Abstract
Background:
Patients undergoing incisional/ventral hernia repair are at risk of developing several postoperative complications particularly venous thromboembolism (VTE), which is a major cause of morbidity and mortality. The aim of this study was to assess 30-day postoperative morbidity and mortality of patients undergoing incisional/ventral hernia repair and to determine the association between component separation and VTE.
Methods:
We reviewed the 2005–2011 American College of Surgeons National Surgical Quality Improvement Program databases to identify patients undergoing incisional/ventral hernia repair. Preoperative variables and postoperative outcomes were compared between a component separation group and a non–component separation group. The χ2 tests and Fisher’s exact test were used for categorical variables and t tests for continuous variables. Logistic regression analysis was performed to determine preoperative predictors for complications in both groups.
Results:
Thirty-four thousand five hundred forty-one patients were included in our study; 501 patients underwent a component separation procedure. A higher rate of wound complications, minor/major morbidity, mortality, and return to the operating room occurred in the component separation group. However, there was no statistically significant difference in deep vein thrombosis/thrombophlebitis and pulmonary embolism rates between the 2 groups (P = 0.780 and P = 0.591, respectively). Several risk factors were significantly associated with postoperative complications in both groups.
Conclusions:
Component separation is used for large and complex incisional/ventral hernia repairs to achieve tension-free midline closure. Although component separation hernia repair is associated with higher incidence of wound complication, morbidity, and mortality, perhaps because of the complexity of the defects, it does not seem to be associated with increased VTE rates.
Over 400,000 patients undergo hernia repair in the United States each year.1 The overall incidence of incisional/ventral hernia following abdominal surgery is reported to be 3–13%.2 Patients undergoing ventral hernia procedures tend to have significant associated comorbidities complicating their repair.3,4 Moreover, operative repair of abdominal wall hernias oftentimes imposes significant physiologic alteration for patients and is associated with major postoperative complications.5 Attempting to repair a large ventral hernia primarily may have potential consequences including alteration of abdominal physiology by increasing abdominal pressure especially if such a repair is performed under tension. Traditional methods of hernia repair have unacceptably high recurrence rates sometimes in excess of 50% when primary suture repair with simple fascial approximation is performed.6–11 The field of complex ventral hernia repair has seen significant advancements since the introduction of the component separation technique which was initially described by Ramirez in 1990.12 Component separation, which allows for repair of abdominal wall defects as wide as 8–10 cm, is based on the concept of recreating a functional dynamic abdominal wall through medialization of the rectus abdominis muscles and recreation of the linea alba.5 This form of muscle advancement allows otherwise inoperable cases to be repaired successfully without tension and is associated with a significant decrease in recurrence rates.12–14 This approach is typically reserved for patients who cannot be treated with conventional hernia repair methods because of the complexity of the abdominal wall defect.
Component separation is not without significant risk. Its extended subcutaneous dissection for exposing anterior abdominal musculature may lead to compromised blood supply of the abdominal wall, raised risk of bleeding and infection, and because of longer operative times may predispose patients to increased risk of venous thromboembolism (VTE). Surgeons must, therefore, be able to balance the risk of a long and aggressive surgical procedure with the patient’s desire for clinical improvement. A variety of conditions associated with ventral hernia also contribute to the development of VTE, including obesity, prior operation, pulmonary disease, corticosteroid dependency, immobilization, and advanced age.15 Closing large fascial defects may result in increased intra-abdominal pressure leading to the development of abdominal compartment syndrome (ACS) and a predisposition for femoral venous stasis with subsequent VTE.16,17 In this study, the authors assess 30-day postoperative morbidity and mortality of patients undergoing incisional/ventral hernia repair and determine the association between component separation and VTE as well as predictors for specific postoperative complications.
METHODS
Patient Identification
We performed a retrospective analysis of patients who underwent incisional/ventral hernia repair using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) databases for the years 2005–2011. The ACS-NSQIP is a well-validated, observational cohort study of patients undergoing non-cardiac procedures under general, spinal, or epidural anesthesia in more than 400 medical centers nationwide. ACS-NSQIP tracks patients for 30 days after their operation, providing a more comprehensive picture of their care.18 Patients were identified using Current Procedural Terminology (CPT) codes for incisional/ventral hernia repair (CPT codes: 49560, 49561, 49565, and 49566). Patients who underwent a concurrent intra-abdominal procedure or one involving another part of the body were excluded to eliminate their confounding effect on outcome. However, panniculectomy (often performed as a concurrent procedure because of the optimized elevation of abdominal pannus), skin closure procedure (frequently coded as a separate procedure but done when repairing large abdominal wound defects), and mesh implantation (may be included in the ventral hernia repair and therefore was incorporated in our analysis) were not used as exclusion criteria. We divided our patient population into 2 groups. The first group consisted of patients who underwent incisional/ventral hernia repair without the use of component separation; the second group consisted of patients with ventral hernias who were managed with component separation. The CPT code 15734 (muscle, myocutaneous, or fasciocutaneous flap) was used to identify component separation procedure in which the aponeurosis of the external oblique muscle is longitudinally incised, and the rectus muscle is mobilized toward the midline to facilitate abdominal fascia closure19 (Fig. 1).
