Supplemental Digital Content is available in the text.
Abstract
Background:
Interpositional jump graft (IPJG) is a nerve graft axonally supercharged from the hypoglossal nerve. However, for using the technique, an autologous nerve, which should contain the great auricular and sural nerves, must be obtained. Depending on the donor site, unavoidable issues such as nerve disorders and postoperative scarring may appear. To reduce the issues, in this study, the authors developed an end-to-side neurorrhaphy technique with the recipient nerve and an artificial nerve conduit and investigated the efficacy of an IPJG with an artificial nerve conduit in a rat facial nerve paresis model.
Methods:
A ligature clip was used to crush the facial nerve trunk, thereby creating a partial facial nerve paresis model. An artificial nerve conduit was then prepared with a 10-mm-long silicone tube containing 10 μL type I collagen and used to create an IPJG between the facial nerve trunk and the hypoglossal nerve (the silicone tube group). Thirteen weeks after the surgery, the outcome was histologically and physiologically compared with conventional IPJG with autograft using the great auricular nerve.
Results:
Retrograde tracer test confirmed a double innervation by the facial and hypoglossal nerve nuclei. In the autograft and silicone tube groups, the regeneration of myelinated axons was observed.
Conclusion:
In this study, the authors successfully developed an end-to-side neurorrhaphy technique with the recipient nerve and an artificial nerve conduit, and revealed that an IPJG in the conduit was effective in the rat facial nerve paresis model.
Facial nerve paresis, such as Bell paralysis and Ramsay-Hunt syndrome, appears in healthy people suddenly, and without effective conservative treatments in the acute stage, the paresis gives serious aftereffects, which spoil their quality of life. Reports describing end-to-side neurorrhaphy for treating facial nerve paresis have became popular from around the end of the 19th century.1 In 1991, May et al2 applied the surgical technique and reported on hypoglossal-facial nerve interpositional jump graft (IPJG) procedure by performing end-to-side neurorrhaphy with transplanting the autologous nerve between the hypoglossal and the facial nerves. IPJG is a nerve graft axonally supercharged from different neural sources including the hypoglossal nerve by creating a bridge between the weakened facial and the normal hypoglossal nerves by end-to-side neurorrhaphy at 2 locations with an autologous nerve.3 A number of reports have unveiled favorable clinical outcomes with autologous nerves as IPJGs by IPJG-improved techniques.4,5 However, for using the technique, an autologous nerve, which should contain the great auricular and sural nerves,2 must be obtained. Depending on the donor site, unavoidable issues such as nerve disorders and postoperative scarring appear.6 For reducing the issues, this study hypothesized that (1) end-to-side neurorrhaphy technique, which was developed in the authors’ laboratory, allowed nerves to be regenerated in an artificial nerve conduit and (2) with the results of animal experiments, this treatment would be applied to the treatment of facial nerve paralysis patients. Thus, the aims of this study were (1) to develop an end-to-side neurorrhaphy technique for regenerating an IPJG between the recipient nerves in an artificial nerve conduit and (2) to perform IPJG procedure for investigating axonal-supercharged nerve. Based on basic research regarding nerve regeneration with an artificial nerve conduit to treat facial nerve deficits in rats,7–9 this study attempted to use regenerated nerves that would become an IPJG as a substitute for an autologous nerve for treating facial nerve paresis. In the previous studies, artificial nerve conduits have been placed and used as an end-to-end neurorrhaphy bridge between 2 locations for facilitating nerve regeneration. This study histologically and physiologically compared 2 IPJGs, which were conventionally transplanted autologous nerve graft and regenerated nerve in the conduit.
METHODS
Surgical Procedure
Lewis rats (8 weeks old, n = 14) (CLEA Japan, Tokyo, Japan) were anesthetized with 4% isoflurane using a nasal mask connected to an Univentor 400 Anesthesia Unit (Univentor, Zejtun, Malta) and placed in a right lateral decubitus position.10 An S-shaped incision was made from behind the left ear to the lower margin of the mandible while the animals were under inhalational anesthesia (Fig. 1A). The great auricular nerve was identified on the sternocleidomastoid muscle after subcutaneous tissue dissection approximately 10 mm ventral to the posterior auricular surface. In this way, the dissection was advanced to the attachment of the sternocleidomastoid muscle, and the facial nerve trunk was identified. Thereafter, the external jugular vein was ligatured and transected, and the digastricus was exposed after subcutaneous tissue dissection. The posterior surface was dissected from the caudal side of the digastricus, and the hypoglossal nerve was identified (Fig. 1B).
Fig. 1.
