Abstract
Background:
Data on premature coronary artery disease (CAD) are scarce. The Tehran Heart Center's Premature Coronary Atherosclerosis Cohort Study (THC-PAC) is the first study of its kind in the Middle East to assess major adverse cardiac events (MACE) in young CAD patients.
Methods:
The cohort consists of CAD patients, males ≤ 45 years old and females ≤ 55 years old. The participants are residents of Tehran or its suburbs and underwent coronary angiography between June 2004 and July 2011. A 10-year follow-up, via either clinical visits or telephone calls at least once a year, was commenced in August 2012. The end point is considered MACE, encompassing death, myocardial infarction, stroke, new coronary involvement, percutaneous coronary intervention, and coronary artery bypass grafting.
Results:
The cohort comprises 1232 eligible patients (613 [49.8%] males) at a mean age of 45.1 years (SD = 5.8). High frequencies of conventional risk factors, including hyperlipidemia (884 [71.8%]), hypertension (575 [46.7%]), positive family history (539 [43.8%]), cigarette smoking (479 [38.8%]), and diabetes mellitus (390 [31.7%]), were seen in the participants. The mean body mass index (BMI) of the enrolled patients was high (29.2 ± 4.8 kg/m2), and 532 (43.3%) and 440 (35.8%) of them were overweight and obese, respectively. The females’ BMI was higher (30.4 ± 5.3 vs. 28.0 ± 3.9 kg/m2; P < 0.001) and they had a greater mean abdominal circumference (99.9 ± 13.5 vs. 98.1 ± 9.3 cm; P = 0.035). Between August 2012 and August 2013, follow-up was successful in 1173 (95.2%) patients (median follow-up duration = 55.3 months, 95%CI: 53.5–57.0 months).
Conclusion:
Our younger patients with CAD had a high frequency of risk factors compared to the same-age general population and all-age CAD patients, which may predispose them to higher incidence of recurrent MACE.
Keywords: Epidemiologic studies, Cohort studies, Coronary artery disease, Young adult
Introduction
Coronary artery disease (CAD) is predominantly seen in the last decades of life. However, it is well known that coronary atherosclerosis begins when individuals are young. Even though it has been estimated that only about 10% of patients with documented CAD are less than 45 years old1 and about 2% to 6% of all infarctions occur in this age group,2 autopsies of young individuals have shown that about 50% of those under 34 years of age have intimal atherosclerosis.3 Therefore, patients with overt premature CAD represent the “tip of the iceberg”. Furthermore, in the coming years, increases in the prevalence of type II diabetes and metabolic syndrome in young adults, related to the increasing frequency of obesity, will result in a rise in the number of premature CAD patients, which will be a critical and challenging medical issue.4
The high prevalence of premature CAD and high mortality rates due to CAD are two important characteristics of this group of diseases in developing countries.5 Unfortunately, while owing to epidemiologic transition, the prevalence of CAD in developed countries is decreasing, this prevalence is notably increasing in low- and mid-income countries.6
This means that developing countries with limited financial resources will face many obstacles in coping with the prevention, screening, and treatment of their CAD patients. This challenge is bound to be even more formidable in the future because the CAD prevalence in a considerable number of developing countries is on the rise in earlier years of life and as such severely impacts workforce productivity.6
Premature CAD can have devastating individual, familial, and social consequences. Despite the alarming rise in premature CAD in developing countries, surprisingly, there is a dearth of relevant high quality data from many of these countries.1, 4 Reducing the prevalence of premature CAD by preventive measures requires knowledge of the relevant risk factors and ways to control them. Unfortunately, most of the knowledge in this regard comes from studies conducted in Western countries. Different societies have diverse lifestyles, nutrition, habits, exposures, socioeconomic status, and cultures which may give rise to dissimilar types of risk factors and differential effects of preventive measures. In other words, the severity and relevance of risk factors may vary from one population to another, and the association between risk factors and disease in different populations needs to be ascertained. Consequently, to have a better understanding of their own risk factors for CAD development and for adverse events thereafter, countries are recommended to conduct their own large-scale cohort studies so as to determine which factors are most important in their own settings and to what extent. Only then will it be possible to make appropriate plans and take suitable preventive measures. Thus, we designed the current cohort study to follow up documented premature CAD patients to assess the relevant risk factors for developing major adverse cardiac events (MACE) after the development of premature atherosclerosis and scrutinize the progress of CAD in young adults.
Methods
Design and Subjects
Tehran Heart Center (THC) is a tertiary heart hospital and one of the largest cardiac centers in the Middle East. This center is affiliated to Tehran University of Medical Sciences and serves people with cardiovascular diseases referred from all around Iran. The participants of the current study (Tehran Heart Center’s Premature Coronary Atherosclerosis Cohort [THC-PAC]) were included from a group of young individuals who had been previously recruited in a genetic study at THC on premature CAD. That group consisted of 3980 individuals (1511 [38.0%] males ≤ 45 years old and 2469 [62%] females ≤ 55 years old) who consecutively underwent coronary angiography between June 2004 and July 2011. Since the aim of the THC-PAC cohort study is to identify factors responsible for developing MACE in premature CAD patients, its participants are premature CAD patients under long-term follow-up. Accordingly, from the above patient population, individuals residing in or near Tehran (the capital city of Iran) with a first abnormal coronary angiography proving CAD were enrolled. CAD was defined as at least ≥ 50% luminal stenosis in individual epicardial vessels. A 10-year follow-up through clinical visits or telephone calls at least once a year of all the participants in the THC-PAC study was commenced in August 2012.