Fig. 1.
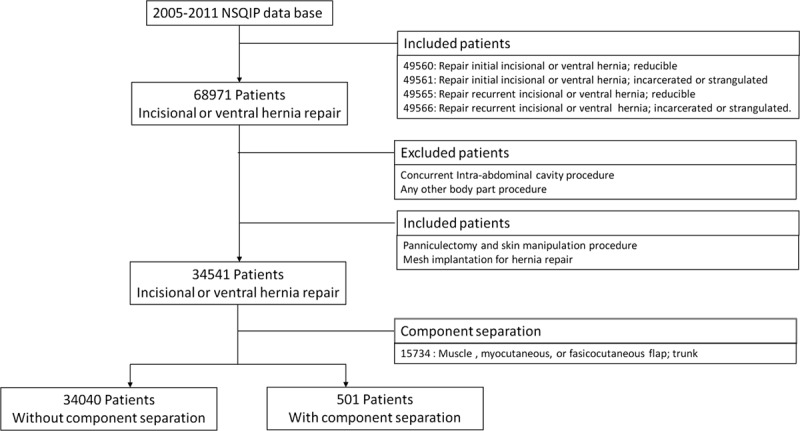
Patient selection process.
Preoperative Variables
Data related to patient demographics, preoperative physical status, medical comorbidity, preoperative laboratory results, VTE risk score, concurrent mesh implantation, and panniculectomy were collected. The VTE risk score (based on the Caprini risk assessment model) was defined as the number of well-known risk factors for VTE that are available in NSQIP database20: older than 40 years, smoking, chronic steroid use, history of prior operation, body mass index greater than 25, concurrent pneumonia/chronic obstructive pulmonary disease, totally dependent functional status, pregnancy, malignancy, sepsis, congestive heart failure, acute myocardial infarction, and history of peripheral vascular disease.
Outcome Variables
Outcome variables were characterized as deep venous thrombosis (DVT)/thrombophlebitis, pulmonary embolism (PE), wound complication, minor/major morbidity, and mortality. Other outcomes of interest included return to the operating room (OR) within 30 days of admission and total operative time. The NSQIP employs Centers for Disease Control definitions for wound infections.21 As such, wound complications were defined by presence of at least one of the following wound related variables in the NSQIP registry: superficial surgical site infection (SSI; above fascia), deep incisional SSI (at or below fascia), organ/space SSI, and wound dehiscence (complete or partial). These individual wound complication variables were assessed separately. Major morbidity was defined by the presence of at least one of the following ACS-NSQIP postoperative complications: being on a ventilator for more than 48 hours, pneumonia, unplanned intubation, progressive renal insufficiency, cerebrovascular accident with neurological deficit, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, transfusion within 72 hours, sepsis, and septic shock. Minor morbidity was defined as a urinary tract infection. Mortality was defined by death within 30 days of admission.
Statistical Analysis
Statistical analyses were performed using IBM SPSS (IBM Corp., Armonk, N.Y.) to determine baseline differences in demographics, comorbidities, preoperative laboratory results, concurrent panniculectomy/mesh implantation, and postoperative complications between the 2 study groups. The χ2 test and Fisher’s exact test were used to analyze categorical variables, and t test was used to analyze continuous variables. Statistical significance was defined as P value less than 0.05. In terms of specific complications, the odds ratio of component separation technique was also assessed. A multivariate logistical regression was performed to determine whether various demographics, comorbidities, and concurrent procedures served as predictors for respective complications in both groups. The data reported are based on 30-day outcomes from incisional/ventral hernia repair with or without component separation.
