Supplemental Digital Content is available in the text.
Abstract
Background:
Negative-pressure wound therapy (NPWT) has been marketed for about 20 years and remains popular. The only real obstacle to NPWT is the cost; therefore, we designed an inexpensive NPWT connected to a wall vacuum. Here, we report the feasibility and safety of this product, which we call PROVACUUM (Z-Biotech, Saint-Avertin, France).
Methods:
As a first step, the constraints imposed on the manufacturer were equipment quality similar to that of commercial NPWT systems, with an average treatment cost of $15/d. Then, we conducted a prospective study of patients with indications for NPWT from September 2013 to January 2015. Data collected included ease of use, quality of materials, and occurrence of complications during treatment.
Results:
We enrolled 23 patients with a mean age of 50.8 years. The average duration of treatment was 8.5 days (range, 3–21 days). The dressings were changed every 3.3 days (range, 2–4 days). Two hematomas occurred that required surgical revision and the transfusion of 2 units after large debridement of pressure ulcer. No other adverse events or infections occurred. The surgeons found that our device was similar to commercial NPWT devices.
Conclusions:
We developed an inexpensive NPWT that costs an average of $15/d. Our process is not intended to replace portable or stand-alone devices with batteries, but rather offers a less expensive alternative for hospitalized patients and makes NPWT accessible to the most precarious countries and institutions.
Although present on the market for about 20 years, the popularity of negative-pressure wound therapy (NPWT) has not decreased [The Vacuum-Assisted Closure (VAC); KCI, San Antonio, Tex.]. NPWT system is known internationally and has revolutionized the way we manage wounds. NPWT has many indications, both acute and chronic, and has brought great comfort to patients, caregivers, doctors, and nurses. The only real obstacle to this useful procedure is the cost, which slightly decreased, but remains expensive for prolonged indications, making it unaffordable in underdeveloped countries where these dressings are needed.
Therefore, we designed a low-cost NPWT connected to a wall vacuum, which we call PROVACUUM; this device was produced by Z-Biotech. The constraint imposed on its manufacture was that it had an average daily cost of less than $15. Here, we report the results of a prospective study evaluating the feasibility and safety of this product.
METHODS
First, we designed an inexpensive NPWT device made of polyurethane foam, transparent adhesive film, tubing, and a 3-way valve, which we had manufactured by Z-Biotech. The constraints imposed on the manufacturer were an equipment quality similar to that of commercial NPWT devices and an average treatment cost of $15/d (Fig. 1).
Fig. 1.
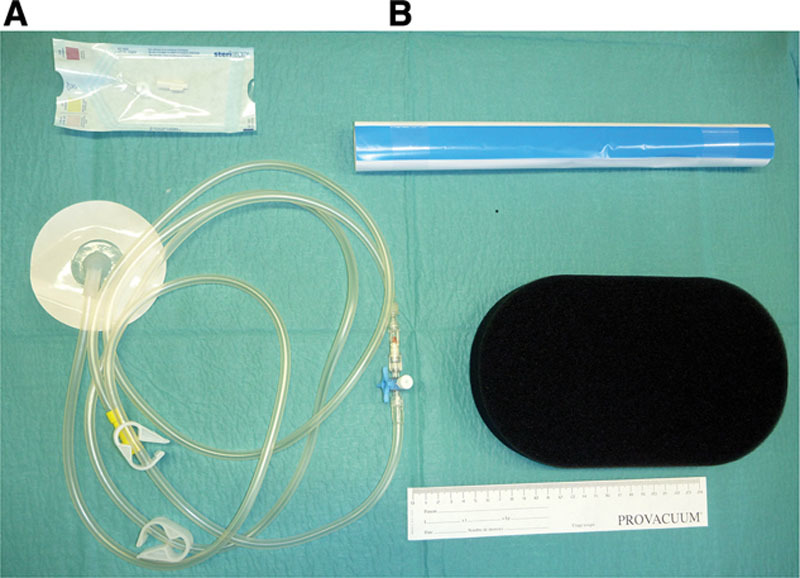
A, NPWT dressing kit in its package. B, Open dressing kit, comprising transparent adhesive film, polyurethane foam, tubing, 3-way valve, and an adapter.