End Points
MACE is the end point of this cohort study and is defined as death (whether cardiac or non-cardiac); documented new myocardial infarction (MI) defined as symptoms of cardiac ischemia associated with either ST elevation on the electrocardiogram (≥ 0.2 mV in leads V1, V2, and V3 and ≥ 0.1 mV in the other leads) or without ST elevation accompanied by a rise in cardiac enzymes (troponin or creatine kinase-MB); new coronary artery involvement defined as at least a new ≥ 50% luminal stenosis in an epicardial vessel besides previously detected lesions confirmed by coronary angiography; stroke (confirmed by a neurologist); candidacy for percutaneous coronary intervention (PCI); or candidacy for coronary artery bypass graft surgery (CABG) during the follow-up period. If a patient suffers more than one event, the one that occurs first is considered the end point. The PCI or CABG procedures performed after the early stages of the first diagnosis of CAD, as the early treatment strategy, are not regarded as MACE.
Follow-Up Methods
To follow up the participants effectively and with the highest response rate, we devised a well-elaborated follow-up protocol. According to the protocol, first, a list of eligible patients was extracted from the Angiography Databank of THC. Then, a trained nurse, who was previously involved in other telephone follow-up studies, was allocated for calling the patients. Based on the experience from several telephone follow-ups having already been done at THC, a team of researchers including a cardiologist, a psychiatrist, and an epidemiologist prepared a protocol for the nurse to teach her how to speak with the patients (or their family members) and how to obtain the most reliable information. We considered various scenarios and prepared a different strategy for every single one of them. The first 20 calls were recorded and were checked by the research team. After analyzing the recorded talks and discussing the results, the researchers and the nurse shared their opinions to improve the telephone call protocol. We called the patients on their home or cell phone lines, collected at the time of recruitment in the initial genetic study, every day between 10.00 and 18.00, excluding weekends. If we failed to reach the patients after three times calling on three different days, we extended the calling time from 8:00 to 22:00 on non-weekend days. We contacted those whom we could not reach by telephone on two different days between 8:00 and 22:00 during the weekends or holidays. We sent an invitation, twice in two successive weeks, in the form of a short text message to the cell phone of those participants who failed to respond to the calls for seven times. If we did not receive any response within a week, we sent a written invitation letter to their home address asking them to call a certain phone number. After a further three weeks, to trace those who did not respond to the telephone calls, cell phone messages, and letters, we checked the THC medical records to find whether they had referred to the outpatient clinics or whether they had been admitted to the hospital. Finally, we referred to the remaining patients’ addresses, registered before they had undergone angiography at THC. For door-to-door follow-up, we thought up different strategies to increase the success rate. Some of the patients missing to follow-up had moved into new residences; to obtain their new addresses, we referred to their neighbors, family members, or adjacent shops. For those who were still living at their previously given addresses but their street names, building names, or building registration numbers had changed, we referred to the municipality or local real estates or inquired their whereabouts from the residents of the same neighborhood. In some instances, we referred to County Offices or Health House Offices to find the patients through their records.
Data Collection
THC enjoys several high-quality databanks, including the Angiography Databank. The patients’ baseline data at the time of the initial diagnosis with premature CAD were retrieved from the databank. At THC, the demographic, clinical, and risk factor data of all the patients who undergo coronary angiography are routinely collected by trained physicians in the outpatient and inpatient settings. The threshold for defining risk factors has already been explained elsewhere.7 The day before catheterization, two expert research nurses check and revise all the collected data via face-to-face interviews with the patients. Then, the collected data are entered by a trained operator via a secure password-protected hospital network. This process is supervised by a databank manager, and at least 5% of the patients’ medical records are randomly selected by the manager to evaluate the accuracy of the data.
Even though efforts are made to obtain all the required data at each follow-up call, all the patients are requested to visit the premature CAD clinic too. According to the protocol of this cohort study, clinical visits are to be done at least once a year as of 2012. At the first visit (2012), informed consent was obtained from all the participants, and a blood sample was drawn for further laboratory studies. At each clinical visit, the patients are to be seen by a cardiologist and a general practitioner. Clinical examinations, medical history takings, and additional measurements of risk factors are conducted and information about how to deal with risk factors is given to the patients. If the individuals need any further diagnostic or therapeutic procedures, proper measures are offered to them or they are referred to relevant specialists (e.g. interventional cardiologists, cardiac surgeons, and psychiatrists) for further management. Useful information on cardiac risk factors, physical activities, different treatment strategies, cardiac procedures, job stress, anxiety, and smoking cessation has been printed in different booklets and are offered to those who visit the clinic. The participants are also asked to fill in different standardized questionnaires for the evaluation of depression, anxiety, job stress, physical activity, and quality of life at baseline and each subsequent clinical visit.