RESULTS
Comparison of Preoperative Variables
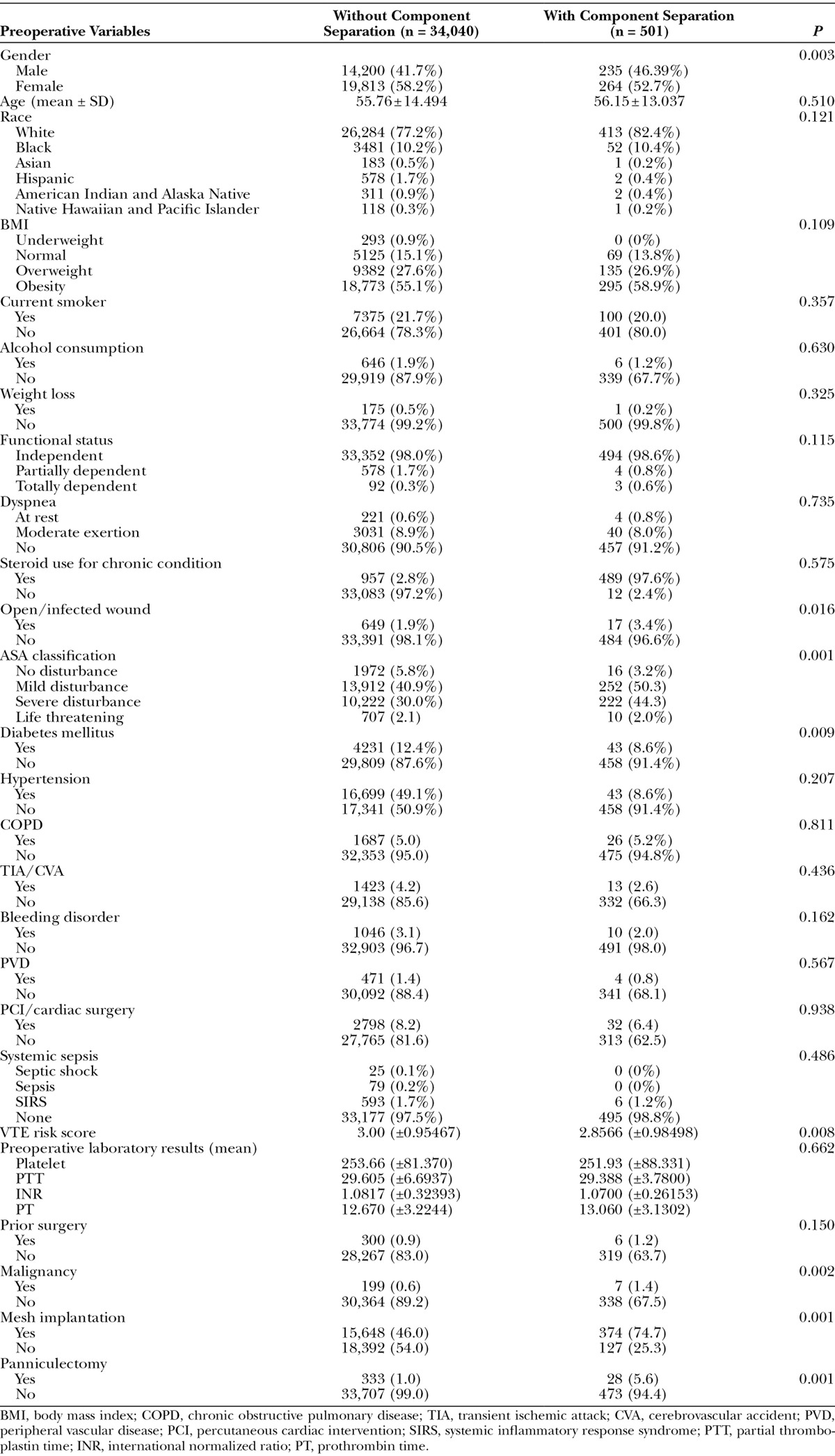
We identified 34,541 patients who underwent incisional/ventral hernia repair between 2005 and 2011. Five hundred and one patients underwent component separation as part of this repair, whereas 34,040 patients did not. One reason for the small number of cases in the component separation group (N = 501) may be attributed to a rigorous patient selection criteria. To eliminate all potential confounding effects of other procedures, patients who underwent concurrent surgeries were excluded from our analyses. Despite the small number, this group represents patients who underwent a component separation procedure only and represents the ideal cohort for analysis. There were more male patients in the component separation group than the non–component separation group (46.39% vs. 41.7%, P = 0.003). The mean ages in the component separation group and non–component separation group were 56.15 ± 13.037 and 55.76 ± 14.494 years, respectively (P = 0.510). There was no statistically significant difference in race between the 2 groups (P = 0.121). Analysis of preoperative comorbidity variables showed a significant difference in open/infected wound (P = 0.016), American Society of Anesthesiologists (ASA) classification (P = 0.001), diabetes mellitus (P = 0.009), VTE risk factors (P = 0.008), and malignancy (P = 0.002) between the 2 cohorts. Mesh implantation and panniculectomy were performed more frequently in component separation group (46.0% vs. 74.7%, P = 0.001 and 1.0% vs. 5.6%, P = 0.001, respectively). Preoperative laboratory results were identical. These data are summarized in Table 1.
Table 1.
