis. (ii) Putative preneoplastic liver foci exhibited severalfold higher rates of DNA replication and apoptosis than unaltered liver. Food restriction lowered DNA replication but increased apoptosis, which reduced the number and volume of putative preneoplastic liver foci by 85% within 3 months. Subsequent return to ad libitum feeding normalized cell replication and apoptosis but clear differences in the volume and number of putative preneoplastic liver foci persisted throughout the following 17 months. Treatment of animals after food restriction with nafenopin, a peroxisome proliferator and potent tumor promoter, produced only half as many hepatocellular adenomas and carcinomas as in animals fed unrestrictedly throughout their lifetime. This indicates that food restriction had actually eliminated a part of the initiated cells. This study demonstrates that food restriction preferentially enhances apoptosis of preneoplastic cells. This effect in combination with lowered cell replication provides protection from carcinogenesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albanes D. Total calories, body weight, and tumor incidence in mice. Cancer Res. 1987 Apr 15;47(8):1987–1992. [PubMed] [Google Scholar]
- Ames B. N., Magaw R., Gold L. S. Ranking possible carcinogenic hazards. Science. 1987 Apr 17;236(4799):271–280. doi: 10.1126/science.3563506. [DOI] [PubMed] [Google Scholar]
- Barbiroli B., Potter V. R. DNA synthesis and interaction between controlled feeding schedules and partial hepatectomy in rats. Science. 1971 May 14;172(3984):738–741. doi: 10.1126/science.172.3984.738. [DOI] [PubMed] [Google Scholar]
- Birt D. F., Salmasi S., Pour P. M. Enhancement of experimental pancreatic cancer in Syrian golden hamsters by dietary fat. J Natl Cancer Inst. 1981 Dec;67(6):1327–1332. [PubMed] [Google Scholar]
- Boissonneault G. A., Elson C. E., Pariza M. W. Net energy effects of dietary fat on chemically induced mammary carcinogenesis in F344 rats. J Natl Cancer Inst. 1986 Feb;76(2):335–338. [PubMed] [Google Scholar]
- Bursch W., Taper H. S., Lauer B., Schulte-Hermann R. Quantitative histological and histochemical studies on the occurrence and stages of controlled cell death (apoptosis) during regression of rat liver hyperplasia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;50(2):153–166. doi: 10.1007/BF02889898. [DOI] [PubMed] [Google Scholar]
- Doll R., Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981 Jun;66(6):1191–1308. [PubMed] [Google Scholar]
- Ferguson D. J., Anderson T. J. Ultrastructural observations on cell death by apoptosis in the "resting" human breast. Virchows Arch A Pathol Anat Histol. 1981;393(2):193–203. doi: 10.1007/BF00431076. [DOI] [PubMed] [Google Scholar]
- Freedman L. S., Clifford C., Messina M. Analysis of dietary fat, calories, body weight, and the development of mammary tumors in rats and mice: a review. Cancer Res. 1990 Sep 15;50(18):5710–5719. [PubMed] [Google Scholar]
- Grasl-Kraupp B., Waldhör T., Huber W., Schulte-Hermann R. Glutathione S-transferase isoenzyme patterns in different subtypes of enzyme-altered rat liver foci treated with the peroxisome proliferator nafenopin or with phenobarbital. Carcinogenesis. 1993 Nov;14(11):2407–2412. doi: 10.1093/carcin/14.11.2407. [DOI] [PubMed] [Google Scholar]
- Hague A., Manning A. M., Hanlon K. A., Huschtscha L. I., Hart D., Paraskeva C. Sodium butyrate induces apoptosis in human colonic tumour cell lines in a p53-independent pathway: implications for the possible role of dietary fibre in the prevention of large-bowel cancer. Int J Cancer. 1993 Sep 30;55(3):498–505. doi: 10.1002/ijc.2910550329. [DOI] [PubMed] [Google Scholar]
- Henderson M. M. Role of intervention trials in research on nutrition and cancer. Cancer Res. 1992 Apr 1;52(7 Suppl):2030s–2034s. [PubMed] [Google Scholar]
- Klurfeld D. M., Weber M. M., Kritchevsky D. Inhibition of chemically induced mammary and colon tumor promotion by caloric restriction in rats fed increased dietary fat. Cancer Res. 1987 Jun 1;47(11):2759–2762. [PubMed] [Google Scholar]
- Kraupp-Grasl B., Huber W., Taper H., Schulte-Hermann R. Increased susceptibility of aged rats to hepatocarcinogenesis by the peroxisome proliferator nafenopin and the possible involvement of altered liver foci occurring spontaneously. Cancer Res. 1991 Jan 15;51(2):666–671. [PubMed] [Google Scholar]