Statistical Analysis
Descriptive methods will be provided for the qualitative and quantitative variables. The continuous data will be reported with mean and standard deviation (SD) or median with 25th and 75th percentiles for the normal and skew-distributed variables. The normality or skewness of the data will be evaluated using the mentioned descriptive statistics as well as applying histograms and box plots. These quantitative variables will be compared between the groups using the Student t or Mann-Whitney U test (for two independent groups) and the one-way analysis of variance (ANOVA) or Kruskal-Wallis test (for more than two independent groups). The qualitative variables will be described with frequency and percentage and will be compared between the groups using the chi-square or Fisher exact test. The incidence rate of MACE will be computed for descriptive purposes. Time to MACE events will be described using survival curves, which will be estimated through the Kaplan-Meier estimator, and 95% confidence intervals (CI) for the survival rates will be computed using the log-transformed method.
The association between the variables and MACE events will be evaluated using the Cox proportional hazards (PH) model and will be reported through the hazards ratio with a 95%CI. A backward stepwise Cox PH model with probabilities 0.05 and 0.1, as entry and removal probabilities, will be used to find the multiple predictors of MACE incidence. All variables with p values less than 0.2 in the univariate analysis will be candidated to enter the prediction model. The PH assumption will be assessed through the chi-square test of correlation coefficient between scaled Schoenfeld residuals and transformed survival time. In case of violation of the PH assumption, parametric models may be drawn upon.
In the presence of time-dependent covariates, an extended Cox PH model will be used. Also, the extended Cox PH model in a multi-state setting will be employed to model the recurrence of some MACE components. The MACE components will be analyzed in a competing risks setting and will be described through cumulative incidence function. A sub-distribution hazard model will be utilized to find multiple predictors of the MACE components in the presence of competing events.
The models stated above will result in valid and unbiased results when the data are “missing completely at random” (MCAR) or “missing at random” (MAR). The existence of missing data for some variables in the analysis, when data are missing not at random, will lead to biased estimates. To evaluate the impact of the missing data, sensitivity analysis will be conducted.
Results
From an initial population source of 3980 individuals, 1946 (48.9%) patients had documented premature CAD. Of these CAD patients, 1447 (74.4%) individuals were residents of Tehran or its suburbs, of whom 215 did not meet the inclusion criteria and were excluded from the study. Therefore, 1232 eligible patients were included in the THC-PAC study for the 10-year follow up according to the study protocol.
At baseline, the mean age of the enrolled patients was 45.1 years (SD = 5.8), and 613 (49.8%) of them were male. The characteristics of the patients based on the baseline visit are depicted in Table 1. Concerning conventional risk factors, hyperlipidemia (884 [71.8%]) was the most frequent risk factor, followed by hypertension (575 [46.7%]), positive family history (539 [43.8%]), cigarette smoking (479 [38.8%]), and diabetes mellitus (390 [31.7%]). Among the patients, 160 (13.0%) individuals reported current opium use. Forty-one (3.3%) patients had no risk factor at all. The initial mean body mass index (BMI) of the enrolled patients was 29.9 kg/m2 (SD = ± 4.8), and 532 (43.3%) and 440 (35.8%) of them were overweight (BMI ≥ 25 and < 30 kg/m2) and obese (BMI ≥ 30 kg/m2), respectively. The female patients had a significantly higher mean BMI than the males (30.4 ± 5.3 vs. 28.0 ± 3.9 kg/m2, mean difference = 2.4, 95%CI: 1.9–2.9; P < 0.001) and their mean abdominal circumference was greater (99.9 ± 13.5 vs. 98.1 ± 9.3 cm, mean difference = 1.8, 95%CI: 0.1–3.4; P = 0.035).
Table 1.