Comparison of Preoperative Variables
Comparison of Complications
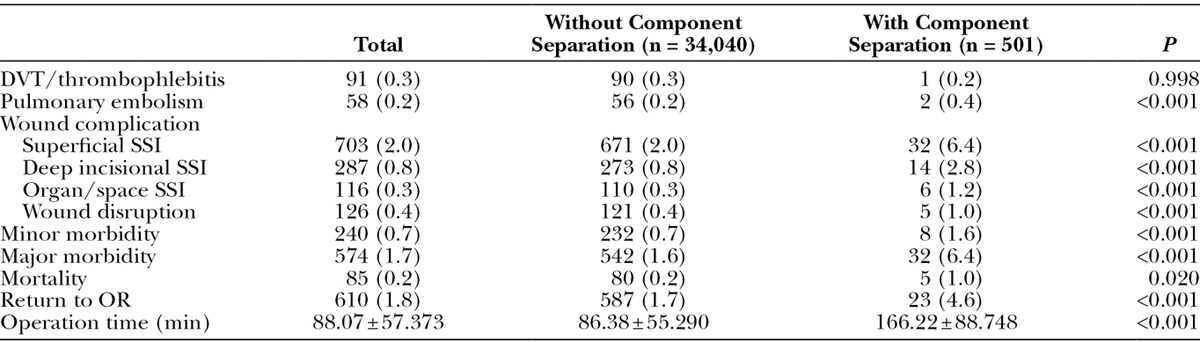
There was no statistical difference in DVT/thrombophlebitis and PE rate between the 2 groups (P = 0.998 and P = 0.591, respectively). With respect to individual wound complications, the rate of superficial SSI (2.0% vs. 6.4%, P < 0.001), deep incisional SSI (0.8% vs. 2.7%, P <0.001), organ/space SSI (0.3% vs. 1.2%, P < 0.001), and wound disruption (0.4% vs. 1.0%, P < 0.001) was significantly higher in the component separation group. The minor/major morbidity, mortality, and return to OR rates were also significantly greater in the component separation group compared with the non-reconstruction group (P < 0.001, P < 0.001, P = 0.020, and P < 0.001, respectively). In addition, operative time was longer in the component separation group (86.38 ± 55.290 vs. 166.22 ± 88.748 minutes, P < 0.001). Complication rates are displayed in Table 2.
Table 2.
Postoperative Complications
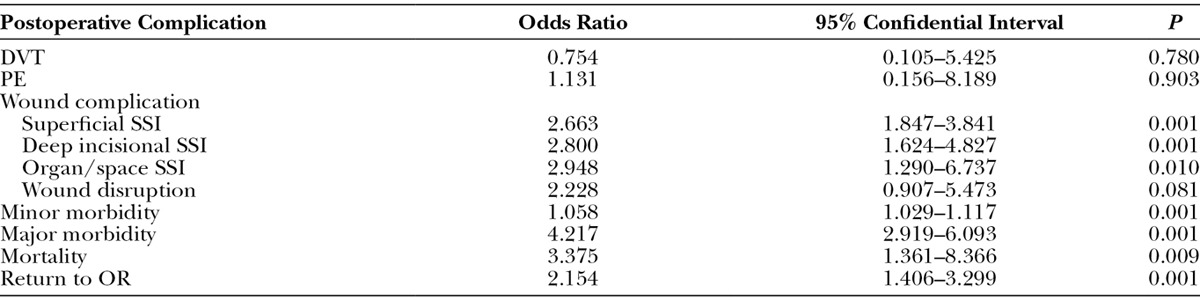
The odds ratios of component separation procedure in terms of specific postoperative complications are shown in Table 3. Superficial SSI, deep incisional SSI, organ/space SSI, wound disruption, minor/major morbidity, mortality, and return to the OR are more likely to occur in component separation group (odds ratio = 2.663, 2.800, 2.948, 2.228, 1.058, 4.217, 3.375, and 2.154, respectively). However, component separation procedure was not associated with an increased probability of DVT/thrombophlebitis and PE.
Table 3.
