Abstract
Background:
AlloDerm, a human acellular dermal matrix, is available in a ready-to-use (RTU) or freeze-dried (FD) form. A limited number of studies have compared complication rates between RTU and FD in implant-based breast reconstruction. The objective of this report was to conduct a meta-analysis of previously reported complication rates between RTU and FD.
Methods:
A systematic literature review was conducted from 2010 to 2014 and supplemented by hand searches. Included studies compared both RTU and FD. Odds ratios and relative risks (RRs) with 95% confidence interval (CI), taking into account study heterogeneity, were calculated. Studies reporting patient-level results as opposed to breast-level results were excluded from the primary analysis but included in subsequent sensitivity analyses. Variable follow-up time within and between studies was also considered in a sensitivity analysis.
Results:
Of the 275 identified studies, 115 studies were eligible for detailed review. Only 5 studies compared RTU with FD, and of these, 2 studies had breast-level data and 1 study had patient-level data appropriate for meta-analysis. The 2 studies included in the primary meta-analysis had a pooled sample size: n = 116 RTU and n = 109 FD patients, or 205 and 186 breasts, respectively. Age and body mass index were similar between groups. Across all meta-analyses, there were no differences in complication rates between RTU and FD: cellulitis (RR = 0.863; 95% CI, 0.272–2.740), seroma (RR = 0.553; 95% CI, 0.026–11.830), and explantation (RR = 0.593; 95% CI, 0.247–1.425). Results remained nonsignificant even after adjustment for variable follow-up time.
Conclusion:
The results suggest that there are no differences in complication rates between RTU and FD forms.
Device-based breast reconstruction following prophylactic or therapeutic mastectomy constitutes the majority of breast reconstructions that are performed today.1 Immediate prosthetic reconstruction is commonly performed using total or partial muscle coverage techniques. Partial muscle coverage for both 1- and 2-stage reconstructions is frequently performed using acellular dermal matrix (ADM). The benefits of ADM include its ability to provide soft-tissue support, provide elasticity within the periprosthetic space, and stabilize the position of the pectoralis major muscle. The use of ADM for 2-stage reconstruction has also been demonstrated to facilitate the expansion process and decrease the time to device exchange.2–4 The most commonly used ADM in the United States is AlloDerm Regenerative Tissue Matrix (LifeCell Corporation, an Acelity company, Bridgewater, N.J.). This ADM is currently available in 2 forms that include sterile “ready-to-use” (RTU) form and an aseptic “freeze-dried” (FD) form. The RTU form requires 2–3 minutes of rehydration, whereas the FD form requires approximately a 30-minute rehydration just before the reconstruction procedure.
There have been many retrospective studies evaluating the benefit of ADM for prosthetic breast reconstruction; however, there has been no prospective randomized clinical trials comparing RTU with FD. Many cohort studies, either prospective5,6 or retrospective7,8 as well as pre-/poststudies,9 have been published with mixed results. Weichman et al5 evaluated 122 women who received either RTU or FD and concluded that the sterile form mitigated the risk of infectious complications. Conversely, Yuen et al8 evaluated 103 patients with either the sterile or aseptic form and found higher complication rates with the sterile form. Similarly, Buseman et al,7 in a sample of 34 patients, reported significantly higher rates of seroma formation in patients who received RTU compared with those who received FD. Interestingly, both Weichman et al5 and Buseman et al7 had a no-ADM group as a control, yet reported different seroma results; Weichman et al5 reported 1.4% versus 1.0% (P = 1.00) and Buseman et al7 reported 8.3% versus 66.6% (P < 0.003) for no-ADM versus sterile, respectively.
In light of the conflicting results, it was postulated that a pooling of results across all eligible studies might give additional insights that would not be evident when evaluating each study independently. The objective of this study was to systematically review the literature with respect to comparisons of sterile and aseptic ADM and conduct a meta-analysis with respect to a hierarchy of primary and secondary outcomes.