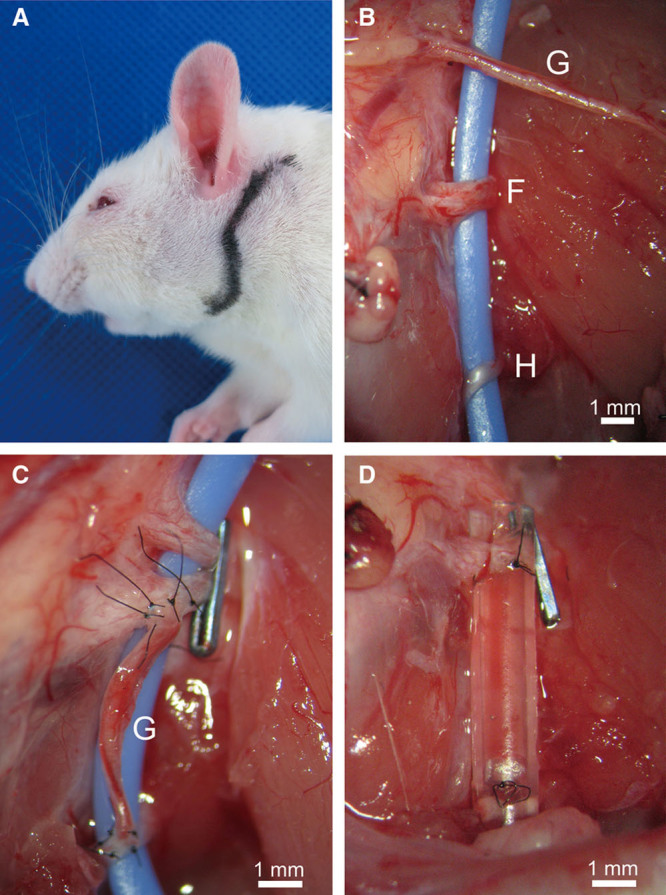
Incision site in rat and nerve transplantation procedures. A, An S-shaped incision was made from behind the left ear to the lower margin of the mandible. B, The facial nerve trunk (F), the hypoglossal nerve (H), and the great auricular nerve (G) were identified. Large blue line was a strip of rubber for visualizing these nerves. C, IPJG prepared with an autologous nerve. After a left partial facial nerve paresis model with a ligature clip placed on the facial nerve trunk was prepared, IPJG was made by end-to-side neurorrhaphy with the ipsilateral great auricular nerve (G) between the hypoglossal nerve and the facial nerve trunk (the autograft group). D, IPJG would be regenerated in an artificial nerve conduit. After a left partial facial nerve paresis model with a ligature clip was prepared, an IPJG was made by end-to-side neurorrhaphy with a silicone tube containing 10 μL type I collagen solution (0.3 wt/vol %) between the hypoglossal nerve and the facial nerve trunk (the silicone tube group).
This study created a left incomplete facial nerve palsy model, which was similar to a model reported by Shichinohe et al,11 by placing a ligature clip (LIGACLIP MCA, Ethicon Endo-Surgery, Ohio) placed over the facial nerve trunk. Then, a silicone tube (length, 10 mm; internal diameter, 1 mm; external diameter, 2 mm) (As-one, Osaka, Japan) containing 10 μL type I collagen solution (0.3 wt/vol %) (Nitta Gelatin, Osaka, Japan) as an artificial nerve conduit was prepared,12 and with a microscope (M651, Leica Microsystems, Wetzlar, Germany), the tube was used as an artificial nerve conduit for holding a regenerated IPJG (the silicone tube group, n = 7) (Fig. 1D) by performing end-to-side neurorrhaphy between the facial nerve trunk and the hypoglossal nerve.
End-to-side neurorrhaphy was performed with the silicone tube of which 2 gaps, 1.5 mm long and 1 mm wide, were made at both ends of the tube (length, 10 mm; internal diameter, 1 mm; external diameter, 2 mm) (Fig. 2). After epineural windows in the facial nerve trunk and hypoglossal nerve were created, neurorrhaphy was performed. The detail of the surgical procedure is given in Figure 2 in a step-by-step manner and in the video. (See Video 1, Supplemental Digital Content 1, which displays end-to-side neurorrhaphy with a silicone tube and facial nerve, http://links.lww.com/PRSGO/A105.) The same procedure was performed for the hypoglossal nerve, resulting in a 7-mm silicone tube bridge.
Fig. 2.
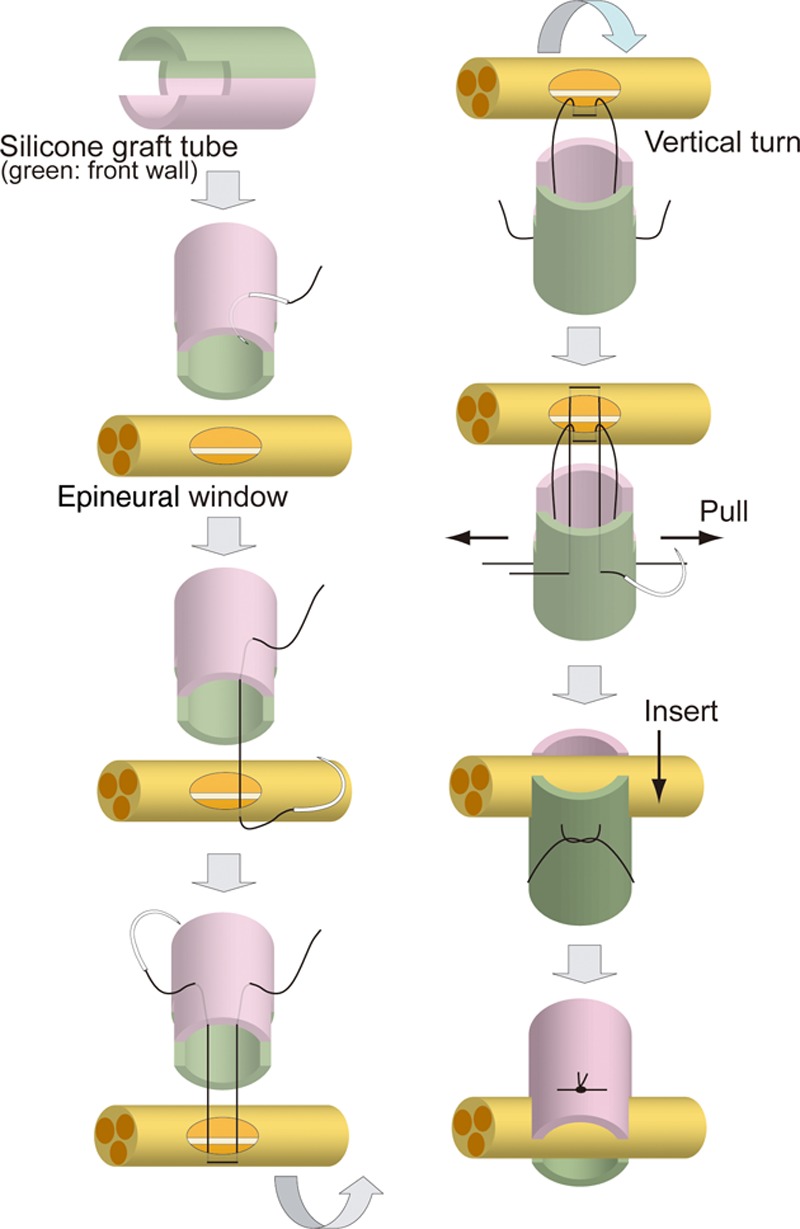
Schematic illustration of end-to-side neurorrhaphy with the silicone tube. End-to-side neurorrhaphy using the silicone tube is described in a step-by-step manner from the top to bottom illustrations in the left and right columns. (1) Gaps (1.5 mm long and 1 mm wide) were created at both ends of the tube (length, 10 mm; internal diameter, 1 mm; external diameter, 2 mm) (left column, first). (2) After epineural windows in the facial nerve trunk and hypoglossal nerve were created, neurorrhaphy was performed as follows. First, a 9-0 nylon suture (Nescosuture, Alfresa, Osaka, Japan) was passed from the external margin of the posterior wall of silicone tube (pink side) into the lumen (left column, second). (3) Then, the suture was passed from the epineural window through the epineurium toward the outside (left column, third). (4) The suture was then passed again from outside the epineural window through the epineurium into the lumen, from the lumen of the silicone tube toward the outside, and left in place without a ligature (left column, fourth). (5) Then, the silicone tube was rotated 180° vertically, and the anterior wall of the silicone tube (green side) was sutured by the same procedure for the posterior wall (right column, first). (6) The anterior and posterior wall sutures were carefully stretched (right column, second). (7) The facial nerve trunk was allowed to insert into the gaps initially created in the silicone tube (right column, third). (8) The facial nerve trunk was confirmed to be firmly inserted into the gaps on the left and right and ligatured with 2 sutures (right column, fourth).