Odds Ratio of Component Separation Procedure
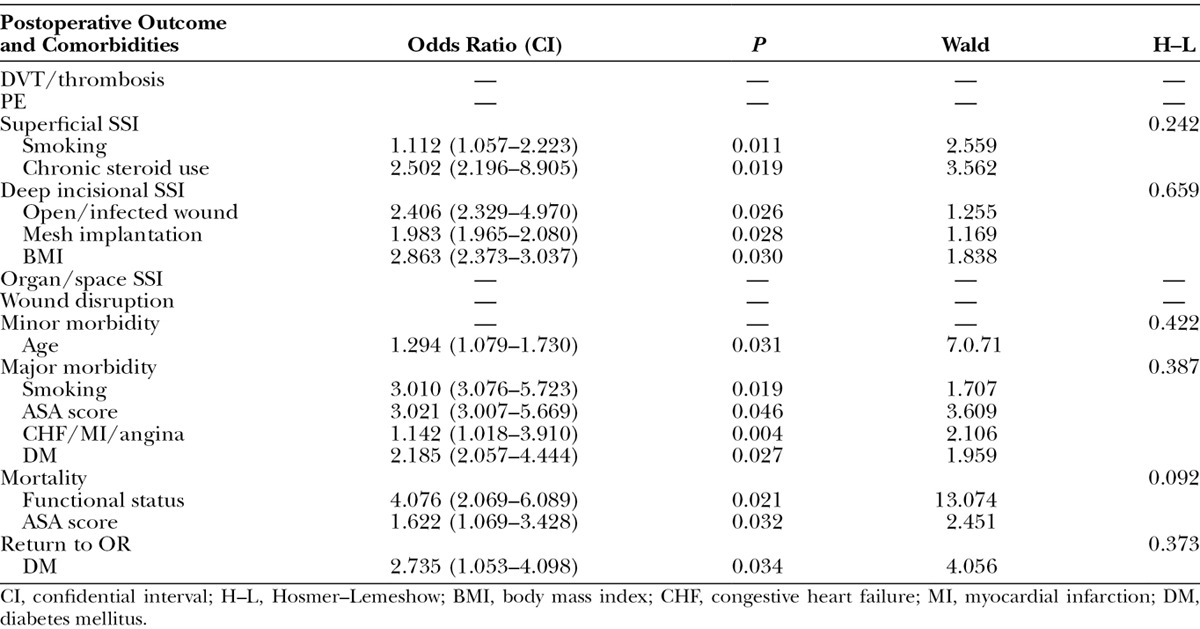
A multivariate logistic regression analysis was used to assess the impact of various patient demographics and comorbidities in the 2 groups (Tables 4 and 5). Several comorbidities served as statistically significant associated factors of complications. In the non–component separation group, functional status and VTE risk score were linked to the occurrence of DVT/thrombosis (odds ratio = 2.447 and 1.451, respectively), and history of prior operation was associated with an increased risk of PE (odds ratio = 1.146). In the component separation group, the number of DVT/thrombosis and PE cases were too small to devise an ideal logistic regression model. Open/infected wound served as a common predictor for superficial SSI, deep incisional SSI, organ/space SSI, and wound disruption in the non–component separation group (odds ratio = 2.598, 4.406, 5.232, and 5.293, respectively) but only for deep incisional SSI in the component separation group (odds ratio = 2.406). In both groups, advanced age was the only predictor for minor morbidity (odds ratio = 1.024 and 1.024, respectively). Smoking, higher ASA score, and history of congestive heart failure/myocardial infarction/angina were common predictors for major morbidity in both groups. In the non–component separation group, functional status, body mass index, ASA score, and VTE score were predictors for mortality (odds ratio = 4.718, 2,729, 1.337, and 2.006, respectively), whereas only functional status and ASA score were predictors in the component separation group (odds ratio = 4.076 and 1.622, respectively). Return to OR was associated with open/infected wound and bleeding disorder in the non–component separation group (odds ratio = 3.441 and 2.091, respectively) and with diabetes mellitus in the component separation group (odds ratio = 2.735).
Table 4.
Statistically Significant Predictors of Postoperative complications without Component Separation
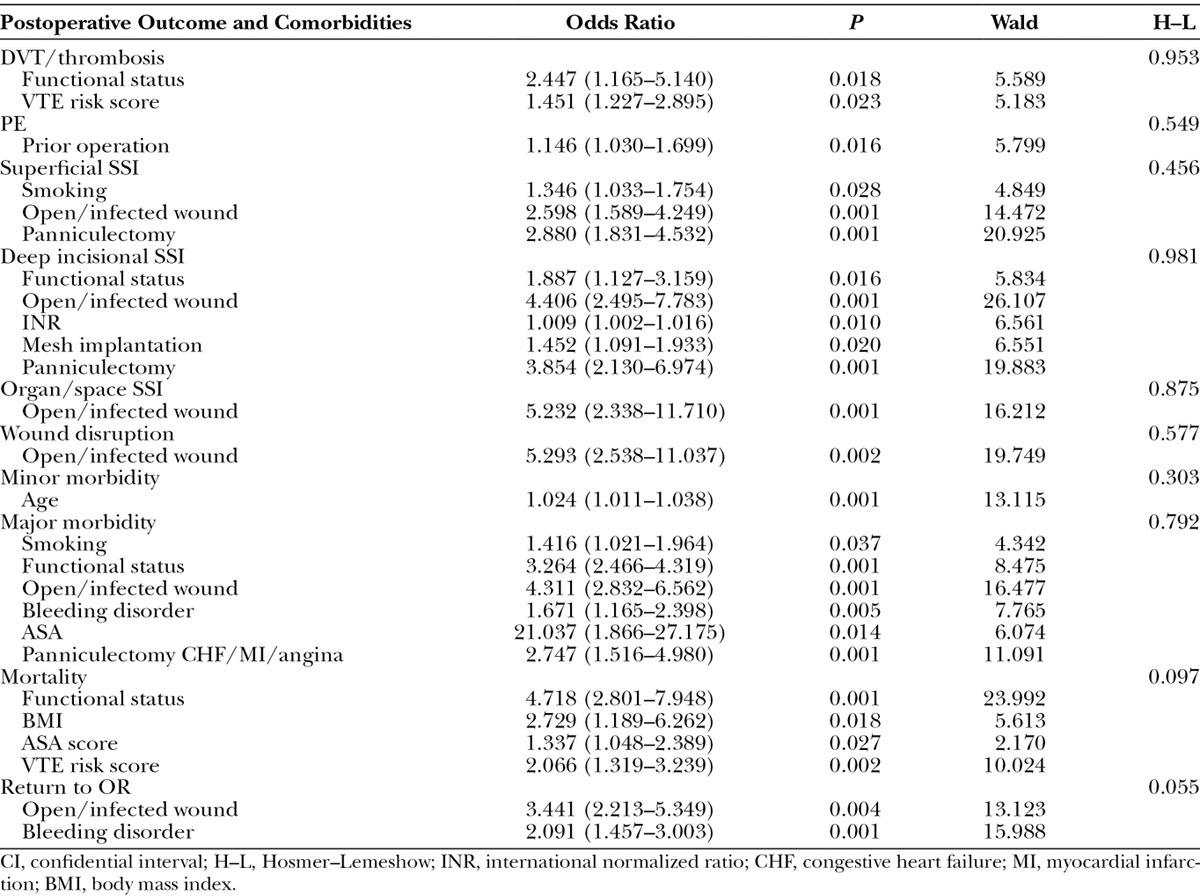
Table 5.
Statistically Significant Predictors of Postoperative Complications with Component Separation
DISCUSSION
Immediate postoperative ACS is a serious complication that can occur following hernia repair. It is defined by an increase of the intra-abdominal pressure over 25 mm Hg (or even over 30 mm Hg), accompanied by impairment of different organs and systemic functions.16, 22-24 The pathophysiology of ACS is considered when intra-abdominal hypertension leads to compression of the inferior vena cava with a subsequent decrease in venous return (preload) and implicitly cardiac output. The ultimate effects of ACS result in diminished systemic perfusion.16 Furthermore, intra-abdominal hypertension is believed to result in venous stasis, a factor in Virchow’s triad and thus serves as a risk factor for VTE development. This is corroborated by several authors who reported femoral venous stasis (and thus VTE) during the study of laparoscopic intra-abdominal surgery.25-27 In addition, a majority of well-known risk factors for VTE can be seen in patients with ventral hernias.15