Patients Characteristics based on their baseline visit (angiography time)*
| All Patient (n = 1232) | Medical Treatment (n = 406) | PCI (n = 530) | CABG (n = 296) | P value | |
|---|---|---|---|---|---|
| Age (y) | 45.11±5.76 | 45.34±6.09 | 44.63±5.80 | 45.64±5.16 | 0.034 |
| Sex | 0.152 | ||||
| Male | 613 (49.8) | 187 (46.1) | 278 (52.5) | 148 (50.0) | |
| Female | 619 (50.2) | 219 (53.9) | 252 (47.5) | 148 (50.0) | |
| Risk Factors | |||||
| Positive Family History | 539 (43.8) | 168 (41.4) | 233 (44.0) | 138 (46.6) | 0.381 |
| Hypertension | 575 (46.7) | 214 (52.7) | 225 (42.5) | 136 (45.9) | 0.007 |
| Hyperlipidemia | 884 (71.8) | 279 (68.7) | 390 (73.6) | 215 (72.6) | 0.242 |
| Diabetes Mellitus | 390 (31.7) | 123 (30.3) | 162 (30.6) | 105 (35.5) | 0.268 |
| Cigarette Smoking | 0.457 | ||||
| Never Smoked | 753 (61.1) | 259 (63.8) | 309 (58.3) | 185 (62.5) | |
| Former Smokers | 93 (7.5) | 26 (6.4) | 45 (8.5) | 22 (7.4) | |
| Current Smokers | 386 (31.3) | 121 (29.8) | 176 (33.2) | 89 (30.1) | |
| Opium Usage | 160 (13.0) | 60 (14.8) | 58 (10.9) | 41 (13.9) | 0.175 |
| No Risk Factor | 41 (3.3) | 13 (3.2) | 20 (3.8) | 8 (2.7) | 0.702 |
| BMI (kg/m2) | 29.18±4.79 | 29.51±5.22 | 28.99±4.52 | 29.07±4.63 | 0.241 |
| BMI Categories | 0.185 | ||||
| Underweight | 12 (1.0) | 5 (1.2) | 5 (0.9) | 2 (0.7) | |
| Normal | 244 (19.9) | 85 (21.1) | 97 (18.3) | 62 (20.9) | |
| Overweight | 532 (43.3) | 153 (38.1) | 251 (47.4) | 128 (43.2) | |
| Obese | 440 (35.8) | 159 (39.6) | 177 (33.4) | 104 (35.1) | |
| Metabloic Syndrome | 590 (47.9) | 190 (32.2) | 248 (42.0) | 152 (25.8) | 0.932 |
| Triglyceride (mg/dl) | 203.37±127.17 | 193.16±110.12 | 207.14±138.40 | 210.47±127.45 | 0.309 |
| Cholesterol (mg/dl) | 196.51±51.67 | 195.48±50.33 | 194.17±53.30 | 202.21±50.16 | 0.092 |
| LDL (mg/dl) | 119.95±42.61 | 119.02±41.96 | 118.10±42.84 | 124.21±42.90 | 0.122 |
| HDL (mg/dl) | 41.09±11.01 | 42.25±11.27 | 40.37±10.95 | 40.75±10.67 | 0.036 |
| FBS (mg/dl) | 127.64±61.01 | 127.35±62.75 | 124.92±56.30 | 132.79±66.01 | 0.580 |
| Creatinine (mg/dl) | 0.97±0.44 | 1.00±0.70 | 0,.95±0.23 | 0.96±0.24 | 0.886 |
| EF (%) | 52.55±10.01 | 52.93±9.99 | 52.40±10.16 | 52.26±9.81 | 0.638 |
| Abdominal Circumference (cm) | 0.360 | ||||
| Male | 98.11±9.33 | 99.34±10.89 | 97.72±8.14 | 97.44±9.42 | |
| Female | 99.87±13.49 | 100.24±12.28 | 100.06±14.71 | 99.00±12.6 | |
| Blood Pressure (mmHg) | |||||
| Systolic | 127.16±18.23 | 128.82±17.01 | 125.30±19.11 | 128.59±17.79 | 0.028 |
| Diastolic | 80.88±18.58 | 81.76±11.13 | 81.21±25.02 | 79.09±9.64 | 0.293 |
| Heart Failure | 5 (0.4) | 2 (0.5) | 1 (0.2) | 2 (0.7) | 0.732 |
| Renal Failure | 6 (0.5) | 5 (1.3) | 0 | 1 (0.4) | 0.024 |
| Recent MI | 533 (45.6) | 167 (43.2) | 251 (49.5) | 117 (42.1) | 0.035 |
| No. of Involved Vessels | < 0.001 | ||||
| SVD | 559 (45.4) | 238 (58.6) | 286 (54.0) | 35 (11.8) | |
| 2VD | 342 (27.8) | 102 (25.1) | 166 (31.3) | 74 (25.0) | |
| 3VD | 331 (26.9) | 66 (16.3) | 78 (14.7) | 187 (63.2) | |
| Left Main Coronary Involvement | 22 (1.8) | 0 | 0 | 22 (7.4) | < 0.001 |
Data are presented as mean±SD or n (%)
PCI, Percutaneous coronary intervention; CABG, Coronary artery bypass grafting; BMI, Body mass index; LDL, Low-density lipoprotein; HDL, High-density lipoprotein; FBS, Fasting blood sugar; EF, Ejection fraction; MI, Myocardial infarction; SVD, Single-vessel disease; 2VD, Two-vessel disease; 3VD, Three-vessel disease
Concerning the initial treatment, as is illustrated in Table 1, from the 1232 enrolled patients, 406 (33.5%) were treated medically, 530 (43.7%) underwent PCI, and 296 (24.4%) underwent CABG. Furthermore, the patients with single-or two-vessel coronary involvement were more frequently treated by PCI, while those with three-vessel or left main coronary involvements were more often treated by CABG.