Video 1.
See video, Supplemental Digital Content 1, which displays end-to-side neurorrhaphy with a silicone tube and facial nerve, http://links.lww.com/PRSGO/A105.
All end-to-side neurorrhaphy was performed only by the epineural window technique, and no axotomy was conducted. For the control group, the ipsilateral great auricular nerve (7 mm long) was obtained, and a conventional IPJG procedure was performed by transplanting the nerve between the facial nerve trunk and the hypoglossal nerve (the autograft group, n = 7) (Fig. 1C). Histological evaluations of the regenerated nerves were performed at postoperative weeks 7 and 13, whereas physiological evaluations were performed at postoperative week 13 only.
All animal care and handling procedures were performed in accordance with the “Principles of Laboratory Animal Care” of the Tokyo Women’s Medical University Animal Experimentation Committee.
Toluidine Blue Staining of Myelinated Fibers
The rats were deeply anesthetized with sodium pentobarbital. The middle portion of each transplanted nerve was subsequently examined. Specimens were prefixed with 2% glutaraldehyde (Distilled EM Grade, EM Science, Gibbstown, N.J.), 2% paraformaldehyde, and 0.1 mol/L cacodylate buffer and postfixed with 2% osmium tetroxide and 0.1 mol/L cacodylate buffer (Wako Pure Chemical, Osaka, Japan) before being embedded in Quetol-812 resin (Nisshin EM, Tokyo, Japan) by polymerization at 60°C for 2 days. The embedded specimens were cut into approximately 1.5-μm-thick sections with a glass knife and heat-stained with 0.5% toluidine blue.
Myelinated fibers in the middle portion (3.5 mm from the proximal end) of the transplanted autograft and regenerated nerve in the silicone tube were counted with a NanoZoomer 2.0-HT scanner (Hamamatsu Photonics, Shizuoka, Japan) and its software (NDP view 1.2.25, Hamamatsu Photonics), and the mean number of myelinated fibers was calculated.
Transmission Electron Microscopy Analysis of the Regenerated Nerves
The polymer-embedded specimens were cut into 70-nm sections, mounted on grids (EM fine-grid F-200, Nisshin EM), stained with 2% uranyl acetate and lead stain solution (Sigma-Aldrich, St. Louis, Mo.), and examined with a JEM1200EX transmission electron microscope (JEOL, Tokyo, Japan) with an accelerating voltage of 80 kV. Fiber diameter, axon diameter, and myelin thickness in randomly selected 5 fields were measured with graphic software, Photoshop CS6 (Adobe Systems, San Jose, Calif.). G-ratio was obtained by dividing the axon diameter by the fiber diameter.
Compound Muscle Action Potential Recordings of the Vibrissal Muscles
Compound muscle action potential (CMAP) was measured as described previously.13 Rats were anesthetized with urethane at a dose of 1.2 g/kg body weight intraperitoneally and placed on a stereotaxic apparatus. Rectal temperature was maintained at 35–36°C by a temperature controller (40-90-8D) (FHC, Bowdoin, Maine). Stages of anesthesia were maintained by confirming the lack of vibrissae movement and eyelid reflex.14 CMAP amplitude was calculated as a difference in voltage between the maximum and baseline CMAP amplitude. CMAP duration was calculated as the time between 2 points where the baseline was crossed by the rising and declining CMAP curves. CMAP latency was estimated as the time between the stimulus artifact and the point where the baseline was crossed by rising CMAP curve.