METHODS
A systematic literature review was conducted to identify publications that compared the use of aseptic and sterile ADM for breast reconstruction. Relevant postsurgical complications were identified and extracted for comparison. Postsurgical complications reported in multiple studies were eligible for comparison. Each complication rate reported by the studies was assessed separately, and a meta-analysis was conducted, which compared each postsurgical complication in terms of an odds ratio (OR) and relative risk (RR) between sterile and aseptic ADM. Our hypothesis was that no difference would be demonstrated between the 2 forms of ADM with respect to postsurgical complications in women undergoing prosthetic breast reconstruction.
This systematic review was conducted using the PubMed database and limited to studies published in English between 2010 and 2014 (RTU was not available prior to 2010) and focused on the use of AlloDerm ADM for prosthetic breast reconstruction. The purpose of the search was to identify all articles that met the selected criteria and compare outcomes. Articles that focused on technique, review, or opinion were excluded. The following search strategy for the above-specified dates was used.
“Mammaplasty”[Mesh] OR “breast reconstruction” OR “breast reconstructions” OR “breast revision” OR “breast surgery” OR “post-mastectomy reconstruction” OR “breast implants” OR “breast implantation” OR “nipple reconstruction” OR mastopexy AND AlloDerm OR “acellular dermal matrix” OR “acellular dermal matrices” OR “biologic matrix” OR “biologic matrices” OR “biological matrix” OR “biological matrices” OR “biologic mesh” OR “biological mesh” OR “bioprosthetic” OR “biomesh” OR “biological scaffold” OR “biologic scaffold” OR “tissue matrix” OR “tissue matrices” OR “allograft” OR “dermis-assisted.”
In order to ensure inclusion of all publications reporting data on AlloDerm ADM use, additional articles were identified manually by reviewing various plastic surgery journals. The references from all eligible articles were also reviewed to include additional articles that may have been missed from the electronic search.
ADM was assumed to be RTU (sterile) if the product name “ready to use” or RTU was mentioned explicitly, the product name was described as “sterile,” or rehydration steps described in article indicate RTU preparation. Conversely, ADM was determined to be FD if product was described as “FD” or “aseptically processed,” rehydration steps described in article indicate FD preparation, or study period before 2010. Analysis of complications was based on individual breasts and not patients. In the event that a study only reported data at the patient level, the reported complications were included as part of a sensitivity analysis.
The sterile and aseptic forms of ADM were considered to be the exposure variable of interest; thus, complications were examined relative to the exposure. Three separate main analyses were conducted to compare the incidence rates of 1) cellulitis, 2) explantation, and 3) seroma between aseptic and sterile ADM. For each complication, RR and OR for the primary exposure (sterile vs aseptic) were reported and used to determine effect sizes for individual studies. A weighted estimator, inversely proportional to the variance in each study, was used to weight the effect sizes. Subsequently, a weighted mean effect size (RR or OR), standard error, and 95% confidence intervals (CIs) were reported for the combined studies.
Homogeneity of the effect sizes across studies was assessed using a chi-square test on Cochran’s Q statistic. For the Q statistic, P < 0.05 was considered to indicate statistically significant heterogeneity. The test for homogeneity determined whether a fixed- or random-effects approach was implemented. A fixed-effects approach considered the set of studies as homogeneous and representing all potential studies of interest, whereas the random-effects approach treated the studies as heterogeneous and considered them to be a sample from a population of comparable studies. The homogeneity test results led to the use of a random-effects model to pool effect-size estimates and compute treatment effects for both the cellulitis and seroma outcomes measures. A fixed-effect model was used to pool effect-size estimates for the explantation analysis. ADM size and surgical technique were not considered. Differences in rehydration time or specific techniques used to prepare the tissue in advance of implantation were not controlled for the analysis. The impact of age and body mass index (BMI) was examined as potential confounders. Patient follow-up was considered for the analysis, given the likelihood of variation among studies. Specifically, the analysis was limited to a comparison of complications provided by at least 2 studies reporting on that particular complication.
