Summary:
Alveolar rhabdomyosarcoma (RMS) has a predilection for the deep soft tissues of the extremities and mainly occurs in children. Although the tumor may originate in other sites, such as the nasal cavity or paranasal sinus, invasion of the orbit is unusual. We describe the clinicopathological features of 2 cases of alveolar RMS of the nasal cavity or paranasal sinus in adult patients with orbital extension. These cases of alveolar RMS of the nasal cavity or paranasal sinuses are described in 2 men, both in the third decade of life. These patients were evaluated with radiological studies. The histological diagnosis was confirmed by immunohistochemical methods. Treatment consisted in a combination of chemotherapy and radiation therapy following excisional biopsy. Alveolar subtype RMS is an extremely aggressive neoplasm that rarely presents in the orbit or paranasal sinuses of adults but should be considered in the differential diagnosis of tumors with this localization. Myoglobin, Myo D1, and myogenin seem to be the most specific markers for RMS.
Alveolar rhabdomyosarcoma (RMS) is a primitive, high-grade malignant, round cell neoplasm, which shows partial skeletal muscle differentiation.1 Alveolar RMS of the orbit and nasal cavity or paranasal sinuses is rare, and most reported cases affect children.1–5 The current report illustrates the clinical and pathological features of alveolar RMS originating in the nasal cavity or paranasal sinuses of 2 male adults.
CASE 1
A 24-year-old male patient with a 2-month history of epistaxis, nasal stuffiness, frontal headache, and decrease of sensitivity in the right side of the face complained of a decrease in visual acuity and proptosis of the right eye. Preauricular, submandibular, and laterocervical lymphadenopathies were noticed. Computed tomography scan showed a mass with a homogeneous destructive pattern in the right nasal cavity that affected the ethmoid sinus and right orbit with bone destruction (Fig. 1). A biopsy showed a mass composed of polygonal to round cells with hyperchromatic nuclei and variably eosinophilic cytoplasm (Fig. 2). The cells were arranged in cohesive clusters or noncohesive aggregates, showing an alveolar pattern. A solid pattern of mononuclear cells without alveolar characteristics was focally present. Some degree of pleomorphism was noted, but it was more apparent in the loosely cellular areas where giant tumor cells were evident. The immunohistochemical study was positive for desmin, vimentin, and myosin fast, and negative for cytokeratins AE1 and AE3, smooth muscle actin 1A4, common muscle actin HHF-35, S-100 protein, CD34 antigen, and myoglobin. The diagnosis of RMS was made. Extension study was negative. The pretreatment classification was III (TNM-UICC). The treatment was initiated with chemotherapy and radiotherapy, to the right middle face. The mass recurred 3 times and new chemotherapies were performed, but the disease continued in progression. Two years later, the patient died due to systemic complications associated with the invasion.
Fig. 1.
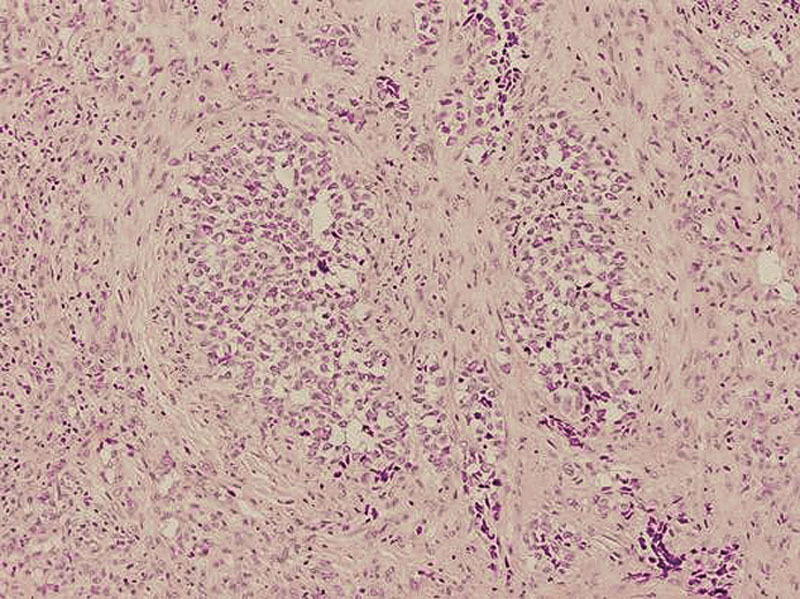
Case 1: An alveolar rhabdomyosarcoma showing diffuse proliferation of round atypic cells, hyperchromatic nuclei, and eosinophilic cytoplasm with a loose alveolar pattern in certain areas (hematoxylin-eosin; original magnification, ×20).