VTE, which includes DVT and PE, is a major cause of morbidity and mortality among hospitalized patients.28 In this study, the rate of VTE was not significantly different between the non–component separation group and the component separation group suggesting that restoration of abdominal wall anatomy and physiology does not vary in terms of vascular compromise regardless of the type of repair. Thus, component separation can be performed safely despite the challenging preoperative conditions and the stress of long and aggressive procedure.
Abdominal wall compliance together with abdominal content determine intra-abdominal pressure.16 Conventional hernia repair techniques entail reducing the contents of the hernia sac into a tight abdominal space, which can increase abdominal pressure; its etiology seems to be related to the loss of abdominal wall compliance.17 On the other hand, release of the external oblique muscles by component separation allows the intra-abdominal volume to be acutely increased.17 Agnew et al17 assessed the effect of component separation on abdominal volume using an abdominal computed tomography scan and reported a 6% increase in intra-abdominal volume after repair from an average of 7640–8166 mL (P = 0.01) implying that component separation actually helps to prevent the issues associated with increased intra-abdominal pressure.
Our logistic regression analysis emphasized the need for proper control and management of preoperative risk factors before surgical intervention. To reduce morbidity and mortality, it is recommended patients undergo glycemic control of diabetes mellitus, strict regulation of hypertension, smoking cessation, loss of weight, and preoperative wound care. Interestingly, concurrent panniculectomy was a statistically significant risk factor for superficial SSI and deep incisional SSI in the non–component separation group, which was not the case in the component separation group. This finding is consistent with a study by Reid and Dumanian4 who showed that panniculectomy and component separation can be a safe and effective approach to hernia repair in morbidly obese patients. Mesh implantation on the other hand was associated with deep incisional SSI in the component separation group. This finding is not uncommon as previous studies have shown the propensity for synthetic mesh to develop complications in hernia repair patients, particularly in a contaminated field.29-32 It is, therefore, important that the surgeon should be attentive to preoperative infection control when performing component separation with mesh implantation.