In order to include as many studies as possible, studies that reported complications at the individual patient level were included and tested using each of the following assumptions:
That all patients underwent unilateral reconstruction and thus the reported complications are at the breast level (i.e., the same as the patient level)
That all patients underwent bilateral reconstruction and the reported complication occurred in both breasts
That all patients underwent bilateral reconstruction and the reported complication occurred in only one breast
The primary analyses assumed that the follow-up between treatment arms and among studies was similar. There was no adjustment for variable follow-up time among studies or for differences in follow-up between treatment arms within the same study for the primary analysis. Additional sensitivity analyses for each reported complication took into account the follow-up time when reported. The sensitivity analyses assessed the robustness of the primary finding by reporting the rate of complication per person-year or person-month.
Where follow-up time was not reported and could not be readily derived, the following assumptions were made:
The study period will be used to derive the total potential follow-up.
Enrollment into the study was assumed to be uniform.
Given the above 2 assumptions, then it follows that the mean follow-up time is the mid-way point of the total study period.
These analyses were a retrospective analytic study and thus noninterventional in nature. Subjects were not exposed to any medical procedure. No individual patient-level data or protected health information was collected. Data collection was limited to the reported literature and limited to data reported in aggregate.
RESULTS
A total of 235 unique citations were retrieved and an additional 40 articles were found by manually searching the aforementioned plastic surgery journals. From the 275 identified citations, 115 were eligible for full review to determine which type of ADM was used and if any comparisons between the 2 types of ADM were made. Ten studies explicitly identified RTU as the product used. Of those, 2 studies were eliminated because their populations were included in a later, more comprehensive report from the same group. Of the remaining 8 studies, only 5 studies contained data that compared outcomes between RTU and FD (Fig. 1).
Fig. 1.
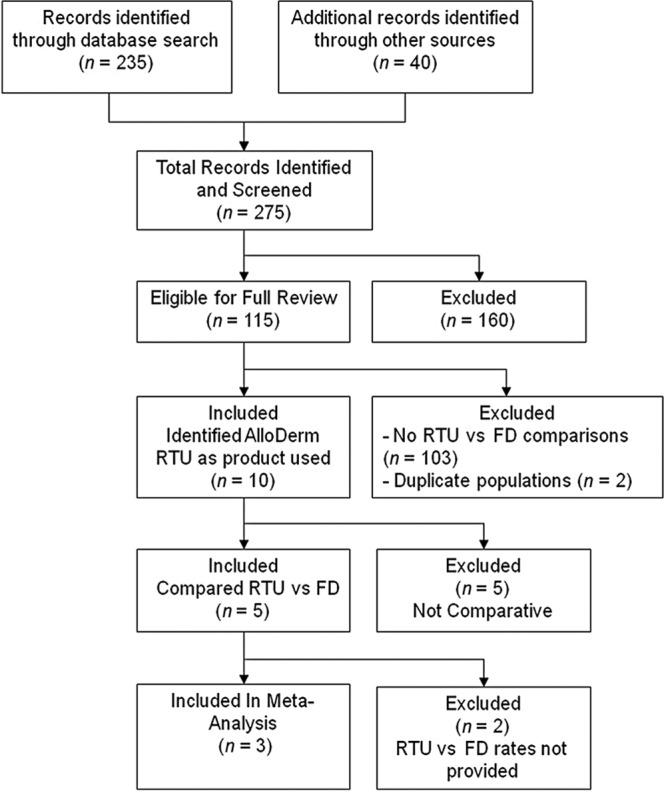
Prisma flow diagram. A total of 275 records were identified through literature searches. The majority of articles (n = 160) did not identify the sterile product explicitly. Of the remaining, 103 did not have comparative data or were duplicates, leaving 5 studies for qualitative synthesis and 3 for meta-analysis.