Retrograde Labeling of Facial and Hypoglossal Motor Neurons
Eleven weeks after the surgery, rats were anesthetized with 4% isoflurane and injected with 100 μL DiI (1%) in ethanol (D-282) (Invitrogen, Carlsbad, Calif.) into the left whisker pad and 200 μL DiO (0.5%) in N,N-demethylformamide (D-275) (Invitrogen) into the tongue through a 25-μL Hamilton syringe (Hamilton, Reno, Nev.). Two weeks later, rats were deeply anesthetized intraperitoneally with urethane or sodium pentobarbital and transcardially perfused with 150 mL physiological saline followed by 300 mL of 4% paraformaldehyde in 0.1 mol/L phosphate buffer at pH 7.4. The brain was then removed and postfixed overnight in the same fixative. Coronal sections (50 μm in thickness) of the brain stem were made with a vibrating blade microtome (VT1000S; Leica Microsystems, Buffalo Grove, Ill.), washed with 0.1 mol/L phosphate buffer saline, mounted on gelatin-coated glass slides, and cover-slipped with an aqueous mounting medium. Images were taken by a Zeiss LSM 710 scanning confocal microscope with a 10× objective lens (Plan-Apochromat 10×/0.45 M27; Carl Zeiss, Oberkochen, Germany) with 0.7× digital zoom.
Statistical Analysis
Values are expressed as means ± SD. All values were compared by unpaired t test. The significance level was set at P < 0.05. Statistical analysis was performed with GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, Calif.).
RESULTS
On macro view, at postoperative week 7, no myelin sheath regeneration was observed in the silicone tube group. At postoperative week 13, the neuronal regeneration rates were 71% (n = 5/7) in the silicone tube group (Fig. 3B) and 100% (n = 7/7) in the autograft group (Fig. 3A). On toluidine blue–stained histological sections, the surrounding area was covered with a thick epineurium, and the regeneration of myelin sheath was observed in the center in the silicone tube group (Fig. 3D). Although, in the autograft group, regenerated nerve was found but covered with granulation tissue, several thick axonal blood vessels appeared to be running alongside the epineurium, and the regeneration of a dense myelin sheath was observed (Fig. 3C).
Fig. 3.
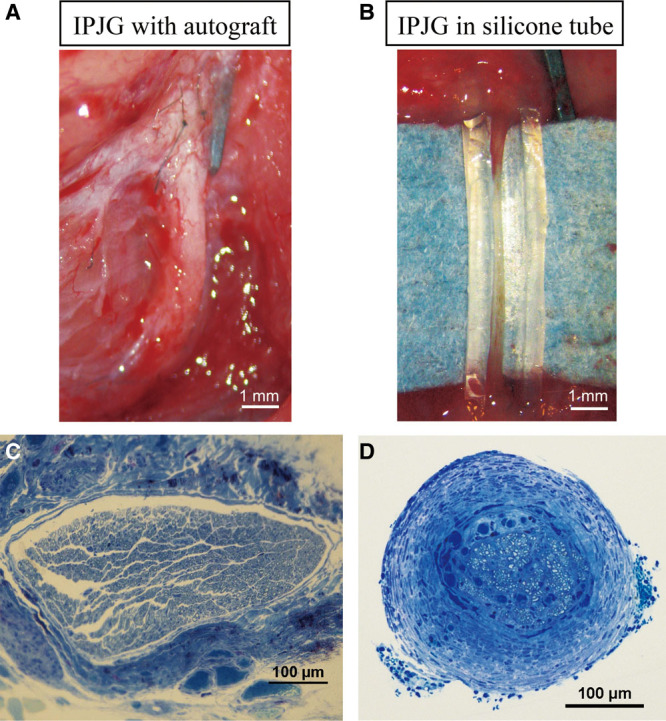
Macroscopical observations of IPJGs with autograft and in a silicone tube conduit, and the toluidine blue staining of their sections at postoperative week 13. A, IPJG with autograft. B, IPJG in a silicone tube conduit. C, Toluidine blue section of IPJG with autograft. D, Toluidine blue section of regenerated IPJG.
Although axonal regeneration was observed with transmission electron microscope in regenerated nerve in the silicone tube group at postoperative week 7 (Fig. 4B), no myelin sheath regeneration was observed. A comparatively thin, however, annular myelin sheath was observed at postoperative week 13 in the group (Fig. 4D). In the autograft group, the regeneration of a thick and substantial annular myelin sheath was observed from postoperative week 7 (Fig. 4A), whereas a thickened, more mature myelin sheath was observed at postoperative week 13 (Fig. 4B). The mean number of myelinated fibers was significantly higher in the autograft group (1980 ± 287) than in the silicone tube group (211 ± 89.2) (P < 0.001; Fig. 5A). The mean fiber diameter, axon diameter, and myelin thickness were significantly higher in the autograft group (7.09 ± 2.4 μm, 5.59 ± 2.12 μm, and 0.75 ± 0.2 μm, respectively) than in the silicone tube group (5.07 ± 1.36 μm, 4.39 ± 1.3 μm, and 0.34 ± 0.08 μm, respectively) (P < 0.001; Fig. 5). The g ratio of the autograft group (0.78 ± 0.05) was significantly lower than that of the silicone tube group (0.86 ± 0.04) (P < 0.0001; Fig. 5).
Fig. 4.