Then, we conducted a prospective study of patients with indications for NPWT from September 2013 to January 2015. The negative pressure was set at 125 mm Hg with a manometer. The exudates were collected in conventional vacuum bottles (See Video 1, Supplemental Digital Content 1, which displays ischial pressure ulcer management with PROVACUUM (Z-Biotech). This video is available in the “Related Videos” section of the full-text article at http://www.PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A106). The dressings were changed every 3–4 days. Data collected included ease of use, quality of materials, and occurrence of complications during treatment. Pain was evaluated at each stage using a visual analogue scale.
Video. 1.
See video, Supplemental Digital Content 1, which displays ischial pressure ulcer management with PROVACUUM. This video is available in the “Related Videos” section of the full-text article at http://www.PRSGO.com or available at http://links.lww.com/PRSGO/A106.
To monitor bleeding and infection, blood count and C-reactive protein were measured twice weekly. All of the patients consented to participate in this study, which had institutional review board approval.
RESULTS
We enrolled 23 patients [20 male, 3 female; mean age, 50.8 years (range, 22–79 years)] in the study from September 2013 to January 2015. The patients had acute or chronic diseases (Table 1). The dressings were changed every 3.3 days (range, 2–4 days). The average treatment lasted 8.5 days (range, 3–21 days). The pain associated with the implementation of NPWT was rated 2 of 10 (range, 0–4 of 10), whereas the pain with dressing changes averaged 3 of 10 (range, 0–6 of 10).
Table 1.
Patients Managed by PROVACUUM
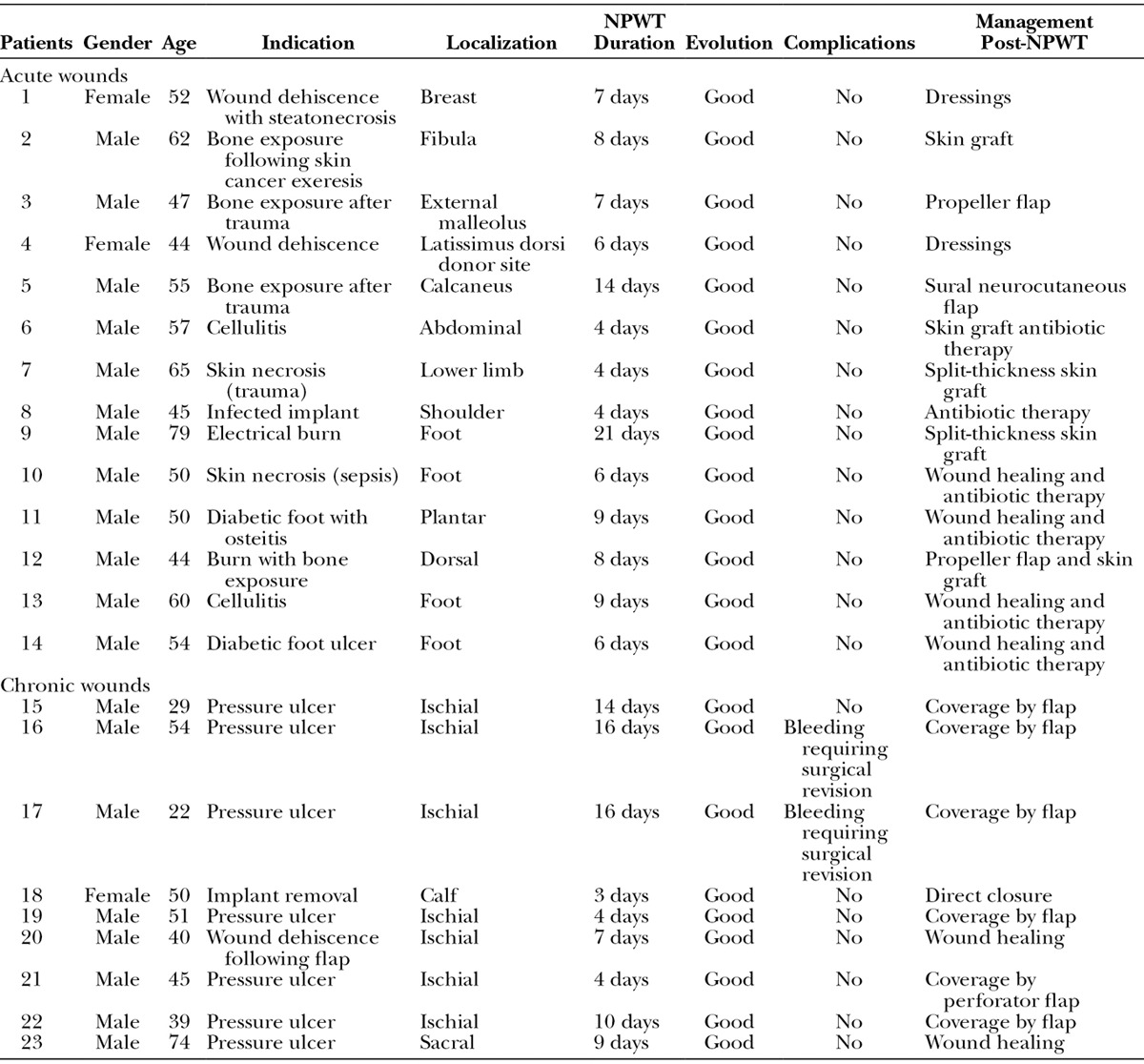
Two serious complications occurred in the first patients; namely, 2 hematomas developed after large pressure ulcer debridement, which required surgical revision and the transfusion of 2 units (Fig. 2). No other adverse events occurred, and no infections were reported. The surgeons who used our device found it as easy to use as commercial NPWT devices, except for the adhesive film (Fig. 3). Indeed, the initial attempts used inadequate adhesive film. We have subsequently improved the quality of the film.
Fig. 2.
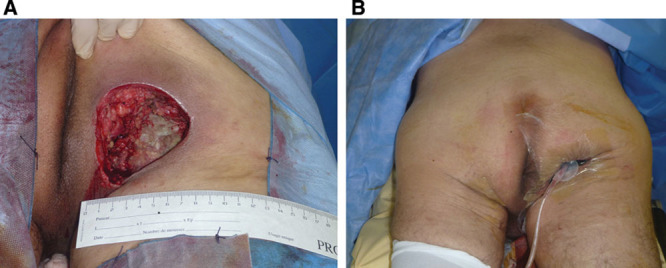
A, Debridement of an infected ischial pressure ulcer in a 29-year-old paraplegic patient. B, Starting NPWT. We can see that the vacuum leads to satisfactory depression of the cavity with 125 mm Hg.
Fig. 3.
