Supplemental Digital Content is available in the text.
Abstract
Background:
Actually, there are 2 main methods to obtain stromal vascular fraction (SVF): enzymatic digestion and mechanical filtration; however, the available systems report heterogeneous and sometimes not univocal results. The aim of this study is to evaluate different procedures for SVF isolation and compare their clinical efficacy in the treatment of soft-tissue defects in plastic and reconstructive surgery. The authors evaluated Celution and Medikhan, enzymatic systems, and Fatstem and Mystem system, mechanical separation systems.
Methods:
Fifty patients affected by breast soft-tissue defects were treated in the Plastic and Reconstructive Surgery Department of Tor Vergata University of Rome. Four groups of 10 patients were managed with enhanced SVF fat grafts using cells obtained by Celution (Cytori Therapeutics, Inc., San Diego, Calif.), Medikhan (Medi-Khan Inc., West Hollywood, Calif.), Fatstem (Fatstem CORIOS Soc. Coop, San Giuliano Milanese, Italy), and Mystem (Mystem evo Bi-Medica, Treviolo, Italy) systems. A control group of 10 patients was treated with only centrifuged fat according to Coleman’s technique.
Results:
In enhanced SVF–treated patients treated with cells obtained by Celution system, we observed a 63% ± 6.2% maintenance of contour restoring after 1 year, compared with 39% ± 4.4% of control group. In patients treated with SVF obtained by Medikhan system, we observed a 39% ± 3.5% maintenance, whereas enhanced SVF with Fatstem and Mystem systems gave a 52% ± 4.6% and 43% ± 3.8% maintenance of contour restoring, respectively. SVF cell counting indicated that Celution and Fatstem were the most efficient systems to obtain SVF cells.
Conclusions:
Celution and Fatstem were the 2 best automatic systems to obtain SVF and to improve maintenance of fat volume and prevent the reabsorption.
The adipose tissue is a highly complex tissue and consists of mature adipocytes, preadipocytes, fibroblasts, vascular smooth muscle cells, endothelial cells, resident monocytes/macrophages,1–4 and lymphocytes.4 The stromal vascular fraction (SVF) of the adipose tissue has come more and more into the focus of stem cell research, as this tissue compartment provides a rich source of multipotent adipose tissue-derived stromal cells.5,6 SVF represents an alternative source of autologous adult stem cells that can be obtained easily and repeatedly in large quantities under general or local anesthesia. Adipose tissue-derived stromal cells have the potential to differentiate into bone, cartilage, tendons, skeletal muscle, and fat when cultivated under lineage-specific conditions.7–10 Tissue engineering of these mesenchymal organs represents an interesting research field for different human diseases, such as inherited, traumatic, or degenerative bone, joint, and soft-tissue defects (skeletal regeneration and cartilage repair). Plastic tissue regeneration after tumor surgery for breast cancer and other malignancies, reconstruction of muscle and adipose tissue after scars, and burn injury represent additional needs for cell-based therapies. In addition, SVF demonstrated to possess the potential for vascular9 and macrophage10 differentiation, an important process for microenvironment tropism and tissue regeneration.
The authors have already published the results obtained from the application of enhanced SVF (e-SVF) in posttraumatic lower extremity ulcers11 and the promising results obtained from the use of fat grafting and e-SVF in breast reconstruction.12,13 These previous findings demonstrated that the application of e-SVF can improve tissue healing and maintenance of fat graft volume. The subsequent questions are as follows: What is the most efficient method to isolate SVF? What is the best system to use considering also the clinical outcome? The purpose of this article is to provide an answer to these questions. In this study, e-SVF was extracted from patient’s adipose tissue at the bedside using enzymatic digestion or mechanical filtration. The authors evaluated, in the first case, Celution and Medikhan system; in the second case, the authors evaluated Fatstem and Mystem system. The enzymatic digestion method separates the SVF cell population from mature adipocytes and extracellular matrix by enzymatic digestion and subsequent centrifugation. The mechanical filtration method separates SVF cell population by centrifugation and subsequent filtration of the solution obtained through 0.2-μm filter. The findings achieved from this study demonstrated that clinical outcome depends on SVF isolation method applied and system used.