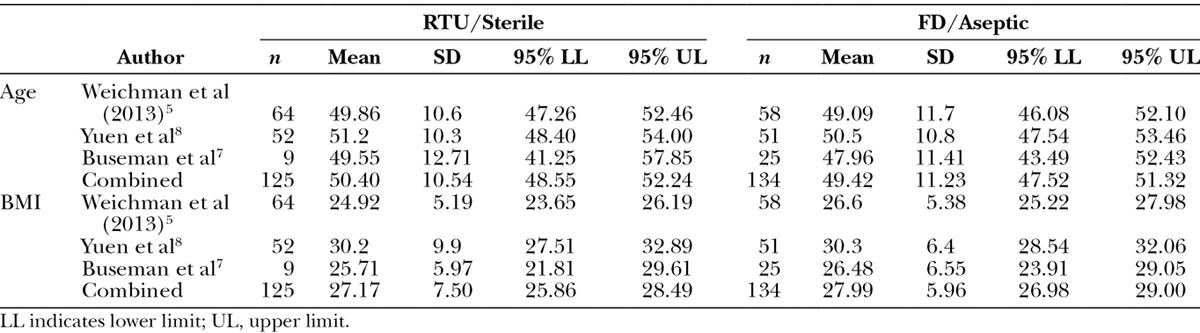
Across the 3 studies that could be included in the meta-analysis, there were a total of 259 patients. Buseman et al7 did not provide data at the breast level; thus, that data were used only for sensitivity analysis. As such, a total of 391 breasts were included in the primary analysis (Table 1). The populations with respect to age were generally comparable between the sterile and aseptic forms both within and between studies (Table 2). For BMI, the groups were similar for sterile and aseptic forms within study. However, between studies, the Yuen et al8 population had slightly higher mean BMI when compared with Weichman et al5 population for both sterile (30.2 kg/m2; 95% CI, 27.51–32.89 vs 24.92 kg/m2; 95% CI, 23.65–26.19) and aseptic (30.3 kg/m2; 95% CI, 28.54–32.06 vs 26.6 kg/m2; 95% CI, 25.22–27.98) forms, respectively. The CI for the Buseman et al7 population included the Weichman et al5 CI and overlapped with Yuen et al8 CI to a significant extent (Table 2). Overall, there was no difference in BMI between sterile and aseptic forms.
Table 1.
Patients and Breasts Numbers by Study and Product
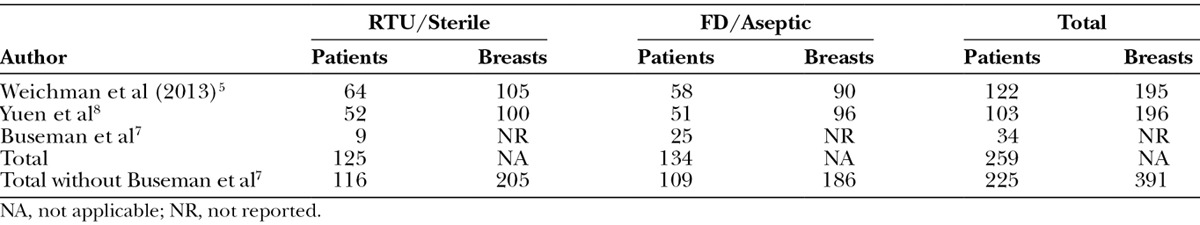
Table 2.
Selected Baseline Characteristics by Study and Product
The results of the current meta-analysis are summarized in Figures 2 and 3. Across all meta-analyses, there were no differences in complication rates measured by OR between RTU and FD: cellulitis (OR = 0.848; 95% CI, 0.220–3.273), seroma (OR = 0.584; 95% CI, 0.047–7.230), and explantation (OR = 0.569; 95% CI, 0.225–1.437). Similarly, there was no difference in any of the complications when measured using RR between the 2 cohorts: cellulitis (RR = 0.863; 95% CI, 0.272–2.740), seroma (RR = 0.553; 95% CI, 0.026–11.830), and explantation (RR = 0.593; 95% CI, 0.247–1.425). None of the sensitivity analyses altered the primary results of the study, including variable follow-up time and inclusion of the study by Buseman et al.7
Fig. 2.