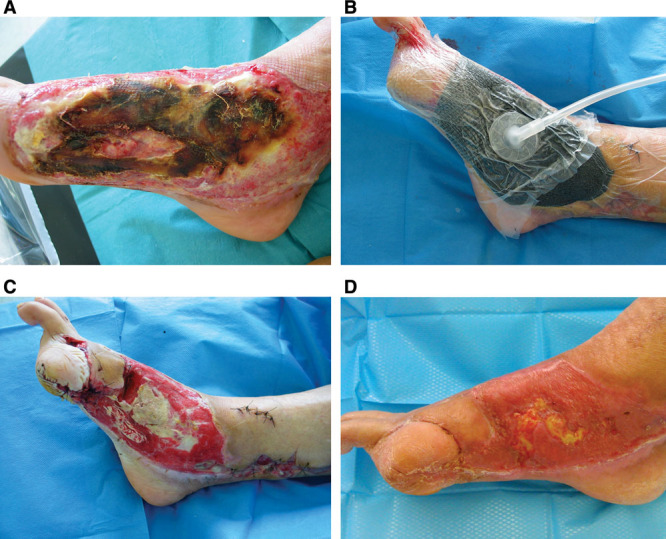
A, Electrical burn in a 79-year-old man with bone exposure on the inner side of the foot. B, Setting up PROVACUUM before attempting a skin graft. C, After 1 week of NPWT, a significant bone exposure persists in the middle of the wound. D, A split-thickness skin graft was performed after 3 weeks of NPWT. The wound healing is almost complete.
DISCUSSION
Negative-pressure therapy is not new, and the VAC system has been marketed internationally by Kinetic Concepts since 1997.1,2 The omnipresence of conflicts of interest in the medical literature dealing with NPWT is harmful because the results of many studies have been minimized because of this problem. There are many innovations in the field of NPWT, including miniaturization,3,4 the development of a fully mechanical system,5,6 and recent indications.7
The marketing of new commercial devices, such as RENASYS (Smith & Nephew, London, United Kingdom) and VivanoTec (Hartmann, Amtsgericht Ulm, Germany), should have led to a significant drop in costs, but this did not happen. Consequently, the cost has limited the accessibility of VAC systems in various institutions. Other low-cost systems have been described that use a suction drain8 or Pleur-Evac (Teleflex Medical, Morrisville, N.C.) system,9 but their low suction power is insufficient for large or complex wounds.
Currently, the French health authorities consider the safety and reliability of equipment using wall vacuum uncertain,10 which is why we performed this preliminary study to assess the feasibility and safety of PROVACUUM (Z-Biotech). NPWT is not risk-free, so any new material must be evaluated and validated. Between 2007 and 2011, the Food and Drug Administration reported 12 deaths and 174 injuries linked to NPWT. Most deaths occurred at home or in long-term care establishments.11,12
Two patients in our study developed bleeding within the first 48 hours, and this seems to be the main complication and requires monitoring. In subsequent patients, after these 2 complications, we waited at least 24 hours before setting the negative pressure to 125 mm Hg. Nonetheless, this problem can occur with all NPWT devices on the market. In general, for NPWT, it seems safer not to start the aspiration the first day following a hemorrhagic debridement. No bleeding occurred in subsequent cases.
As evaluated by Dorafshar et al,13 this system uses a wall vacuum and must be restricted to hospital use to ensure regular monitoring and good functioning. In practice, we require monitoring every 4 hours to confirm good depression of the dressing and to ensure that the exudate in the bottle is not bloody, which could indicate bleeding or discharge, suggestive of infection. We included a 3-way valve that allows manual instillations, similar to the VAC Ulta (KCI, San Antonio, Tex.). Instillation with NPWT has led to interesting results in acutely infected wounds,14 but the real role of this procedure in the treatment algorithm is not fully defined.
Ultimately, the low cost of PROVACUUM (Z-Biotech) makes this procedure available in less wealthy institutions and countries, with similar safety and comfort of use as the NPWT systems marketed for hospital use.
CONCLUSIONS
NPWT has completely changed our management of wounds, even in the most complex or desperate situations. The development of a NPWT dressing kit accessible to all, costing an average of $15/d, is a step in the democratization of NPWT. This process is not intended to replace miniature or autonomous devices with batteries, but rather offers a less expensive alternative, especially during the first weeks of hospitalization, and makes NPWT accessible in the most precarious regions.