MATERIALS AND METHODS
Patients and Clinical Procedures
A total of 50 patients aged 19–60 years were treated from January 2013 to March 2014 at the Plastic and Reconstructive Surgery Department of “Tor Vergata” University, Rome. Ten patients affected by breast soft-tissue defects (1 patient affected by unilateral breast hypoplasia, 6 patients affected by outcomes of radiotherapy in breast cancer reconstruction, and 3 patients after prosthesis removal) were treated with SVF-enhanced autologous fat grafts obtained by Medikhan for breast reconstruction (Fig. 1). The fat (80 ml) was centrifuged at 4000 rpm for 8 minutes (Fig. 1A) and placed in 60-ml syringes. At the end of centrifugation, the authors obtained 25 ml of condensated fat (Fig. 1B) that was enzymatically digested in the TP102 syringe Celltibator, containing 1 ml of collagenase and 24 ml of saline solution (Fig. 1C). The syringe was aseptically inserted in the incubator for the isolation of SVF by a slow centrifugation for 30 minutes at 200 rcf (Fig. 1D). At the end of this procedure, the authors extracted the syringe and put the digestion obtained in a new 60-ml syringe. After extensive washing and subsequent centrifugation cycles (200 rcf for 4 minutes), the authors obtained 5 ml of solution containing SVF and added it to fat graft. To implant the fat tissue, small tunnels were previously created, forcing the cannulas of 1.5 mm diameter with accurate and controlled movements. Once the fat tissue was implanted at different levels, the access incisions were closed using 5-0 nylon stitches, and no compressive bandage was applied. It was then reinjected aseptically with a specific microcannula, using the drop-to-drop technique in small pulses (0.2–1 ml), in a radial retrograde manner, on different planes into multiple areas of the breast.
Fig. 1.

Medikhan procedure. A, Medikhan centrifuge. B, The fat (80 ml) was centrifuged at 4000 rpm for 8 minutes and placed in 60-ml syringes; extraction of syringes at the end of centrifugation. C, Collagenase, 1 ml of collagenase and 24 ml of saline solution and 25 ml of condensated fat. D, The syringe with condensated fat was enzymatically digested in the TP102 syringe Celltibator, containing 1 ml of collagenase and 24 ml of saline solution and was aseptically inserted in the incubator for the isolation of SVF by a slow centrifugation for 30 minutes at 200 rcf.
According to the patient’s needs, in each session, 50–150 ml (average, 93.54 ml) was injected, for a total of 187 ml (range, 110–250 ml) per patient.
Ten patients (2 patients affected by unilateral breast hypoplasia, 7 patients affected by outcomes of radiotherapy in breast cancer reconstruction, and 1 patient after prosthesis removal) were treated with SVF-enhanced autologous fat grafts, obtained using the Celution system (Fig. 2). Patient’s adipose tissue (80 ml) was automatically centrifuged (Fig. 2A) and subjected to enzymatic digestion (Fig. 2B), additional wash (Fig. 2C), and centrifugation cycles. At the end, 5 ml of the e-SVF suspension was extracted from the system (Fig. 2D). The e-SVF suspension was added and mixed with the washed fat graft. Using specific microcannulas for implantation, the SVF-enhanced fat graft was transferred into 10-ml syringes and aseptically reinjected into the soft-tissue defect.
Fig. 2.
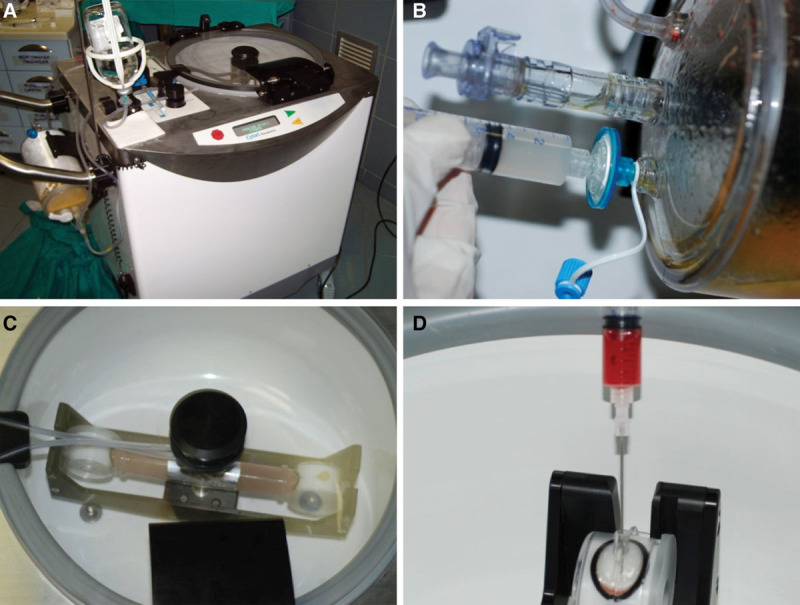
Celution procedure. A, Celution system. B, The Celase 835/CRS Reagent was added to enzymatically digest the tissue that released SVF. C, Enzymatic digestion. D, 5 ml of the SVF suspension was extracted from the system.