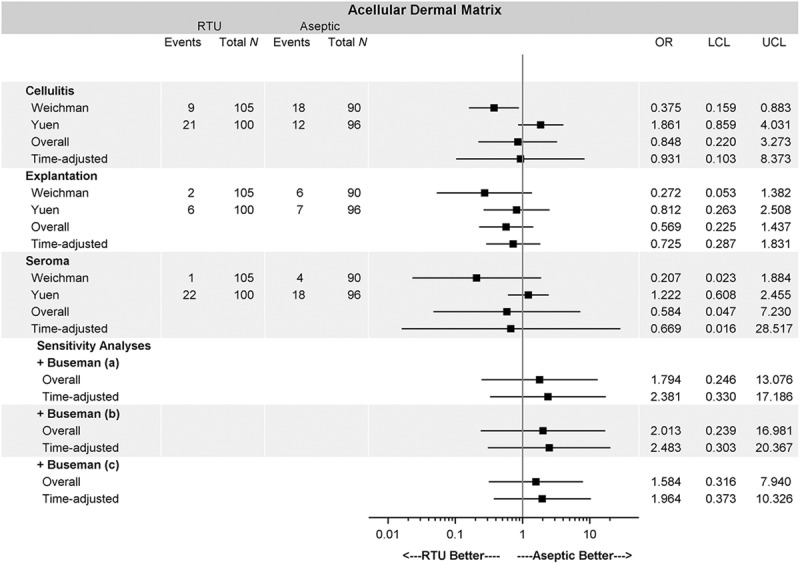
OR for baseline analysis and sensitivity analysis. The overall OR for each complication shows no difference between sterile vs aseptic AlloDerm. The inclusion of patient-level data and time adjustment did not alter the conclusion under a variety of assumptions. OR or RR less than 1 favors sterile AlloDerm over aseptic AlloDerm. Buseman et al7 (a) assumed that all patients had unilateral reconstruction and reported complications are at the breast level. Buseman et al7 (b) assumed that all patients had bilateral reconstruction and the reported complication occurred in both breasts. Buseman et al7 (c) assumed that all patients had bilateral reconstruction and the reported complication occurred in only one breast.
Fig. 3.
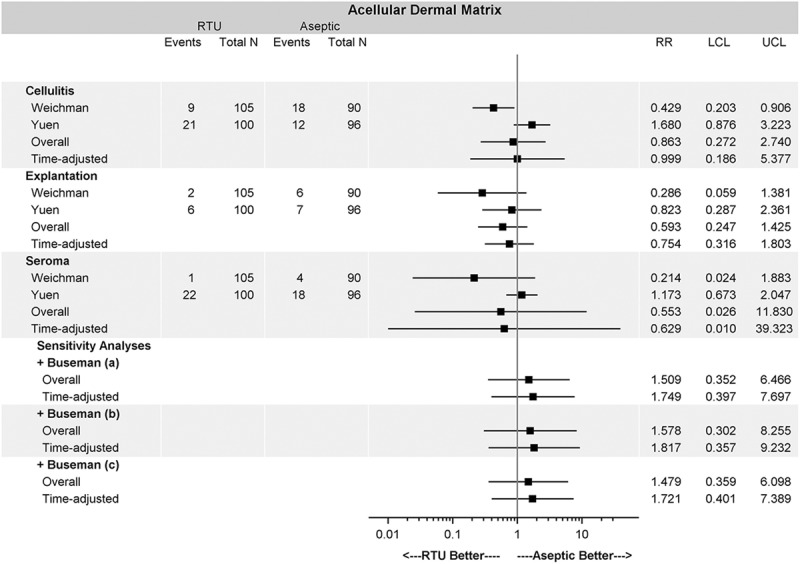
Relative risk for baseline and sensitivity analyses. The overall RR for each complication shows no difference between sterile vs aseptic AlloDerm. The inclusion of patient-level data and time adjustment did not alter the conclusion under a variety of assumptions. OR or RR less than 1 favors sterile AlloDerm over aseptic AlloDerm. Buseman et al7 (a) assumed that all patients had unilateral reconstruction and reported complications are at the breast level. Buseman et al7 (b) assumed that all patients had bilateral reconstruction and the reported complication occurred in both breasts. Buseman et al7 (c) assumed that all patients had bilateral reconstruction and the reported complication occurred in only one breast.