Supplementary Material
Footnotes
Presented at the French Plastic Surgery Meeting “Symposium Vidéo Plastie”, July 4, 2014, Toulouse, France.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Morykwas MJ, Argenta LC, Shelton-Brown EI, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553–562. doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Danino AM, Weber ID. [A short history of negative pressure therapy in wound healing: “to avoid the Frigidaire syndrome”]. Ann Chir Plast Esthet. 2007;52:624–625. doi: 10.1016/j.anplas.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Sposato G, Molea G, Di Caprio G, et al. Ambulant vacuum-assisted closure of skin-graft dressing in the lower limbs using a portable mini-VAC device. Br J Plast Surg. 2001;54:235–237. doi: 10.1054/bjps.2000.3537. [DOI] [PubMed] [Google Scholar]
- 4.Payne C, Edwards D. Application of the single use negative pressure wound therapy device (PICO) on a heterogeneous group of surgical and traumatic wounds. Eplasty. 2014;14:e20. [PMC free article] [PubMed] [Google Scholar]
- 5.Fong KD, Hu D, Eichstadt S, et al. The SNaP system: biomechanical and animal model testing of a novel ultraportable negative-pressure wound therapy system. Plast Reconstr Surg. 2010;125:1362–1371. doi: 10.1097/PRS.0b013e3181d62b25. [DOI] [PubMed] [Google Scholar]
- 6.Isaac AL, Rose J, Armstrong DG. Mechanically powered negative pressure wound therapy as a bolster for skin grafting. Plast Reconstr Surg Glob Open. 2014;2:e103. doi: 10.1097/GOX.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riot S, de Bonnecaze G, Garrido I, et al. Is the use of negative pressure wound therapy for a malignant wound legitimate in a palliative context? “The concept of NPWT ad vitam”: a case series. Palliat Med. 2015;29:470–473. doi: 10.1177/0269216314560009. [DOI] [PubMed] [Google Scholar]
- 8.Bekara F, Herlin C, Ayestaray B, et al. Suction drain-assisted split-thickness skin grafting: a simple procedure to improve skin graft take. Plast Reconstr Surg. 2015;135:240e–241e. doi: 10.1097/PRS.0000000000000782. [DOI] [PubMed] [Google Scholar]
- 9.Dorafshar AH, Agarwal S, Franczyk M, et al. Low-pressure negative-pressure wound therapy using the Pleur-evac system in hemodynamically unstable patients. Plast Reconstr Surg. 2010;125:179e–181e. doi: 10.1097/PRS.0b013e3181d45e9d. [DOI] [PubMed] [Google Scholar]
- 10.Traitement des plaies par pression négative (TPN), des utilisations spécifiques et limitées. Recommendations HAS Janvier 2011. Available at: http://www.has-sante.fr/portail/upload/docs/application/pdf/2010-02/fiche_de_bon_usage_traitement_des_plaies_par_pression_negative.pdf. Accessed February 22, 2010. [DOI] [PubMed]
- 11.UPDATE on Serious Complications Associated with Negative Pressure Wound Therapy Systems: FDA Safety Communication. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm244211.htm. Accessed February 24, 2011.
- 12.Kim PJ, Attinger CE, Steinberg JS, et al. The impact of negative-pressure wound therapy with instillation compared with standard negative-pressure wound therapy: a retrospective, historical, cohort, controlled study. Plast Reconstr Surg. 2014;133:709–716. doi: 10.1097/01.prs.0000438060.46290.7a. [DOI] [PubMed] [Google Scholar]
- 13.Dorafshar AH, Franczyk M, Gottlieb LJ, et al. A prospective randomized trial comparing subatmospheric wound therapy with a sealed gauze dressing and the standard vacuum-assisted closure device. Ann Plast Surg. 2012;69:79–84. doi: 10.1097/SAP.0b013e318221286c. [DOI] [PubMed] [Google Scholar]
- 14.Orgill DP, Bayer LR. Update on negative-pressure wound therapy. Plast Reconstr Surg. 2011;127(Suppl 1):105S–115S. doi: 10.1097/PRS.0b013e318200a427. [DOI] [PubMed] [Google Scholar]