Ten patients affected by breast soft-tissue defects (1 patient affected by unilateral breast hypoplasia, 5 patients affected by outcomes of radiotherapy in breast cancer reconstruction, and 4 patients after prosthesis removal) were treated with SVF-enhanced autologous fat grafts obtained by Fatstem for breast reconstruction (Fig. 3). Fat (80 ml) was subjected to automatic filtration (Fig. 3B) and centrifugation cycles at 1700 rpm per 10 minutes (Fig. 3C), after which 40 ml of the suspension was extracted from the bag. The suspension was further filtered through 0.2-μm filter, and 20 ml of the e-SVF suspension was obtained (Fig. 3D). Subsequently, the e-SVF suspension was added and mixed with the centrifuged fat graft. Using specific microcannulas for implantation, the SVF-enhanced fat graft was transferred into 10-ml syringes and aseptically reinjected into the soft-tissue defect.
Fig. 3.
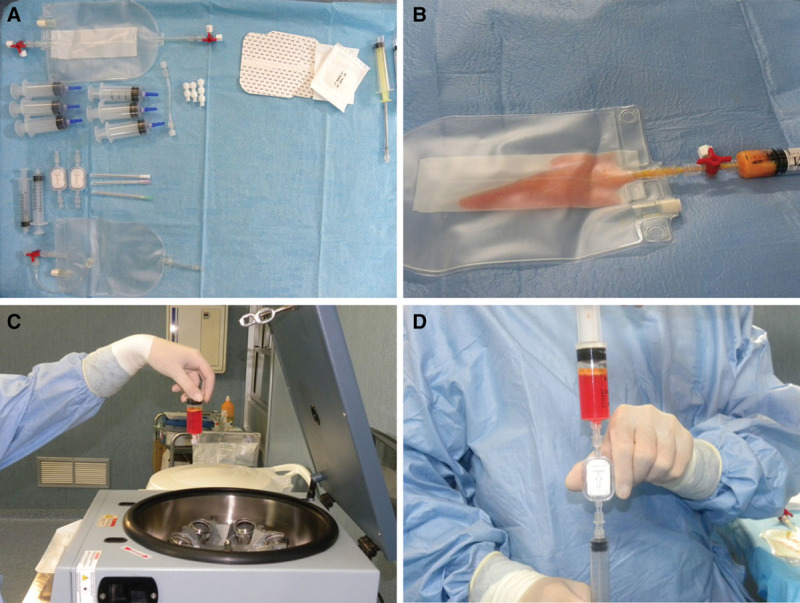
Fatstem procedure. A, Fatstem kit. B, Fat (80 ml) was inserted in the bag and subjected to automatic filtration. C, The fat harvested was inserted in a centrifuge at 1700 rpm per 10 minutes. D, The suspension obtained after centrifugation was further filtered through 0.2-μm filter, and 20 ml of the e-SVF suspension was obtained.
Ten patients affected by breast soft-tissue defects (2 patient affected by unilateral breast hypoplasia, 6 patients affected by outcomes of radiotherapy in breast cancer reconstruction, and 2 patients after prosthesis removal) were treated with SVF-enhanced autologous fat grafts obtained by Mystem (Fig. 4) for breast reconstruction. Fat (80 ml) was subjected to automatic wash and filtration cycles (Fig. 4B) based on the direct passage through 0.2-μm filter, after which 10 ml of the residual fluid was extracted from the system (Fig. 4C). The e-SVF suspension (20-ml average) was extracted from the filter (Fig. 4D). Subsequently, the e-SVF suspension was added and mixed with the centrifuged fat graft. This system does not include a centrifuge, and so the authors centrifuged and purified fat grafting using the Coleman procedure. Then, using specific microcannulas for implantation, the SVF-enhanced fat graft was transferred into 10-ml syringes and aseptically reinjected into the soft-tissue defect.