Fig. 2.

Case 1: Computed tomography scan showed a mass with homogeneous destructive pattern that affected the paranasal sinuses and extended into the right orbit.
CASE 2
A 26-year-old man with a 3-month history of nasal stuffiness and epistaxis also complained of orbital pain and decrease of visual acuity in the right eye. The physical examination discovered 2 submandibular lymph nodes of 2 cm in diameter, severe proptosis, inferior and lateral displacement of the eyeball, and complete ophthalmoplegia. The right fundus showed optic nerve avulsion. The magnetic resonance imaging documented a large mass of the right nasal cavity with infiltration into the orbit, ethmoidal and frontal sinuses. The extraconal component of the mass showed extradural extension into the anterior cranial fossa. However, there were no intraparenchymal cerebral lesions (Fig. 3). The pretreatment classification was III (TNM-UICC). A biopsy of the neoplasm in the nasal cavity was performed. Grossly, the tumor tissue showed a pale red soft-tissue tumor. Hematoxylin and eosin–stained section demonstrated a tumor composed of solid sheets of small, round cells with scant cytoplasm and hyperchromatic nuclei. The centers of the nests were solid and filled with polygonal cells with more abundant eosinophilic cytoplasm. Immunohistochemical studies showed that the tumor cells were negative to cytokeratins and S-100 protein, and they were positive for markers of skeletal muscle differentiation, desmin (Fig. 4), vimentin, and antibodies against Myo D1, which are highly specific and sensitive for RMS. The diagnosis of RMS was made. The extension study was negative. The treatment was initiated with chemotherapy and radiotherapy to the right middle face. The patient died 5 months later due to systemic complications associated with the invasion.
Fig. 3.

Case 2: Magnetic resonance imaging showing a large mass in the right sinuses with orbital invasion and extension into anterior cranial fossa.
Fig. 4.

Case 2: Immunostain showing significant positive staining for desmin (original magnification, ×20).
DISCUSSION
RMSs are soft-tissue tumors of myogenous differentiation. Although rare in adults, they are the most common soft-tissue malignancy of childhood and adolescence.1–5 Alveolar RMS may arise on the extremities and head and neck regions, as in our patients.1,4 The pathologist should consider age at presentation and the anatomic site of the tumor in the formulation of a differential diagnosis when the histology of the biopsy shows an undifferentiated or poorly differentiated neoplasm. If the patient is younger than 15, with a tumor composed of small round cells, RMS is one of the limited number of neoplasms with this morphologic appearance. But in adult patients, RMS is relatively uncommon. As far as we know, it has been reported in only few cases arising in the paranasal sinuses and orbit.3–13 In adults, these tumors may be confused with other soft-tissue malignancies. When most of the tumor consists of solid nest cells of small rounded cells as in our case reports, tumors of the Ewing family, neuroblastoma, malignant rhabdoid tumor,1 poorly differentiated carcinoma, leukemia, malignant lymphoma, melanoma, and neuroendocrine carcinoma2–5 should be considered in the differential diagnosis. Often, a large battery of immunostains is required. To exclude other entities, myoglobin, Myo D1, and myogenin seem to be the most specific markers for RMS. The most common staging systems are the TNM-UICC System and the System of the Intergroup Rhabdomyosarcoma Study. The TNM classification is a pretreatment staging system. Systemic chemotherapy followed by radiotherapy provides better local-regional control than other treatment modalities.