There are limitations that stem from the inherent characteristics of the database used in this study. In terms of preoperative conditions, the database does not offer information on the size of the defect nor does it quantify the number of prior hernia repair attempts. In addition, because this database is organized by CPT code, various modifications of the component separation technique could not be assessed. Functional assessment of the abdominal wall and pain also could not be analyzed. Another factor is that the NSQIP data set only reflects outcomes within 30 days after an operation; it is, therefore, difficult to assess long-term results or quality of life. Despite these, previously published studies have been limited by study design and a small patient population; furthermore, they have not specifically investigated the association between component separation and DVT.33-36 The lack of specificity of with regard to certain CPT codes in the ACS-NSQIP database may represent a flaw leading to inaccurate conclusions. However, by implementing rigorous inclusion criteria our final analyses were optimized. The use of a nationwide, well-validated, and large data set may serve as an addition to the current literature.
CONCLUSIONS
The goal of abdominal wall reconstruction is to restore functional integrity and thereby provide support, protect the abdominal viscera, and minimize complications and recurrences. The component separation technique aids in the management of complex hernias. It improves abdominal wall physiology and thus may not contribute to the development of VTE. Given the multitude of factors and complexities inherent in ventral hernia patients, surgeons should make efforts to identify and correct preoperative risk factors that have been shown to predispose to complications.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The ACS-NSQIP databases are the source of information used in this study. Data extrapolated, statistical analysis performed, and conclusions reached have not been verified by the ACS-NSQIP but rather are the result of the work done by authors of this study. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Lomanto D, Iyer SG, Shabbir A, et al. Laparoscopic versus open ventral hernia mesh repair: a prospective study. Surg Endosc. 2006;20:1030–1035. doi: 10.1007/s00464-005-0554-2. [DOI] [PubMed] [Google Scholar]
- 2.DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. National Center for Health Statistics. In: Vital and Health Statistics. 2007;vol. 13:1–209. [PubMed] [Google Scholar]
- 3.Paajanen H, Laine H. Operative treatment of massive ventral hernia using polypropylene mesh: a challenge for surgeon and anesthesiologist. Hernia. 2005;9:62–67. doi: 10.1007/s10029-004-0283-9. [DOI] [PubMed] [Google Scholar]
- 4.Satterwhite TS, Miri S, Chung C, et al. Outcomes of complex abdominal herniorrhaphy: experience with 106 cases. Ann Plast Surg. 2012;68:382–388. doi: 10.1097/SAP.0b013e31823b68b1. [DOI] [PubMed] [Google Scholar]
- 5.Blatnik JA, Krpata DM, Pesa NL, et al. Predicting severe postoperative respiratory complications following abdominal wall reconstruction. Plast Reconstr Surg. 2012;130:836–841. doi: 10.1097/PRS.0b013e318262f160. [DOI] [PubMed] [Google Scholar]
- 6.Cobb WS, Kercher KW, Heniford BT. Laparoscopic repair of incisional hernias. Surg Clin North Am. 2005;85:91–103, ix. doi: 10.1016/j.suc.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Jin J, Rosen MJ. Laparoscopic versus open ventral hernia repair. Surg Clin North Am. 2008;88:1083–1100, viii. doi: 10.1016/j.suc.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Cassar K, Munro A. Surgical treatment of incisional hernia. Br J Surg. 2002;89:534–545. doi: 10.1046/j.1365-2168.2002.02083.x. [DOI] [PubMed] [Google Scholar]
- 9.Paul A, Korenkov M, Peters S, et al. Unacceptable results of the Mayo procedure for repair of abdominal incisional hernias. Eur J Surg. 1998;164:361–367. doi: 10.1080/110241598750004391. [DOI] [PubMed] [Google Scholar]
- 10.Flum DR, Horvath K, Koepsell T. Have outcomes of incisional hernia repair improved with time? A population-based analysis. Ann Surg. 2003;237:129–135. doi: 10.1097/00000658-200301000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vries Reilingh TS, van Goor H, Charbon JA, et al. Repair of giant midline abdominal wall hernias: “components separation technique” versus prosthetic repair: interim analysis of a randomized controlled trial. World J Surg. 2007;31:756–763. doi: 10.1007/s00268-006-0502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86:519–526. doi: 10.1097/00006534-199009000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Lowe JB, Garza JR, Bowman JL, et al. Endoscopically assisted “components separation” for closure of abdominal wall defects. Plast Reconstr Surg. 2000;105:720–729; quiz 730. doi: 10.1097/00006534-200002000-00039. [DOI] [PubMed] [Google Scholar]