Fig. 4.
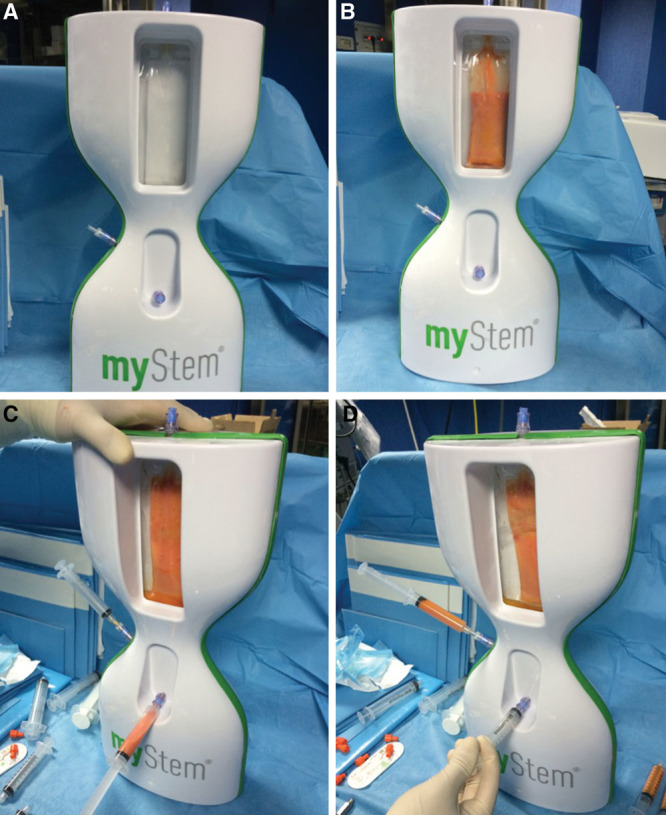
Mystem procedure. A, Mystem kit. B, Fat (80 ml) was added to Mystem and it was contained in the bag. C, After filtration and washed cycles, the residual fluid and oil was removed. D, 10 ml of the e-SVF suspension was extracted from the system (see Supplemental Fig. 1, Supplemental Digital Content 1, which shows a patient affected by breast hypoplasia treated with e-SVF fat graft obtained by Fatstem, http://links.lww.com/PRSGO/A99).
The control group comprised 10 women aged 21–65 years, all affected by breast soft-tissue defects (3 patients affected by unilateral breast hypoplasia, 5 patients affected by outcomes of radiotherapy in breast cancer reconstruction, and 2 patients after prosthesis removal). The fat (80 ml) of control group was treated only with centrifuged fat grafting injection according to the Coleman procedure.
Before and after each procedure, we performed a careful anamnesis and a clinical examination and took photographs to document improvement or the disappearance of defects. Before the first lipofilling session, mammography, ultrasound, and magnetic resonance imaging (MRI) were performed to rule out signs of tumor recurrence and to have an initial point of comparison to identify new lesions. Only patients who showed no signs of malignancy were included in this study. All procedures were performed under general anesthesia at least 3 months after surgery and at least 6 months after the end of radiotherapy/chemotherapy.
In all patients, the procedure was carried out in 2 sessions. According to the patient’s needs, in each of the 2 sessions, 50–150 ml (average, 93.54 ml) was injected, for a total of 187 ml (range, 110–250 ml) per patient.
This study is part of a research project approved by Tor Vergata University, and all procedures were performed under written patient informed consent and according to the guidelines of the local committee on human research.
Exclusion Criteria
The exclusion criteria were divided into 2 types: systemic and local. The systemic criteria included platelet disorders, thrombocytopenia, antiaggregating therapy, bone marrow aplasia, uncompensated diabetes, sepsis, and cancer. The local criteria included cancer loss of substance. We did not consider tobacco use or genetic disorders as exclusion criteria.
Clinical Evaluation Methods
Three methods for the evaluation of outcomes were used: (1) team evaluation, (2) MRI and ultrasound, and (3) patient self-evaluation.