Between August 2012 and August 2013, from the 1232 enrolled patients, 1173 (95.2%) individuals were followed up successfully. A schematic presentation of the follow-up process is given in Figure 1. Telephone follow-up was successful in 1110 (90.1%) individuals. Among the non-responders to telephone calls, 10 (0.1%), 17 (1.4%), 17 (1.4%), and 19 (1.5%) patients were successfully followed up via short messages to cell phones, invitation letters by mail, door-to-door survey, and checking hospital records, respectively. Of 95 patients who were followed up by door-to door survey, 17 (17.9%) individuals were found at their given addresses, 35 (36.9%) patients had moved to other places, and 43 (45.3%) patients could not be found.
Figure 1.
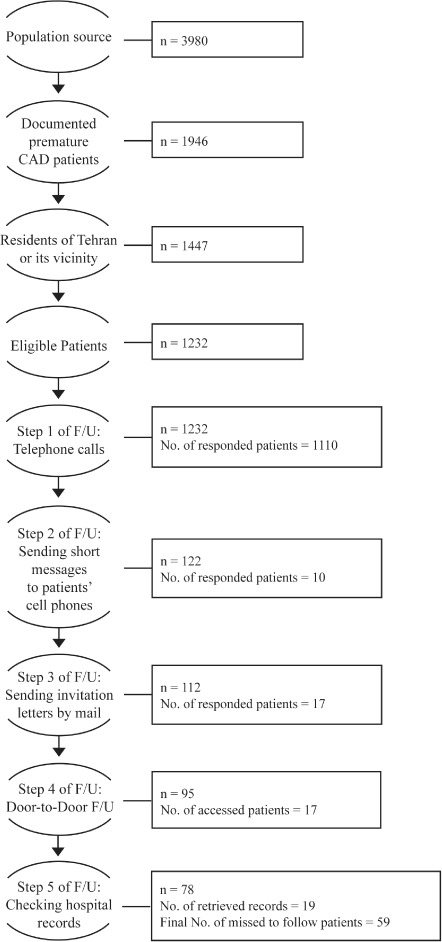
Scheme of the follow-up (F/U) process
A comparison between the initial recommendations by treating physicians following coronary angiography and the treatment which the patients received is presented in Table 2. As is shown in this table, in 79.7% of the patients in the medical treatment, 84.0% of the PCI, and 78.2% of CABG groups, the initial recommendations were the same as the initially given treatments.
Table 2.
Comparison between initial recommendations by treating physicians and initial treatment
| Initial Treatment (n=1232) | TOTAL | P value | |||
|---|---|---|---|---|---|
| Medical Treatment (n=406) | PCI (n=530) | CABG (n=296) | |||
| Recommendations by Treating Physicians | < 0.001 | ||||
| Medical Treatment | 310 (79.9) | 52 (13.4) | 27 (6.9) | 389 | |
| PCI | 63 (12.0) | 442 (84.0) | 21 (4.0) | 526 | |
| CABG | 33 (10.4) | 36 (11.4) | 248 (78.2) | 317 | |
Data are presented as n (%)
PCI, Percutaneous coronary intervention; CABG, Coronary artery bypass grafting
Discussion
In developing countries, higher prevalence of premature CAD and higher mortality due to cardiovascular disorders warrant special consideration.5 In comparison with the Western population, it has been estimated that CAD occurs at least 10 years earlier in South Asians.8 The current literature contains a number of studies on cardiovascular risk factors in young adults, with the bulk of the research being confined to Western countries. Some important studies in this regard are the Bogalusa Heart Study, started in 1972 in Bogalusa, Louisiana, United States, with 3524 children ages 5–14 years;9 Coronary Artery Risk Development in Young Adults (CARDIA), started in 4 field centers in the United States in 1985–86 with 5115 normal participants aged between 18 and 30 years;10 Atherosclerosis Risk in Young Adults (ARYA), comprising two cohorts of young adults in the Netherlands (the Utrecht cohort including 750 young adults born between 1970 and 1973 and the Hague cohort including 262 young adults born between 1963 and 1968);11 Muscatine study, initiated in 1970 in Muscatine, Iowa, United States, participating 11377 students from Muscatine in 6 biennial school survey examinations;12 Atherosclerosis Risk Factors in Male Youngsters (ARMY), commenced in Austria in 2001, in a sample of 141 white males homogenous in age and sex (ages 17–18 years);13 National Health and Nutrition Examination Survey (NHANES), started in early 1960s in the United States, examining a nationally representative sample of about 5000 persons each year;14 Pathological Determinants of Atherosclerosis in Young (PDAY), initiated in 1985 in the United States, investigating the arteries of about 3000 young people who died between 15 and 34 years of age in 15 centers;15 the Northern Ireland Young Heart Project, started in 1989 in Northern Ireland, recruiting 1015 school children ages 12–15 years;16 Cardiovascular Risk in Young Finns, initiated in late 1970s in Finland, including 3596 boys and girls in 6 age cohorts (ages 3, 6, 9, 12, 15, and 18 years);17 and Minneapolis Children’s Blood Pressure Study, launched in 1978 in Minneapolis, United States, surveying 9977 black and white children between 6 and 9 years of age.18 Unfortunately, since most of these studies have been mainly confined to the Western population, their results cannot always be generalized to other populations. Furthermore, the settings of all these cohorts are different from that of the THC-PAC study inasmuch as the age of their participants was different and none of their recruited individuals had angiographically documented CAD.