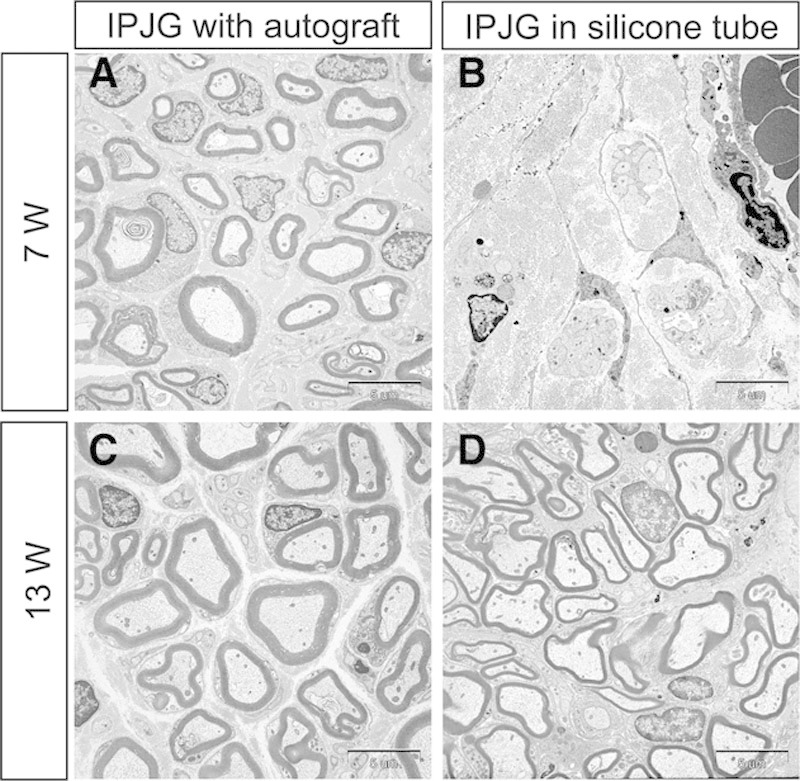
Transmission electron microscope observations of IPJGs at postoperative weeks 7 (A and B) and 13 (C and D). The left and right columns show IPJGs with autograft and in a silicone tube conduit, respectively. The upper and lower rows show the observations at postoperative weeks 7 and 13, respectively. B, Although axonal regeneration was observed in the regenerating nerve in the silicone tube group at week 7, no regeneration of the myelin sheath was observed. D, A comparatively thick and substantial annular myelin sheath was observed in the silicone tube group at week 13. A and C, Regeneration of mature myelin sheath was observed in the autograft group at postoperative week 7 and 13.
Fig. 5.
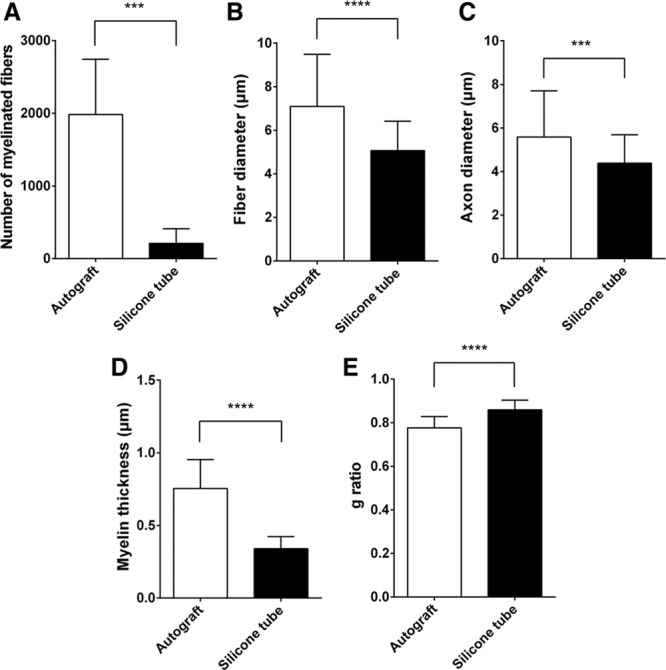
Number of myelinated fibers, fiber diameter, axon diameter, myelin thickness, and g ratio (axon diameter/fiber diameter) of the regenerated nerves in the autograft and silicone tube groups at postoperative week 13. A, The mean number of myelinated fibers was significantly larger in the autograft group (1980 ± 287) than in the silicone tube group (211 ± 89.2). The mean fiber diameter (B), axon diameter (C), and myelin thickness (D) were significantly larger in the autograft group (7.09 ± 2.4 μm, 5.59 ± 2.12 μm, and 0.75 ± 0.2 μm, respectively) than those in the silicone tube group (5.07 ± 1.36 μm, 4.39 ± 1.3 μm, and 0.34 ± 0.08 μm, respectively). E, The g ratio was significantly smaller in the autograft group (0.78 ± 0.05) than that in the silicone tube group (0.86 ± 0.04). The number of myelinated fibers, fiber diameter, axon diameter, myelin thickness, and g ratio were compared by unpaired t test. ***P < 0.001. ****P < 0.0001.
In CMAP physiological tests, the contraction signal of facial muscles for expression supplied by the buccal branch of the facial nerve was observed after the electrical stimulation of this branch in both groups (Figs. 6A, B). Although no difference was observed in CMAP duration between the autograft and silicone tube groups (0.89 ± 0.63 ms and 1.08 ± 0.30 ms) (Fig. 6D), the amplitude values were significantly higher (P < 0.01; Fig. 6C), and the latency values were significantly lower (P < 0.05; Fig. 6E) in the autograft graft group (4.19 ± 1.02 mV and 2.93 ± 0.85 ms) (n = 3) than in the silicone tube group (1.77 ± 1.43 mV and 5.56 ± 2.65 ms) (n = 4). These results indicated that rat facial muscles contracted by the stimuli of regenerated nerve in the silicone tube group.
Fig. 6.