The team (plastic surgeons involved in the study) evaluation is an evaluation method based on clinical observation, using a scale of 5 values (excellent, good, discreet enough, poor, and inadequate). The patient-based self-evaluation uses the same 5 values mentioned above. The factors/variables that were taken into account were pigmentation, vascularization, pliability, thickness, itching, and pain.
The percentage of maintenance restored was clinically evaluated with 2 different criteria. The first was the subjective evaluation, and the second one was the objective evaluation.
The subjective evaluation was based on the personal score of each patient focused on the following parameters: (1) presence of asymmetry, deformity, irregularity, dyschromia, dysesthesia, paraesthesia, and pain; (2) results of the superoexternal quadrant, inferoexternal quadrant, superointernal quadrant, and inferointernal quadrant; (3) resorption of fat in one or more regions; (4) time of stabilization of the transplanted fat; and (5) need for retreatment.
For each parameter, patients gave a yes/no or positive/negative evaluation, and percentage of maintenance of restored was calculated as the mean of all calculated single percentages.
The objective evaluation was made on the analysis of the preoperative and postoperative photographs. The photographs were of the same size, brightness, and even contrast. According to parameters reported above, the operator similarly calculated the percentage of restoration.
Postoperative follow-up took place at 2, 7, 15, 21, and 36 weeks and then annually.
Finally, the mean between patient and operator evaluations was calculated.
SVF Nucleated Cells Counting
A small sample of SVF obtained by the automatic systems was resuspended in erythrocyte lysis buffer (155 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA) and incubated for 5 minutes at room temperature. After centrifugation at 1100 rpm for 5 minutes, the pellet was resuspended in a few microliters of growth medium and passed through a 100-μm Falcon strainer (Becton and Dickinson, Sunnyvale, Calif.) and cellular population counted by using a hemocytometer with trypan blue staining exclusion. Cell viability by trypan blue exclusion was consistently more than 98%. Cell yield was referred to cell number/ml of fat graft volume.
Statistical Analysis
Values were expressed as mean plus standard error and analyzed by Student’s t test. Differences were considered statistically significant for P < 0.05.
RESULTS
Clinical Outcome
In patients treated with SVF-enhanced autologous fat grafts obtained by Celution (see Supplemental Fig. 2A, Supplemental Digital Content 2, which shows the preoperative situation of patient affected by bilateral breast hypoplasia in frontal projection, http://links.lww.com/PRSGO/A100), the authors observed a 63% + 6.2% maintenance of contour restoring and 3-dimensional volume after 1 year (see Supplemental Fig. 2C, Supplemental Digital Content 2, which shows the postoperative situation after 12 months and after 1 treatment based on the use of e-SVF in frontal projection, http://links.lww.com/PRSGO/A100) compared with only 39% + 4.4% of the control group treated with fat graft centrifuged (P < 0.0001). In patients treated with SVF-enhanced autologous fat grafts obtained by Medikhan, the authors observed the same maintenance of contour restoring and 3-dimensional volume of the control group (39% + 3.5%). In patients treated with SVF-enhanced autologous fat grafts obtained by Fatstem, a 52% + 4.6% maintenance of contour restoring and 3-dimensional volume was noted (P < 0.001)(see Supplemental Fig. 1A, Supplemental Digital Content 1, which shows a preoperative situation of patient affected by bilateral breast hypoplasia in frontal projection; Supplemental Fig. 1B shows the postoperative situation after 12 months and after 1 treatment based on the use of e-SVF in frontal projection; Supplemental Fig. 1C shows the preoperative situation in ¾ right projection; Supplemental Fig. 1D shows the postoperative situation in ¾ right projection after 12 months, http://links.lww.com/PRSGO/A99). In patients treated with SVF-enhanced autologous fat grafts obtained by Mystem, the authors observed a 43% + 3.8% maintenance of contour restoring and 3-dimensional volume. The authors reported the results in Figure 5B. Transplanted fat tissue reabsorbing was analyzed with instrumental imaging (MRI-ultrasound). In patients affected by soft-tissue defects of the breast, reconstruction with SVF-enhanced fat tissue obtained by Celution and Fatstem showed a lower fat reabsorbing. All patients were satisfied with the resulting texture, softness, contour, and MRI (see Supplemental Fig. 2B, Supplemental Digital Content 2, which shows the MRI, preoperative situation; Supplemental Fig. 2D, which shows the MRI, postoperative situation after 12 months, http://links.lww.com/PRSGO/A100) and confirmed the percentage of resorption. The images were acquired on axial and sagittal planes. Based on MRI scans acquired, volumetric assessments of the breasts were also calculated, taking as edges the anterior axillary line, anterior margin of the pectoral muscle, mediosternal line, skin, and nipple.