For all complications and tested scenarios (with and without Buseman et al,7 using different assumptions regarding Buseman’s data, and adjustment for variable follow-up time), there was no difference with respect to complications between sterile and aseptic ADM (Figs. 2 and 3).
DISCUSSION
The reported benefits of ADM stem from its regenerative potential and its ability to revascularize and recellularize with host erythrocytes and fibroblasts. ADM has been demonstrated to incorporate into the host tissues and provide tissue support. The success of this regenerative tissue matrix has been attributed to the specialized processing of the native skin and the preservation of its extracellular matrix.
The use of ADM for 1- and 2-stage prosthetic breast reconstruction was initially reported in 2006 by Salzberg10 when it was realized that surgical outcomes could be enhanced. Since 2006, there have been a plethora of articles demonstrating the indications and benefits of AlloDerm for breast surgery.11–14 The majority of these articles reported the use of the FD ADM. FD ADM is processed aseptically and requires hydration prior to implantation. It was not until 2010 that the sterile or RTU form was introduced. Although utilization of the sterile ADM has increased, there have been few studies comparing outcomes between the RTU and FD varieties. Thus, the question that warrants inquiry is whether the processing differences associated with RTU and FD ADM have affected the performance of the materials.
Only 3 studies provided sufficient data for inclusion in meta-analysis (Table 3).5,7,8 The studies by Khansa et al9 and Weichman et al6 either reported data in aggregate or with limited detail to permit inclusion. This systematic review and meta-analysis has tabulated outcomes from the remaining 3 studies that when analyzed individually demonstrated differing results. The meta-analysis comparison of RTU and FD ADM has demonstrated a similar performance profile based on the occurrence of cellulitis, seroma, and explantation. Across all meta-analyses, there were no differences in complication rates between the 2 cohorts. The seroma results remained similar between the 2 groups after including the study by Buseman et al.7 Results remained nonsignificant even after adjustment for variable follow-up time. Patient demographics based on BMI and age was similar and not significantly different.
Table 3.