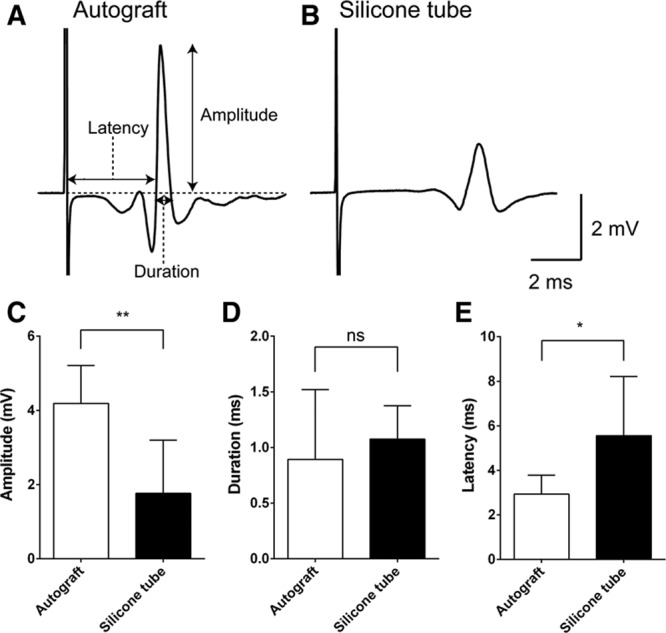
CMAP analysis. For recording CMAP, a stainless steel microelectrode (9–12 MΩ at 1 kHz) (Uesmgcselnnm-type) (FHC, Bowdoin, Maine) was inserted into the vibrissal muscles between the middle vibrissal rows C and D. A reference electrode (TN204-089B) (Unique Medical, Tokyo, Japan) was placed in the caudal position of the skull. For stimulating the regenerated facial nerve, a tandem hook-shaped stimulation electrode (IMC-220224) (InterMedical, Aichi, Japan) was connected to the exfoliated nerve of the buccal branch of facial nerve in the autograft (n = 3) and silicone tube groups (n = 4), and stimulation pulses at a supramaximal level of 2 mA (100 μs monopolar pulses) were delivered at 0.2 Hz via an isolator (SS-202J) (Nihon Kohden, Tokyo, Japan). Recorded signals were processed with a multichannel amplifier (MEG-6100) (Nihon Kohden) at 15–10,000 Hz and digitized at 40 kHz using a PowerLab4/30 and LabChart7 system (ADInstruments, Dunedin, New Zealand). Data were analyzed in an off-line manner with Igor Pro software (Wavemetrics, Lake Oswego, Oreg.). The upward trace of CMAP gave a negative deflection (depolarization), and 10 consecutive traces were averaged. CMAP traces recorded from a whisker pad after supramaximal stimulation IPJG with autograft (A) and in silicone tube (B). Although amplitude (C) values were significantly larger in the autograft group (4.19 ± 1.02 mV) (n = 3) than those in the silicone tube group (1.76 ± 1.43 mV) (n = 4), latency (E) values were significantly lower in the autograft graft group (2.93 ± 0.85 ms) (n = 3) than those in the silicone tube group (5.56 ± 2.65 ms) (n = 4). D, No significant difference in duration was observed (0.89 ± 0.63 ms vs 1.08 ± 0.30 ms). CMAP amplitude, duration, and latency were compared by unpaired t test. *P < 0.05. **P < 0.01. ns indicates not significant.
To investigate bidirectional nerve regeneration, DiI and DiO, which are fluorescent retrograde tracers, were injected into the whisker pads and tongue, respectively. At postoperative week 13, the retrograde tracer findings showed a mixture of DiI-positive (red) motor neurons and DiO-positive (green) motor neurons (white arrow) in the facial nerve nucleus (7N) in the autograft group (Fig. 7A). In the hypoglossal nerve nucleus (12N), DiI-positive (red) motor neurons (white arrowhead), which were mixed with DiO-positive (green) motor neurons, had an irregular arrangement (Fig. 7C). Similarly, in the silicone tube group, a mixture of DiI-positive motor neurons and DiO-positive motor neurons (white arrow) was found in 7N (Fig. 7B). In 12N, DiI-positive motor neurons (white arrowhead) mixed with DiO-positive motor neurons had an irregular arrangement (Fig. 7D). These results confirmed the double innervation of the facial muscles of expression and the tongue by 7N and 12N.
Fig. 7.
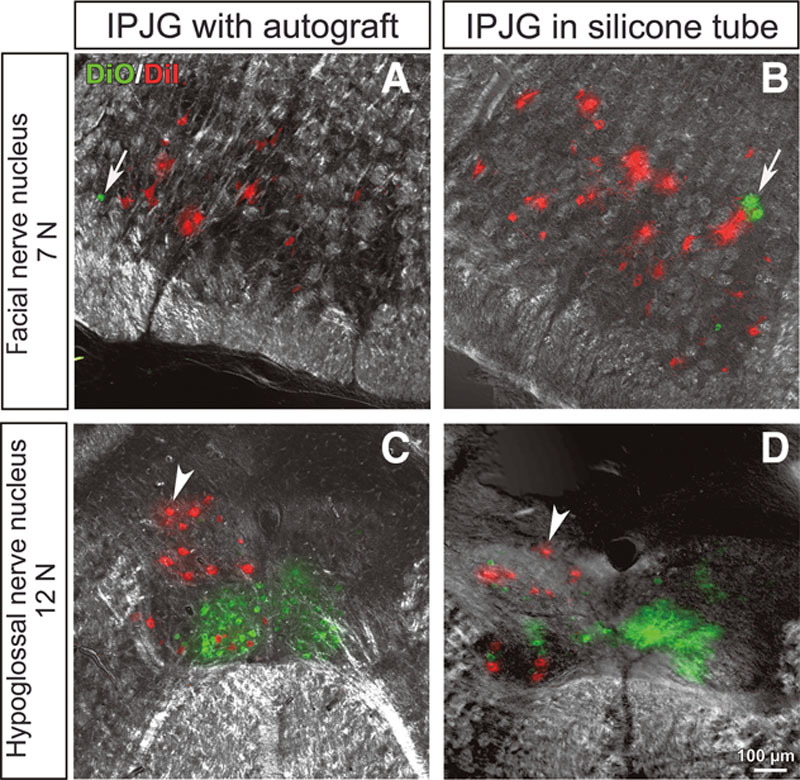
Retrograde tracer findings of IPJGs at postoperative week 13 in the autograft (A and C) and silicone tube (B and D) groups. DiI and DiO, fluorescent retrograde tracers, were injected into the whisker pads and tongue, respectively. The left and right columns show IPJGs with autograft and in silicone tube conduit, respectively. The upper and lower rows show the observations at facial nerve nucleus (7N) and hypoglossal nucleus (12N), respectively. A, DiO-positive (green) motor neurons (white arrow) were found to be mixed with DiI-positive (red) motor neurons in 7N of the autograft group. C, In the hypoglossal nerve nucleus, DiI-positive (red) motor neurons (white arrowhead), which were mixed with DiO-positive (green) motor neurons, had an irregular arrangement (white arrowhead). B, Similarly, a mixture of DiI-positive (red) motor neurons and DiO-positive (green) motor neurons (white arrow) in 7N were found in the silicone tube group. D, In the hypoglossal nerve nucleus, DiI-positive (red) motor neurons (white arrowhead) mixed with DiO-positive (green) motor neurons had an irregular arrangement. The results confirmed the double innervation of the facial muscles of expression and the tongue by 7N and 12N. Unusual labelings after IPJG treatments were indicated with white arrows and white arrowheads.