There is also a study by Cole and colleagues,2 who followed up 843 CAD patients 40 years of age who underwent coronary angiography between 1975 and 1985. Although their study is the closest study to ours and they followed up premature CAD patients too, our settings differ in several aspects. First, the ages of the participants are not the same: whereas we recruited CAD patients consisting of males ≤ 45 years old and females ≤ 55 years old, Cole et al. followed up CAD patients under 40 years of age whether male or female. Second, while we considered MACE as the end point of our study and also assessed some other variables aside from MACE, they considered only death as the end point. Third, the Cole et al. study followed up patients who underwent angiography between 1975 and 1985, since which period the treatment of cardiovascular disorders has advanced a great deal. That our study is more recent means that the findings are closer to present treatment measures. Fourth, they included all premature CAD patients, while we included only those with first-time premature CAD presentation. Therefore, some of their patients may already have had CAD, but since their end point was only death, this would not affect their study. In contrast, what we probed into was first-time MACE after CAD diagnosis and, accordingly, we recruited only first-time CAD patients to ensure that our cohort had not already developed post-CAD MACE. Fifth, that study was a cross-sectional study, while the THC-PAC study has a cohort design and we are to follow up the participants at yearly intervals. Sixth, that study was done in a Western setting and we studied on Eastern individuals. Western and Eastern societies have considerably diverse genetic backgrounds and dissimilar exposures, and these differences may give rise to a noticeable variation in the development and progression of CAD. Hence, studies on the Eastern population are of great importance and are highly recommended. To the best of our knowledge, not only is the THC-PAC study the largest cohort study of its kind in the Middle East, but also it is the only cohort in which the participants are young adults with angiographically documented CAD.
For the THC-PAC study, MACE was considered as the end point. The term “MACE” is almost the most commonly used composite end point in cardiovascular studies. Although the term “MACE” originated in the mid 1990s and it has been strictly used to indicate in-hospital complications due to PCI and then used in other cardiovascular settings, there is no standard definition for it. Even in studies with a very specific question, there is no consensus definition of MACE.19 With the differently defined MACE, it is likely that widely different results and conclusions are obtained even within a single study. This end point is used to reflect both the safety and effectiveness of various treatment approaches. “Safety” signifies security, and “effectiveness” denotes the risk for lesion recurrence. For “safety”, different variables focused on by various studies are comprised of death, MI, acute coronary syndrome, stroke, and heart failure; and for “effectiveness” they comprise new coronary involvement or stent thrombosis in angiographic follow-up evaluation, PCI, and CABG. Depending on the designs of the various studies, researchers have employed different combinations of these variables. In our study, we defined MACE as the most commonly used definition in the literature, which includes death, MI, stroke, new coronary involvement, PCI, or CABG. Concerning death, the majority of the studies on PCI complications have only included mortalities which are obviously due to cardiac problems, while in general cardiovascular settings, they have mainly included all-cause mortality. In our study, we considered all-cause mortality as MACE.
An important feature of our study is its inclusion of a large proportion of women. Much of our current knowledge on preventive measures, diagnostic plans, and treatment strategies is based on studies conducted predominantly in men. A large number of analyses have underscored the underrepresentation of women in cardiovascular studies, and the proportion of women enrolled in many studies fails to reflect their actual representation in the disease populations receiving treatment accurately.20 Even so, these studies have resulted in guidelines which in reality are appropriate for men only and whether this constitutes sufficient support for guidelines in women is open to debate.
Concerning conventional risk factors, the premature CAD patients in this study had a high prevalence rate. When we compared our data to the same-aged Iranian general population data,21 there was a large excess of individuals who were hypertensive (46.7% vs. 13.2%), were smokers (38.8% vs. 9.4%), and had diabetes (31.7% vs. 9.8%). In our study, hyperlipidemia was defined as either hypercholesterolemia or hypertriglyceridemia. Official reports of the risk factor surveillance in Iran21 suggest that 33.1% of the same-aged Iranian population has hypercholesterolemia and 19.3% has hypertriglyceridemia. In our study, we found an even higher prevalence rate compared to the summation of those types of dyslipidemia in the general population (71.8% vs. 52.4%). Furthermore, when we compared the current data with those from another study done on 37358 CAD patients from all-age groups who underwent coronary angiography at THC between 2005 and 2010,22 there were also some differences in the prevalence of risk factors. Premature CAD patients in the THC-PAC study were less hypertensive (46.7% vs. 53.3%) and had higher prevalence of a positive family history of CAD (43.8% vs. 21.7%) but had largely the same frequency of cigarette smoking and diabetes histories (38.8% vs. 39.5%, and 31.7% vs. 31.4%, respectively) and had a lower prevalence of opium usage (13.0% vs. 18.6%). The most noteworthy observation here is that our premature CAD patients had twice more often a positive family history than the CAD patients of all ages combined. It implies the great impact of this risk factor on developing CAD in younger age and the importance of conducting relevant genetic studies. Family history is representative of the interaction between genetic and environmental factors and its relevant studies are the most accessible means to measure the inherited component of CAD. Therefore, a considerable part of the THC-PAC study is bound to be focused on a detailed family history and genetic assessment of the participants.