Fig. 5.
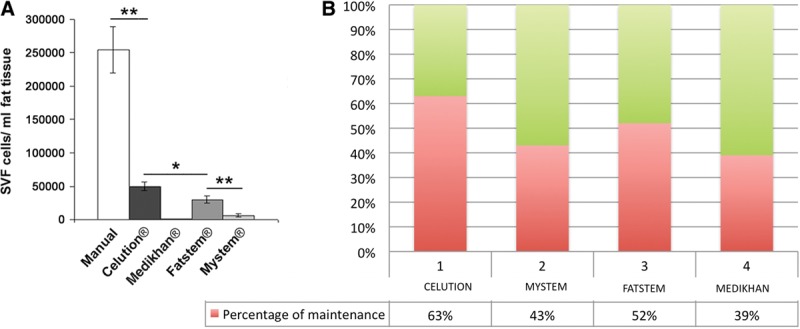
SVF cell counting. A, Graph bar showing SVF cell yield obtained by different isolation methods: Celution, Medikhan, Fatstem, and Mystem systems. B, Graph bar of fat graft maintenance obtained by different isolation methods: Celution, Medikhan, Fatstem, and Mystem systems.
They were assessed using a 3-dimensional reconstruction on a separate workstation (ADW 4.0; GE Medical Systems, Milwaukee, Wis.).
Finally, at 12 months after the last lipofilling session, the resorption percentage for each breast was evaluated. Then, we calculated their overall average and the average for the breasts with oily cyst resorption and for those remaining without resorption. All examinations were performed and analyzed in a blinded fashion by 2 radiologists experienced in interpreting breast imaging.
Ultrasound showed oily cysts in 45.83% at 12 months after the last lipofilling, At the same time, cytosteatonecrotic areas were observed with ultrasound in 12.5%.
There were no complications in any patient, and the results were lasting in all cases (the mean follow-up period was 0 months). As reported, Celution and Fatstem were the 2 best systems to improve the maintenance of fat volume.
SVF Nucleated Cells from Automatic Systems
As reported in Figure 5A, SVF obtained by Celution system gave the higher cell recovery (P < 0.05 vs Fatstem system). The automatic SVF extraction by Fatstem and Mystem systems (P < 0.01 vs Fatstem) gave intermediate values. Medikhan system gave irrelevant amounts of SVF cells whose adhesion to plastic supports for culture was negligible.
DISCUSSION
The potential benefit of e-SVF supplementation could be explained by the ability of cells, which exist within the e-SVF population,14 to secrete various growth factors that improve survival and increased vascularization,15,16 leading to an increased survival of the graft as shown by a study on a rodent.17 The authors previously published the results obtained from the application of e-SVF in posttraumatic lower extremity ulcers13 and the promising results obtained by the use of fat grafting and e-SVF in breast reconstruction.12,13,18 These previous findings demonstrated that the application of e-SVF can improve tissue healing and maintenance of fat graft volume. The term SVF refers to a heterogeneous cell population isolated from adipose tissue that also contained multipotent stem cells named adipose-derived stem cells. Actually, there are 2 methods to obtain SVF: enzymatic digestion and mechanical filtration. In this work, the authors evaluated different SVF isolation methods to verify the best technique for clinical application.
We documented that Celution and Fatstem automatic systems gave the best SVF cell recovery. Aronowitz and Ellenhorn19 demonstrated that the Celution system shows the highest and most reproducible cell output, better cell viability, compared with other commercial systems. Moreover, the Celution system shows low residual enzyme activity.
Recently, Markarian et al20 described an alternative method for SVF isolation, based on the use of trypsin, with interesting results. Actually, there are no studies of nonenzymatic SVF isolation, without adipose tissue digestion. Rada et al21 described a method that provided the isolation of rat SVF by antibodies using immunomagnetic beads, but the use of animal antibodies might lead to contamination with viruses or prions.