DISCUSSION
The authors in this study successfully developed the end-to-side neurorrhaphy between facial and hypoglossal nerves with an artificial nerve conduit for the first time. Furthermore, with the same suture method, this study prepared an IPJG, which was made for the facial and hypoglossal nerves with a silicone tube as an artificial nerve conduit. This study confirmed a bidirectional nerve regeneration in the tube. The most important point during peripheral nerve regeneration with an artificial nerve conduit is how to induce the sufficient number of Schwann cells from the bilateral severed nerve stump as the first steps toward nerve regeneration.15 Regarding this point, in this study, 2 factors leading to the success of IPJG by end-to-side neurorrhaphy were (1) the effect of width of enlarged epineural window at both ends of the silicone tube, and (2) definitely the adequate direct of epineural window toward the lumen of silicone tube. The careful adjustments of 2 factors would allow IPJG to be highly effective for the induction of Schwann cell growth into the tube lumen. The facial nerve trunk and the hypoglossal nerve were able to be firmly inserted into the gap of silicone tube by the careful manipulation in this study, although there were 2 concerns that (1) the distance between the hypoglossal nerve and facial nerve trunk would increase as rats grew and (2) the silicone tube might be lost due to the mechanical strain caused by neck exercises. Fortunately, after suturing, the stability of silicone tube was found to be high without dropout. However, because (1) the hypoglossal nerve is extremely thin with a thickness of 1 mm or less, (2) the manipulation for epineural incision in uninjured axons is difficult, and (3) the hypoglossal nerve exists deep within the neck, the use of an artificial nerve conduit in end-to-side anastomosis is complex, and an IPJG with an artificial nerve conduit in rats requires a skillful microsurgery technique.
This study performed no axotomy of the facial nerve trunk and hypoglossal nerve during the end-to-side neurorrhaphy. In experiments with rats, Viterbo et al16 have reported end-to-side neurorrhaphy without axotomy. Similarly, Ueda et al5 have reported favorable results with IPJGs with autologous nerves without axotomy.5 In the future, differences in the degree of nerve regeneration after axotomy and the degree of decreased lingual motor function due to the incomplete transection of the hypoglossal nerve should be evaluated.
The retrograde tracer test in the silicone tube group found the double innervation of the muscles of facial expression by 7N and 12N,3,17,18 and the result was similar to those of the autograft group. Shichinohe et al11 have confirmed “a bidirectional nerve regeneration,” which is previously known to be the regeneration of axons extending outward from both the ends of a single autologous transplanted nerve. In addition to the previous study, this study found the bidirectional nerve regeneration from the facial and hypoglossal nerves after using an artificial nerve conduit, indicating that signals were bidirectionally transmitted from the hypoglossal to facial nerves and vice versa. This study first succeeded in making an IPJG in an artificial nerve conduit. Therefore, an axonal supercharge appeared effectively even after an IPJG regenerated in an artificial nerve conduit, similar to the result after an autologous nerve was used as an IPJG. After performing end-to-side neurorrhaphy, this study attempted to elucidate the nerve regeneration mechanism that causes collateral sprouting at the contact surface between the recipient nerves and the artificial nerve conduit.16,19
Although the findings at postoperative week 7 showed no myelin sheath regeneration, a good regeneration of myelin sheath was observed in the silicone tube group at postoperative week 13. However, the results in the silicone tube group were incomparable with those in the autograft group. In the autologous group, nerve regeneration could have been promoted by angiogenesis from the graft bed and by the supplies of oxygen and nutrients as previously suggested.20 In the silicone tube group, because the tube has no tissue permeability, regenerating nerves could require more time to reach maturity. A semipermeable and biodegradable artificial nerve conduit could provide an adequate nerve regeneration condition, which can be comparable with that of autologous nerves.
Although original IPJG technique performed clinically requires 50–70 mm autologous nerve,2 in this study, the length of silicone tube for IPJG was up to 7 mm in rats. For clinical applications, the suitable maximum length of artificial nerve conduit for IPJG technique needs to be investigated in the future with large experimental animals.
In the CMAP measurement, although the autograft group showed better results than the silicone tube group, autograft treatment requires an autograft nerve, which must be obtained from the donors with their sacrifice spirits, inducing possible ethical problems. From this viewpoint, regenerated nerves in the conduits might be better choice despite their slightly poor physiological functions. New artificial nerve conduits, which had stem cells or basic fibroblast growth factor for allowing regenerated nerves to become more similar to autograft, should be investigated in the future.