In another study, by Reibis et al.,23 risk factors between 5725 young (men aged < 55 years and women aged < 65 years) and 9656 older CAD patients were compared. The cut-off point of age for defining young patients in that study was 10 years higher than ours. In their study, cigarette smoking, family history, and hyperlipidemia in the young and hypertension and diabetes in the older CAD patients stood out. Also in that study, 92.7% of the young patients were hyperlipidemic and 71.4%, 43.6%, 31.5%, and 23.5% of them had a history of hypertension, positive family history, cigarette smoking, and diabetes, respectively.
In our study, the mean BMI of our patients was high. While the mean BMI of the same-aged general population in Iran is 24.9 kg/m2, it was 29.2 kg/m2 in the TCH-PAC patients. An official report21 showed that 43.7% of the same-aged Iranians were either overweight or obese, but this figure was substantially higher in our patients (79.1%). Iranian women have a higher BMI than men,21 which was also evident in our patients. However, both men and women in our study had a significantly higher BMI than their counterparts in the general population (males = 28.0 vs. 23.9 kg/m2; females = 30.4 vs. 25.7 kg/m2). Similar to most countries in the Middle East,24 Iranian women are less physically active than men and are more obese, which may predispose them to CAD development.22 The frequency of low physical activity in the Iranian population is 24.3% in men and 46.3% in women. The mean daily physical activity has been estimated to be 189.0 minutes in men and 78.8 minutes in women.21
Another significant finding in the THC-PAC study is the high prevalence of central obesity. In the same-aged Iranian population, the mean abdominal circumference has been estimated to be 83.8 cm in men and 82.8 cm in women,21 while they were 98.1 cm and 99.9 cm in our study, respectively.
Some limitations of the THC-PAC study need due consideration. The participants of this study were recruited from a source already enrolled for a genetic study in young CAD patients, and different age criteria were applied for men and women to define “premature”, based on the known differences in age-related incidence rates between the sexes. This has led to an overrepresentation of women, which, however, can be adjusted for by stratification by sex, and has the advantage of yielding reliable estimates of risk in women. Also, we recruited only patients who were referred to THC and were the residents of Tehran or its vicinity. Iran is a multicultural country with different ethnic groups. It is probable that most of the recruited patients belong to some particular ethnicities, which can affect the results of the study. Tehran is a metropolitan city the residents of which are from different parts of the country with different ethnicity backgrounds. Loss to follow-up is a drawback in cohort studies but was low in our study thanks to our elaborate methods to locate patients.
Conclusion
The prevalence rates of all conventional risk factors for CAD were significantly higher in our sample of young Iranian CAD patients than in the Iranian general population. This included a high prevalence of hyperlipidemia, hypertension, positive family history, cigarette smoking, diabetes mellitus, and obesity. Our finding implies that these young CAD patients are at great risk for developing MACE, which is the subject of subsequent analyses. It also emphasizes the urgent need for dedicated care for these patients, which should particularly focus on weight management and exercise in women.
Acknowledgements
The authors would like to thank all the staff of the THC Research Center and THC Clinics, Mrs. Somayeh Kalaei, and Ms. Fatemeh Esmaeili Darabi for their sincere collaboration. This study was approved and was supported by Tehran Heart Center, Tehran University of Medical Sciences.