Complications Reported by Study
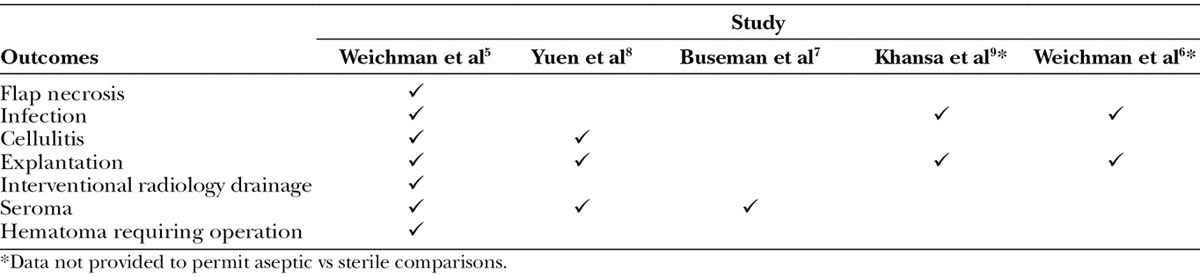
Although the data reported by Khansa et al9 and Weichman et al6 were excluded from the analyses, both studies did report similar complication rates between the RTU and FD groups. Khansa et al9 noted that the rate of infection was no different with respect to sterile or aseptic (sterile, 11.8%; aseptic, 14.0%; P = 0.74). The 2014 report by Weichman et al6 appears to be a progress report of the 2013 publication by Weichman et al,5 with more reconstructed breasts, 790 breast reconstructions compared with the 195 reported in the 2013 publication. Although infection and explantation are reported in 2014 report by Weichman et al,6 rates are not provided for sterile or aseptic ADM. Nevertheless, the report does suggest that major infectious complications [10.3% (n = 56) to 5.6% (n = 8); P = 0.112] and explantation [7.7% (n = 50) to 2.8% (n = 4); P = 0.004] have decreased since the introduction of sterile ADM. It is important to note that the decline reported for major infectious complications was not significant.
The clinical relevance of these findings is important because of the potential advantages of sterile RTU compared with FD ADM. RTU ADM has been terminally sterilized to achieve a sterility assurance level (SAL) of 10–3. Although the SAL of FD ADM is also 10–3, it is considered aseptic because it has not been terminally sterilized according to the regulations set forth by the Food and Drug Administration. The process of cryopreservation is bacteriocidal to achieve an SAL of 10–3. The sterility associated with terminal radiation can be adjusted to achieve a SAL of 10–3 or higher. However, excessive radiation of a biologically material that is heat sensitive such as skin can result in excessive damage to the extracellular matrix, resulting in a greater inflammatory response associated with resorption and less regenerative potential.15
The other benefit of RTU ADM is that it is prehydrated and thus requires only a 2- to 3-minute soak when removed from the pouch and before implantation. The FD ADM typically requires 20–30 minutes of hydration to reverse the cryopreservation and to adequately remove the cryopreservatives. Thus, it may increase operative times while waiting for the rehydration step to complete. Failure to adequately hydrate the ADM has the potential to cause “red breast syndrome” that has been linked to the retained cryopreservatives.16
Clinical observations using RTU and FD ADM corroborate the findings of this systematic review. Having performed nearly 1000 AlloDerm breast reconstructions (performed between 2005 and 2015) with almost equal RTU and FD cohorts, surgical outcomes have been unchanged between the 2 (MY Nahabedian, unpublished data, 2015). Tissue integration and the incidence of infection, seroma formation, and explantation are similar.
The limitation of this study includes a lack of a randomized clinical trial or level 1 study in the publications used within this meta-analysis. A meta-analysis of retrospective data inherits potential confounders that can skew results preferentially toward one arm of a comparison group. For example, confounding variables such as radiation or neoadjuvant chemotherapy may increase complications on one arm of the study masking the overall beneficial effect in that arm. Additionally, although the RTU ADM is used extensively, the literature rarely specifies RTU and instead makes reference only to ADM; as a result, the number of studies available for inclusion were limited. Given this, the lack of statistical difference may be attributable to the low power of the study, leading to a type II error. Finally, there was no way to ensure common definition among studies for any of the outcomes.
CONCLUSIONS
The results of this meta-analysis have demonstrated that RTU and FD ADM have similar outcomes when used for prosthetic breast reconstruction based on infection, seroma, and explantation. In light of the fact that RTU is the most widely used repair material, this study demonstrates no difference from FD. Future outcome studies should be randomized, controlled, and prospective to provide level 1 evidence supporting the use of RTU ADM. Additionally, future studies examining potential differences in the economic impact between RTU and FD ADM use in breast reconstruction are also warranted.
ACKNOWLEDGMENT
We thank Julie M. Robertson, PhD (Acelity), for her assistance with medical editing.