Most cases of orbital RMS have an excellent prognosis with a 10-year event-free period and overall survival rates of 77% in metastatic and 87% in nonmetastatic patients. Factors associated with a less favorable outcome include the alveolar subtype,3–12 parameningeal involvement, and advanced stage of disease. The unfavorable prognosis of the rare alveolar subtype of RMS, at least in older children and adults, is related to the tumor tendency toward early and widespread dissemination, often involving bone marrow with poor response to the chemotherapy.11 Although in our patients the tumor originated in the paranasal sinus, morbidity would be increased due to the involvement of the orbit.10
CONCLUSION
It is important that alveolar RMS should be diagnosed at an early stage of its development and be aggressively treated.10–13
Footnotes
The research project has been approved by a suitably constituted Ethics Committee of the institutions within which the work was undertaken; it conforms to the provisions of the Declaration of Helsinki in 1995 (as revised in Edinburgh 2000). The subjects gave informed consent and patient anonymity should has been preserved.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Shields JA, Shields CL. Rhabdomyosarcoma: review for the ophthalmologist. Surv Ophthalmol. 2003;48:39–57. doi: 10.1016/s0039-6257(02)00415-0. [DOI] [PubMed] [Google Scholar]
- 2.Nakhleh RE, Swanson PE, Dehner LP. Juvenile (embryonal and alveolar) rhabdomyosarcoma of the head and neck in adults. A clinical, pathologic, and immunohistochemical study of 12 cases. Cancer. 1991;67:1019–1024. doi: 10.1002/1097-0142(19910215)67:4<1019::aid-cncr2820670426>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Lee MS, Lee BH, et al. Rhabdomyosarcoma of the head and neck in adults: MR and CT findings. AJNR Am J Neuroradiol. 1996;17:1923–1928. [PMC free article] [PubMed] [Google Scholar]
- 4.Pardo RJ, Acosta RE, Espaillat J, et al. Alveolar rhabdomyosarcoma arising in the nasal cavity of a 3-year-old child. Pediatr Dermatol. 1988;5:254–256. doi: 10.1111/j.1525-1470.1988.tb00898.x. [DOI] [PubMed] [Google Scholar]
- 5.Friling R, Marcus M, Monos T, et al. Rhabdomyosarcoma: invading the orbit in an adult. Ophthal Plast Reconstr Surg. 1994;10:283–286. [PubMed] [Google Scholar]
- 6.Hayashi Y, Kikuchi F, Oka T, et al. Rhabdomyosarcoma with bone marrow metastasis simulating acute leukemia. Report of two cases. Acta Pathol Jpn. 1988;38:789–798. doi: 10.1111/j.1440-1827.1988.tb02350.x. [DOI] [PubMed] [Google Scholar]
- 7.Ihara T, Okamura D, Takahashi N, et al. Alveolar rhabdomyosarcoma mimicking nasal lymphoma at the initial presentation. J Clin Exp Hematop. 2008;48:61–64. doi: 10.3960/jslrt.48.61. [DOI] [PubMed] [Google Scholar]
- 8.Manucha V, Castellani R, Sun CC. Alveolar rhabdomyosarcoma of the paranasal sinuses in a 57-year-old woman with 1:16 translocation. Int J Surg Pathol. 2006;14:238–242. doi: 10.1177/1066896906290560. [DOI] [PubMed] [Google Scholar]
- 9.Callender TA, Weber RS, Janjan N, et al. Rhabdomyosarcoma of the nose and paranasal sinuses in adults and children. Otolaryngol Head Neck Surg. 1995;112:252–257. doi: 10.1016/S0194-59989570246-6. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed AA, Tsokos M. Sinonasal rhabdomyosarcoma in children and young adults. Int J Surg Pathol. 2007;15:160–165. doi: 10.1177/1066896906299122. [DOI] [PubMed] [Google Scholar]
- 11.Sultan I, Quaaoumi I, Yaser S, et al. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009;27:3391–3397. doi: 10.1200/JCO.2008.19.7483. [DOI] [PubMed] [Google Scholar]
- 12.Seregard S. Management of rhabdomyosarcoma of the orbit. Acta Ophthalmol Scand. 2002;80:660–664. doi: 10.1034/j.1600-0420.2002.800619.x. [DOI] [PubMed] [Google Scholar]
- 13.Parikh D, Spindle J, Linden C, et al. Adult rhabdomyosarcoma of the maxillary sinus with orbital extension. Orbit. 2014;33:302–304. doi: 10.3109/01676830.2014.902480. [DOI] [PubMed] [Google Scholar]