- Kritchevsky D., Weber M. M., Klurfeld D. M. Dietary fat versus caloric content in initiation and promotion of 7,12-dimethylbenz(a)anthracene-induced mammary tumorigenesis in rats. Cancer Res. 1984 Aug;44(8):3174–3177. [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Isaacs J. T. Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation. Cancer Res. 1990 Jun 15;50(12):3748–3753. [PubMed] [Google Scholar]
- Lok E., Scott F. W., Mongeau R., Nera E. A., Malcolm S., Clayson D. B. Calorie restriction and cellular proliferation in various tissues of the female Swiss Webster mouse. Cancer Lett. 1990 May 15;51(1):67–73. doi: 10.1016/0304-3835(90)90232-m. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G. Liver regeneration and growth factors: old puzzles and new perspectives. Lab Invest. 1992 Oct;67(4):413–415. [PubMed] [Google Scholar]
- Miller J. A., Miller E. C. Carcinogens occurring naturally in foods. Fed Proc. 1976 May 1;35(6):1316–1321. [PubMed] [Google Scholar]
- Moolgavkar S. H., Luebeck E. G., de Gunst M., Port R. E., Schwarz M. Quantitative analysis of enzyme-altered foci in rat hepatocarcinogenesis experiments--I. Single agent regimen. Carcinogenesis. 1990 Aug;11(8):1271–1278. doi: 10.1093/carcin/11.8.1271. [DOI] [PubMed] [Google Scholar]
- Oberhammer F. A., Pavelka M., Sharma S., Tiefenbacher R., Purchio A. F., Bursch W., Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5408–5412. doi: 10.1073/pnas.89.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitot H. C. Altered hepatic foci: their role in murine hepatocarcinogenesis. Annu Rev Pharmacol Toxicol. 1990;30:465–500. doi: 10.1146/annurev.pa.30.040190.002341. [DOI] [PubMed] [Google Scholar]
- Pollard M., Luckert P. H., Snyder D. Prevention of prostate cancer and liver tumors in L-W rats by moderate dietary restriction. Cancer. 1989 Aug 1;64(3):686–690. doi: 10.1002/1097-0142(19890801)64:3<686::aid-cncr2820640320>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Prentice R. L., Sheppard L. Dietary fat and cancer: consistency of the epidemiologic data, and disease prevention that may follow from a practical reduction in fat consumption. Cancer Causes Control. 1990 Jul;1(1):81–109. doi: 10.1007/BF00053187. [DOI] [PubMed] [Google Scholar]
- Préat V., Lans M., de Gerlache J., Taper H., Roberfroid M. Comparison of the biological effects of phenobarbital and nafenopin on rat hepatocarcinogenesis. Jpn J Cancer Res. 1986 Jul;77(7):629–638. [PubMed] [Google Scholar]
- Schulte-Hermann R., Schuppler J., Timmermann-Trosiener I., Ohde G., Bursch W., Berger H. The role of growth of normal and preneoplastic cell populations for tumor promotion in rat liver. Environ Health Perspect. 1983 Apr;50:185–194. doi: 10.1289/ehp.8350185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Hermann R., Timmermann-Trosiener I., Barthel G., Bursch W. DNA synthesis, apoptosis, and phenotypic expression as determinants of growth of altered foci in rat liver during phenobarbital promotion. Cancer Res. 1990 Aug 15;50(16):5127–5135. [PubMed] [Google Scholar]
- Schulte-Hermann R., Timmermann-Trosiener I., Schuppler J. Promotion of spontaneous preneoplastic cells in rat liver as a possible explanation of tumor production by nonmutagenic compounds. Cancer Res. 1983 Feb;43(2):839–844. [PubMed] [Google Scholar]
- Schulte-Hermann R. Two-stage control of cell proliferation induced in rat liver by alpha-hexachlorocyclohexane. Cancer Res. 1977 Jan;37(1):166–171. [PubMed] [Google Scholar]
- Sylvester P. W., Aylsworth C. F., Van Vugt D. A., Meites J. Influence of underfeeding during the "critical period" or thereafter on carcinogen-induced mammary tumors in rats. Cancer Res. 1982 Dec;42(12):4943–4947. [PubMed] [Google Scholar]
- TANNENBAUM A., SILVERSTONE H. Nutrition in relation to cancer. Adv Cancer Res. 1953;1:451–501. doi: 10.1016/s0065-230x(08)60009-3. [DOI] [PubMed] [Google Scholar]
- Welsch C. W., House J. L., Herr B. L., Eliasberg S. J., Welsch M. A. Enhancement of mammary carcinogenesis by high levels of dietary fat: a phenomenon dependent on ad libitum feeding. J Natl Cancer Inst. 1990 Oct 17;82(20):1615–1620. doi: 10.1093/jnci/82.20.1615. [DOI] [PubMed] [Google Scholar]
- Willett W. C., Stampfer M. J., Colditz G. A., Rosner B. A., Speizer F. E. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med. 1990 Dec 13;323(24):1664–1672. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]
- Youngman L. D., Park J. Y., Ames B. N. Protein oxidation associated with aging is reduced by dietary restriction of protein or calories. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9112–9116. doi: 10.1073/pnas.89.19.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]