Footnotes
Disclosure: Mr. Macarios and Dr. Lee are employees of LifeCell, an Acelity company; Ms. Griffin is an Acelity employee; Dr. Chatterjee is a consultant for LifeCell, an Acelity company; Ms. Milburn is a former employee of LifeCell, an Aceltiy company; Dr. Nahabedian is a consultant for LifeCell, an Acelity company, Allergan Inc., and Sientra. The Article Processing Charge was paid for by LifeCell, an Acelity company.
REFERENCES
- 1.American Society of Plastic Surgeons. 2012 Plastic Surgery Statistics Report. Arlington Heights, Ill.: American Society of Plastic Surgeons; 2013. [Google Scholar]
- 2.Weichman KE, Wilson SC, Weinstein AL, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:1049–1058. doi: 10.1097/PRS.0b013e31824a2acb. [DOI] [PubMed] [Google Scholar]
- 3.Liu AS, Kao HK, Reish RG, et al. Postoperative complications in prosthesis-based breast reconstruction using acellular dermal matrix. Plast Reconstr Surg. 2011;127:1755–1762. doi: 10.1097/PRS.0b013e31820cf233. [DOI] [PubMed] [Google Scholar]
- 4.Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg. 2009;124:1735–1740. doi: 10.1097/PRS.0b013e3181bf803d. [DOI] [PubMed] [Google Scholar]
- 5.Weichman KE, Wilson SC, Saadeh PB, et al. Sterile “ready-to-use” AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;132:725–736. doi: 10.1097/PRS.0b013e31829fe35b. [DOI] [PubMed] [Google Scholar]
- 6.Weichman K, Wilson S, Broer PN, et al. LOP17: an outcomes based evolution of 800 implant based breasts reconstructions with acellular dermal matrix. (Presented at the Sixth Annual Meeting of the European Plastic Surgery Research Council, August 21–24, 2014, Hamburg, Germany) Plast Reconstr Surg. 2014;134:386–387. [Google Scholar]
- 7.Buseman J, Wong L, Kemper P, et al. Comparison of sterile versus nonsterile acellular dermal matrices for breast reconstruction. Ann Plast Surg. 2013;70:497–499. doi: 10.1097/SAP.0b013e31827f52c8. [DOI] [PubMed] [Google Scholar]
- 8.Yuen JC, Yue CJ, Erickson SW, et al. Comparison between freeze-dried and ready-to-use AlloDerm in alloplastic breast reconstruction. Plast Reconstr Surg Glob Open. 2014;2:e119. doi: 10.1097/GOX.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khansa I, Hendrick RG, Jr, Shore A, et al. Breast reconstruction with tissue expanders: implementation of a standardized best-practices protocol to reduce infection rates. Plast Reconstr Surg. 2014;134:11–18. doi: 10.1097/PRS.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 10.Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg. 2006;57:1–5. doi: 10.1097/01.sap.0000214873.13102.9f. [DOI] [PubMed] [Google Scholar]
- 11.Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg. 2011;128:403e–410e. doi: 10.1097/PRS.0b013e31822b6637. [DOI] [PubMed] [Google Scholar]
- 12.Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg. 2011;128:1170–1178. doi: 10.1097/PRS.0b013e318230c2f6. [DOI] [PubMed] [Google Scholar]
- 13.Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg. 2011;127:514–524. doi: 10.1097/PRS.0b013e318200a961. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy CM, Lee CN, Halvorson EG, et al. The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg. 2012;130(5 Suppl 2):57S–66S. doi: 10.1097/PRS.0b013e31825f05b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Woedtke T, Kramer A. The limits of sterility assurance. GMS Krankenhaushygiene Interdisziplinar. 2008;3:Doc19. [PMC free article] [PubMed] [Google Scholar]
- 16.Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg. 2009;124:1743–1753. doi: 10.1097/PRS.0b013e3181bf8087. [DOI] [PubMed] [Google Scholar]