CONCLUSION
In this study, the authors successfully developed end-to-side neurorrhaphy between facial and hypoglossal nerves with an artificial nerve conduit and also found that a conventional IPJG technique with an autologous nerve can be replaced with an artificial nerve conduit in a rat model with partial facial nerve paresis.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study was supported by the Formation of Innovation Center for Fusion of Advanced Technologies project sponsored by the Special Coordination Funds for Promoting Science and Technology “Cell Sheet Tissue Engineering Center” and by the Global Center of Excellence program at the Multidisciplinary Education and Research Center for Regenerative Medicine, funded by the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by the Japan Science and Technology Agency, Precursory Research for Embryonic Science and Technology program. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Al-Qattan MM. Terminolateral neurorrhaphy: review of experimental and clinical studies. J Reconstr Microsurg. 2001;17:99–108. doi: 10.1055/s-2001-12698. [DOI] [PubMed] [Google Scholar]
- 2.May M, Sobol SM, Mester SJ. Hypoglossal-facial nerve interpositional-jump graft for facial reanimation without tongue atrophy. Otolaryngol Head Neck Surg. 1991;104:818–825. doi: 10.1177/019459989110400609. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto Y, Sekido M, Furukawa H, et al. Surgical rehabilitation of reversible facial palsy: facial–hypoglossal network system based on neural signal augmentation/neural supercharge concept. J Plast Reconstr Aesthet Surg. 2007;60:223–231. doi: 10.1016/j.bjps.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto Y, Sasaki S, Sekido M, et al. Alternative approach using the combined technique of nerve crossover and cross-nerve grafting for reanimation of facial palsy. Microsurgery. 2003;23:251–256. doi: 10.1002/micr.10115. [DOI] [PubMed] [Google Scholar]
- 5.Ueda K, Akiyoshi K, Suzuki Y, et al. Combination of hypoglossal-facial nerve jump graft by end-to-side neurorrhaphy and cross-face nerve graft for the treatment of facial paralysis. J Reconstr Microsurg. 2007;23:181–187. doi: 10.1055/s-2007-974654. [DOI] [PubMed] [Google Scholar]
- 6.Harii K, Asato H, Yoshimura K, et al. One-stage transfer of the latissimus dorsi muscle for reanimation of a paralyzed face: a new alternative. Plast Reconstr Surg. 1998;102:941–951. doi: 10.1097/00006534-199809040-00001. [DOI] [PubMed] [Google Scholar]
- 7.Matsumine H, Sasaki R, Yamato M, et al. A polylactic acid non-woven nerve conduit for facial nerve regeneration in rats. J Tissue Eng Regen Med. 2014;8:454–462. doi: 10.1002/term.1540. [DOI] [PubMed] [Google Scholar]
- 8.Matsumine H, Sasaki R, Tabata Y, et al. Facial nerve regeneration using basic fibroblast growth factor-impregnated gelatin microspheres in a rat model. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1884. Apr 16. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Sasaki R, Aoki S, Yamato M, et al. PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. J Tissue Eng Regen Med. 2011;5:823–830. doi: 10.1002/term.387. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki R, Matsumine H, Watanabe Y, et al. Anesthesia for research on reconstructive facial surgery in rats. J Reconstr Microsurg. 2013;29:209–210. doi: 10.1055/s-0032-1331147. [DOI] [PubMed] [Google Scholar]
- 11.Shichinohe R, Furukawa H, Sekido M, et al. Direction of innervations after interpositional nerve graft between facial and hypoglossal nerves in individuals with or without facial palsy: a rat model for treating incomplete facial palsy. J Plast Reconstr Aesthet Surg. 2012;65:763–770. doi: 10.1016/j.bjps.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Matsumine H, Sasaki R, Takeuchi M, et al. Surgical procedure for transplanting artificial nerve conduits for peripheral nerve regeneration. Plast Reconstr Surg. 2011;128:95e–97e. doi: 10.1097/PRS.0b013e31821ef380. [DOI] [PubMed] [Google Scholar]
- 13.Matsumine H, Sasaki R, Takeuchi Y, et al. Vascularized versus nonvascularized island median nerve grafts in the facial nerve regeneration and functional recovery of rats for facial nerve reconstruction study. J Reconstr Microsurg. 2014;30:127–136. doi: 10.1055/s-0033-1357500. [DOI] [PubMed] [Google Scholar]
- 14.Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol. 1999;81:2243–2252. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- 15.Widmer MS, Gupta PK, Lu L, et al. Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials. 1998;19:1945–1955. doi: 10.1016/s0142-9612(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 16.Viterbo F, Trindade JC, Hoshino K, et al. End-to-side neurorrhaphy with removal of the epineurial sheath: an experimental study in rats. Plast Reconstr Surg. 1994;94:1038–1047. doi: 10.1097/00006534-199412000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara T, Matsuda K, Kubo T, et al. Axonal supercharging technique using reverse end-to-side neurorrhaphy in peripheral nerve repair: an experimental study in the rat model. J Neurosurg. 2007;107:821–829. doi: 10.3171/JNS-07/10/0821. [DOI] [PubMed] [Google Scholar]
- 18.Isaacs JE, Cheatham S, Gagnon EB, et al. Reverse end-to-side neurotization in a regenerating nerve. J Reconstr Microsurg. 2008;24:489–496. doi: 10.1055/s-0028-1088230. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Soucacos PN, Bo J, et al. Evaluation of collateral sprouting after end-to-side nerve coaptation using a fluorescent double-labeling technique. Microsurgery. 1999;19:281–286. doi: 10.1002/(sici)1098-2752(1999)19:6<281::aid-micr5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Terzis JK, Kostopoulos VK. Vascularized ulnar nerve graft: 151 reconstructions for posttraumatic brachial plexus palsy. Plast Reconstr Surg. 2009;123:1276–1291. doi: 10.1097/PRS.0b013e31819f2afd. [DOI] [PubMed] [Google Scholar]