References
- 1. Choudhury L, Marsh JD. Myocardial infarction in young patients. Am J Med 1999; 107: 254–261. [DOI] [PubMed] [Google Scholar]
- 2. Cole JH, Miller JI, 3rd, Sperling LS, Weintraub WS. Long-term follow-up of coronary artery disease presenting in young adults. J Am Coll Cardiol 2003; 41: 521–528. [DOI] [PubMed] [Google Scholar]
- 3. Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman WP, 3rd, Herderick EE, Cornhill JF. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA 1999; 281: 727–735. [DOI] [PubMed] [Google Scholar]
- 4. Cole JH, Sperling LS. Premature coronary artery disease: clinical risk factors and prognosis. Curr Atheroscler Rep 2004; 6: 121–125. [DOI] [PubMed] [Google Scholar]
- 5. Gupta R, Misra A, Vikram NK, Kondal D, Gupta SS, Agrawal A, Pandey RM. Younger age of escalation of cardiovascular risk factors in Asian Indian subjects. BMC Cardiovasc Disord 2009; 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma M, Ganguly NK. Premature coronary artery disease in Indians and its associated risk factors. Vasc Health Risk Manag 2005; 1: 217–225. [PMC free article] [PubMed] [Google Scholar]
- 7. Sadeghian S, Karimi AA, Salarifar M, Lotfi Tokaldany M, Hakki Kazzazi E, Sheikh Fathollahi M. Using workload to predict left main coronary artery stenosis in candidates for coronary angiography. J Teh Univ Heart Ctr 2007; 3: 145–150. [Google Scholar]
- 8. Motlagh B, O›Donnell M, Yusuf S. Prevalence of cardiovascular risk factors in the Middle East: a systematic review. Eur J Cardiovasc Prev Rehabil 2009; 16: 268–280. [DOI] [PubMed] [Google Scholar]
- 9. Lai CC, Sun D, Cen R, Wang J, Li S, Fernandez-Alonso C, Chen W, Srinivasan SR, Berenson GS. Impact of long-term burden of excessive adiposity and elevated blood pressure from childhood on adulthood left ventricular remodeling patterns: the Bogalusa heart study. J Am Coll Cardiol 2014; 64: 1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spring B, Moller AC, Colangelo LA, Siddique J, Roehrig M, Daviglus ML, Polak JF, Reis JP, Sidney S, Liu K. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study Circulation 2014; 130: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vos LE, Oren A, Bots ML, Gorissen WH, Grobbee DE, Uiterwaal CS. Birth size and coronary heart disease risk score in young adulthood. The Atherosclerosis Risk in Young Adults (ARYA) study. Eur J Epidemiol 2006; 21: 33–38. [DOI] [PubMed] [Google Scholar]
- 12. Burns TL, Letuchy EM, Paulos R, Witt J. Childhood predictors of the metabolic syndrome in middle-aged adults: the Muscatine study. J Pediatr 2009; 155: S5.e17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knoflach M, Kiechl S, Kind M, Said M, Sief R, Gisinger M, van der Zee R, Gaston H, Jarosch E, Willeit J, Wick G. Cardiovascular risk factors and atherosclerosis in young males: ARMY study (Atherosclerosis Risk-Factors in Male Youngsters). Circulation 2003; 108: 1064–1069. [DOI] [PubMed] [Google Scholar]
- 14. Murphy RS, Michael GA. Methodologic considerations of the National Health and Nutrition Examination Survey. Am J Clin Nutr 1982; 35(5 Suppl): 1255–1258. [DOI] [PubMed] [Google Scholar]
- 15. Bressler J, Shimmin LC, Boerwinkle E, Hixson JE. Global DNA methylation and risk of subclinical atherosclerosis in young adults: the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Atherosclerosis 2011; 219: 958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Lenthe FJ, Boreham CA, Twisk JW, Strain JJ, Savage JM, Smith GD. Socio-economic position and coronary heart disease risk factors in youth. Findings from the Young Hearts Project in Northern Ireland. Eur J Public Health 2001; 11: 43–50. [DOI] [PubMed] [Google Scholar]
- 17. Nuotio J, Oikonen M, Magnussen CG, Jokinen E, Laitinen T, Hutri-Kähönen N, Kähönen M, Lehtimäki T, Taittonen L, Tossavainen P, Jula A, Loo BM, Viikari JS, Raitakari OT, Juonala M. Cardiovascular risk factors in 2011 and secular trends since 2007: The Cardiovascular Risk in Young Finns Study. Scand J Public Health 2014; 42: 563–671. [DOI] [PubMed] [Google Scholar]
- 18. Munger RG, Prineas RJ, Gomez-Marin O. Persistent elevation of blood pressure among children with a family history of hypertension: the Minneapolis Children›s Blood Pressure Study. J Hypertens 1988; 6: 647–653. [DOI] [PubMed] [Google Scholar]
- 19. Kip KE, Hollabaugh K, Marroquin OC, Williams DO. The problem with composite end points in cardiovascular studies: the story of major adverse cardiac events and percutaneous coronary intervention. J Am Coll Cardiol 2008; 51: 701–707. [DOI] [PubMed] [Google Scholar]
- 20. Abbasi SH, Kassaian SE. Women and coronary artery disease. Part I: basic considerations. J Teh Univ Heart Ctr 2011; 6: 109–116. [PMC free article] [PubMed] [Google Scholar]
- 21. STEP wise approach to chronic disease risk factor surveillance. http://www.who.int/chp/steps/iran/en/ (20 June 2014).
- 22. Abbasi SH, De Leon AP, Kassaian S, Karimi A, Sundin O, Soares J, Macassa G. Gender differences in the risk of coronary artery disease in Iran. Iran J Public Health 2012; 41: 36–47. [PMC free article] [PubMed] [Google Scholar]
- 23. Reibis R, Treszl A, Wegscheider K, Bestehorn K, Karmann B, Völler H. Disparity in risk factor pattern in premature versus late-onset coronary artery disease: a survey of 15,381 patients. Vasc Health Risk Manag 2012; 8: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shara NM. Cardiovascular disease in Middle East Women. Nutr Metab Cardiovasc Dis 2010; 20: 412–418. [DOI] [PubMed] [Google Scholar]