- 14.DiBello JN, Jr, Moore JH., Jr Sliding myofascial flap of the rectus abdominus muscles for the closure of recurrent ventral hernias. Plast Reconstr Surg. 1996;98:464–469. doi: 10.1097/00006534-199609000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Thorbjarnarson B, Goulian D. Complications from use of surgical mesh in repair of hernias. N Y State J Med. 1967;67:1189–1192. [PubMed] [Google Scholar]
- 16.Mavrodin CI, Pariza G, Ion D, et al. Abdominal compartment syndrome – a major complication of large incisional hernia surgery. Chirurgia (Bucur) 2013;108:414–417. [PubMed] [Google Scholar]
- 17.Agnew SP, Small W, Jr, Wang E, et al. Prospective measurements of intra-abdominal volume and pulmonary function after repair of massive ventral hernias with the components separation technique. Ann Surg. 2010;251:981–988. doi: 10.1097/SLA.0b013e3181d7707b. [DOI] [PubMed] [Google Scholar]
- 18.Hall BL, Hamilton BH, Richards K, et al. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250:363–376. doi: 10.1097/SLA.0b013e3181b4148f. [DOI] [PubMed] [Google Scholar]
- 19.Senkowski C, Jackson J. Coding hernia and other complex abdominal repairs. Bull Am Coll Surg. 2011;96:42–44. [PubMed] [Google Scholar]
- 20.Pannucci CJ, Bailey SH, Dreszer G, et al. Validation of the Caprini risk assessment model in plastic and reconstructive surgery patients. J Am Coll Surg. 2011;212:105–112. doi: 10.1016/j.jamcollsurg.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606–608. [PubMed] [Google Scholar]
- 22.Papavramidis TS, Marinis AD, Pliakos I, et al. Abdominal compartment syndrome—intra-abdominal hypertension: defining, diagnosing, and managing. J Emerg Trauma Shock. 2011;4:279–291. doi: 10.4103/0974-2700.82224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malbrain ML, Cheatham ML. Definitions and pathophysiological implications of intra-abdominal hypertension and abdominal compartment syndrome. Am Surg. 2011;77(Suppl 1):S6–S11. [PubMed] [Google Scholar]
- 24.Dries DJ. Abdominal compartment syndrome: toward less-invasive management. Chest. 2011;140:1396–1398. doi: 10.1378/chest.11-2350. [DOI] [PubMed] [Google Scholar]
- 25.Beebe DS, McNevin MP, Crain JM, et al. Evidence of venous stasis after abdominal insufflation for laparoscopic cholecystectomy. Surg Gynecol Obstet. 1993;176:443–447. [PubMed] [Google Scholar]
- 26.Sobolewski AP, Deshmukh RM, Brunson BL, et al. Venous hemodynamic changes during laparoscopic cholecystectomy. J Laparoendosc Surg. 1995;5:363–369. doi: 10.1089/lps.1995.5.363. [DOI] [PubMed] [Google Scholar]
- 27.Ido K, Suzuki T, Kimura K, et al. Lower-extremity venous stasis during laparoscopic cholecystectomy as assessed using color Doppler ultrasound. Surg Endosc. 1995;9:310–313. doi: 10.1007/BF00187775. [DOI] [PubMed] [Google Scholar]
- 28.Arnold DM, Kahn SR, Shrier I. Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines. Chest. 2001;120:1964–1971. doi: 10.1378/chest.120.6.1964. [DOI] [PubMed] [Google Scholar]
- 29.Xourafas D, Lipsitz SR, Negro P, et al. Impact of mesh use on morbidity following ventral hernia repair with a simultaneous bowel resection. Arch Surg. 2010;145:739–744. doi: 10.1001/archsurg.2010.144. [DOI] [PubMed] [Google Scholar]
- 30.Chu CC, Welch L. Characterization of morphologic and mechanical properties of surgical mesh fabrics. J Biomed Mater Res. 1985;19:903–916. doi: 10.1002/jbm.820190803. [DOI] [PubMed] [Google Scholar]
- 31.Leber GE, Garb JL, Alexander AI, et al. Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg. 1998;133:378–382. doi: 10.1001/archsurg.133.4.378. [DOI] [PubMed] [Google Scholar]
- 32.Grevious MA, Cohen M, Jean-Pierre F, et al. The use of prosthetics in abdominal wall reconstruction. Clin Plast Surg. 2006;33:181–97, v. doi: 10.1016/j.cps.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Pauli EM, Rosen MJ. Open ventral hernia repair with component separation. Surg Clin North Am. 2013;93:1111–1133. doi: 10.1016/j.suc.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Singhal V, Szeto P, VanderMeer TJ, et al. Ventral hernia repair: outcomes change with long-term follow-up. JSLS. 2012;16:373–379. doi: 10.4293/108680812X13427982377067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hood K, Millikan K, Pittman T, et al. Abdominal wall reconstruction: a case series of ventral hernia repair using the component separation technique with biologic mesh. Am J Surg. 2013;205:322–327; discussion 327. doi: 10.1016/j.amjsurg.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Clarke JM. Incisional hernia repair by fascial component separation: results in 128 cases and evolution of technique. Am J Surg. 2010;200:2–8. doi: 10.1016/j.amjsurg.2009.07.029. [DOI] [PubMed] [Google Scholar]