The SVF extraction must comply with 2 fundamental concepts: traceability and qualification.22 We hypothesize that the mechanism of regeneration of the tissue is the following: targeting of damaged areas, release of angiogenic and antiapoptotic factors for the hypoxic stress, and then, the formation of new vessels and oxygenation.
Implanted adipose tissue must survive by a simple diffusion mechanism until an active blood supply is re-established. Prosurvival factors may therefore promote long-term retention and consequently durability of the graft. In an animal study, this effect was achieved by using gene therapy to deliver vascular endothelial growth factor (a potent proangiogenic factor) to the graft. This resulted in increased blood vessel density within the graft and a significant improvement in graft retention at 15 weeks.16 Neoangiogenesis was also confirmed from a histopathologically point of view highlighting the abundance in capillaries sprout within the healing tissue, leading to a complete re-epithelialization of the ulcers.13
e-SVF can favor neoangiogenic vascularization and fibrogenic activity of fibroblasts that favor adipose tissue survival and 3-dimensional organization.12 Compared with traditional fat grafting, the survival of the graft is more probable and fat necrosis is potentially reduced due to improved vascular development in the implanted area.12,13,18 This study demonstrated that e-SVF improved maintenance and function of adipose tissue graft. One of the main reasons why the technique of fat graft injection was questioned is that there may be lipofilling resorption. In the literature, the resorption rate reported over the first year is highly variable (20–90%), most evidently between the fourth and sixth months.23–25 However, so far, in many studies, the evidence of breast lipofilling survival was based on patient satisfaction and plastic surgeons’ evaluations.26,27 In a study where mammary volumes were calculated by computed tomography using a 3-dimensional program, a resorption rate of 47.5% at 9 months was reported.28
Serial MRI offers a quantitative measure of fat resorption and survival.
In a previous study,12 the authors found a total average resorption percentage of 15.36% at 6 months after the last lipofilling session and of 28.23% at 12 months after the last lipofilling session in a patient with soft-tissue defects of the breasts treated with fat graft mixed with platelet rich plasma (platelet rich lipotransfert). The authors previously discussed the effect of PRP in vitro and in clinical fat graft maintenance.29
To prevent, or rather minimize, resorption, it is crucial to perform each step of the procedure carefully, paying close attention to the technical details. The authors injected 200–600 ml of modified Klein solution (1fl of adrenalin in 500 ml of cold physiological solution + 3fl of Naropine 7.5 mg/ml) into the donor site before liposuction. The lipoaspirate must then be purified in various ways: washed with different solutions, filtered, centrifuged, and in this study enriched with e-SVF obtained by enzymatic digestion or mechanical filtration.
In our patients, the lipoaspirate was purified by centrifugation and combined with SVF. SVF improves lipofilling results and reduces the resorption rate, increasing fat graft survival.30 Then, the lipoaspirate was injected using the drop-to-drop technique and in multiple sessions to maximize the contact surface between the lipoaspirate and the host’s capillaries.24 Finally, the problem of fat resorption may be resolved by injecting a total volume of fat not greater than the desired volume, such as suggested actually, but with injection of a volume of fat balanced and studied on the dimension of the defects to correct. In particular, the authors suggest that the volume of fat graft that will need to be used will be the same of the defect volume.
CONCLUSIONS
Clinical outcome was supported by SVF cell counting that indicated Celution and Fatstem as the best automatic systems to obtain SVF cells starting from the same amount of adipose tissue. Consequently, we reported that clinical results depend on the SVF isolation method used and the system applied.
Supplementary Material
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Franchini M. [Mesenchymal stem cells: from biology to clinical applications]. Recenti Prog Med. 2003;94:478–483. [PubMed] [Google Scholar]
- 2.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 3.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz AJ, Tholpady A, Tholpady SS, et al. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez AM, Elabd C, Amri EZ, et al. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87:125–128. doi: 10.1016/j.biochi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 9.Urbich C, Dimmeler S. Endothelial progenitor cells functional characterization. Trends Cardiovasc Med. 2004;14:318–322. doi: 10.1016/j.tcm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Charrie`re G, Cousin B, Arnaud E, et al. Preadipocyte conversion to macrophage. Evidence o plasticity. J BiolChem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 11.Cervelli V, Gentile P, De Angelis B, et al. Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post-traumatic lower extremity ulcers. Stem Cell Res. 2011;6:103–111. doi: 10.1016/j.scr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Fiaschetti V, Pistolese CA, Fornari M, et al. Magnetic resonance imaging and ultrasound evaluation after breast autologous fat grafting combined with platelet-rich plasma. Plast Reconstr Surg. 2013;132:498e–509e. doi: 10.1097/PRS.0b013e3182a00e57. [DOI] [PubMed] [Google Scholar]
- 13.Gentile P, Orlandi A, Scioli MG, et al. A comparative translational study: the combined use of enhanced stromal vascular fraction and platelet-rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cells Transl Med. 2012;1:341–351. doi: 10.5966/sctm.2011-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–1422; discussion 1423. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55; discussion 56. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lendeckel S, Jödicke A, Christophis P, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 17.García-Olmo D, García-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–1423. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 18.Gentile P, Di Pasquali C, Bocchini I, et al. Breast reconstruction with autologous fat graft mixed with platelet-rich plasma. Surg Innov. 2013;20:370–376. doi: 10.1177/1553350612458544. [DOI] [PubMed] [Google Scholar]
- 19.Aronowitz JA, Ellenhorn JD. Adipose stromal vascular fraction isolation: a head-to-head comparison of four commercial cell separation systems. Plast Reconstr Surg. 2013;132:932e–939e. doi: 10.1097/PRS.0b013e3182a80652. [DOI] [PubMed] [Google Scholar]
- 20.Markarian CF, Frey GZ, Silveira MD, et al. Isolation of adipose-derived stem cells: a comparison among different methods. Biotechnol Lett. 2014;36:693–702. doi: 10.1007/s10529-013-1425-x. [DOI] [PubMed] [Google Scholar]
- 21.Rada T, Gomes ME, Reis RL. A novel method for the isolation of subpopulations of rat adipose stem cells with different proliferation and osteogenic differentiation potentials. J Tissue Eng Regen Med. 2011;5:655–664. doi: 10.1002/term.364. [DOI] [PubMed] [Google Scholar]
- 22.Bourin P, Peyrafitte JA, Fleury-Cappellesso S. A first approach for the production of human adipose tissue- derived stromal cells for therapeutic use. Methods Mol Biol. 2011;702:331–343. doi: 10.1007/978-1-61737-960-4_24. [DOI] [PubMed] [Google Scholar]
- 23.Delay E. Lipomodeling of the reconstructed breast. In: Spear SL, Willey SC, Robb GL, et al., editors. In: Surgery of the Breast: Principles and Art. 2nd ed. Vol. 1. Philadelphia, Pa.: Lippincott Williams & Wilkins; 2006. pp. 930–946. [Google Scholar]
- 24.Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg. 2007;119:775–785; discussion 786. doi: 10.1097/01.prs.0000252001.59162.c9. [DOI] [PubMed] [Google Scholar]
- 25.Chan CW, McCulley SJ, Macmillan RD. Autologous fat transfer—a review of the literature with a focus on breast cancer surgery. J Plast Reconstr Aesthet Surg. 2008;61:1438–1448. doi: 10.1016/j.bjps.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Coleman SR. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg. 1995;19:421–425. doi: 10.1007/BF00453875. [DOI] [PubMed] [Google Scholar]
- 27.Zheng DN, Li QF, Lei H, et al. Autologous fat grafting to the breast for cosmetic enhancement: experience in 66 patients with long-term follow up. J Plast Reconstr Aesthet Surg. 2008;61:792–798. doi: 10.1016/j.bjps.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 28.Amar O, Bruant-Rodier C, Lehmann S, et al. [Fat tissue transplant: restoration of the mammary volume after conservative treatment of breast cancers, clinical and radiological considerations]. Ann Chir Plast Esthet. 2008;53:169–177. doi: 10.1016/j.anplas.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Cervelli V, Gentile P, Scioli MG, et al. Application of platelet-rich plasma in plastic surgery: clinical and in vitro evaluation. Tissue Eng Part C Methods. 2009;15:625–634. doi: 10.1089/ten.TEC.2008.0518. [DOI] [PubMed] [Google Scholar]
- 30.Cervelli V, Scioli MG, Gentile P, et al. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Transl Med. 2012;1:206–220. doi: 10.5966/sctm.2011-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]


