Abstract
Chordomas are rare malignant bone tumours with a predilection for the axial skeleton, especially the sacrum and skull base. Median survival in patients with metastatic disease is usually dismal. Treatment is challenging due to the propensity for local recurrence, metastatic disease as well as lack of clear consensus regarding the optimal management. Our case report highlights two cases of sacral chordoma with locally recurrent and widespread metastatic disease, stable on molecular targeted therapy.
Keywords: Chordoma, metastasis, imatinib, positron emission tomography
Keywords: Kordoma, metastaz, imatinib, pozitron emisyon tomografi
Özet
Kordomalar özellikle sakrum ve kafa tabanı ile eksenel iskelet yerleşme eğiliminde olan, nadir görülen malign kemik tümörleridir. Metastatik hastalığı olan hastalarda sağkalım ortanca değeri genellikle umutsuzdur. Tedavi, lokal nüks, metastatik hastalık yanı sıra optimum tedaviye ilişkin net konsensus eksikliği nedeniyle zordur. Bizim olgu sunumumuzda moleküler hedefli tedavinin istikrarlı yerel tekrarlayan ve yaygın metastatik hastalığı olan sakral kordomayla ilgili iki olgu bulunmaktadır.
Introduction
Chordomas are slow growing, locally aggressive malignant neoplasms with a predilection for the sacrococcygeal region (50%), clivus (35%) and vertebral bodies (15%) [1]. Treatment is challenging because of their unique location, close vicinity to key structures, contiguous spread and relapse. The reported incidence of metastases in the literature vary widely from 3 to 48% with the lungs, liver, bone and lymph nodes being the commonly involved sites [1–4]. We describe two cases of metastatic sacral chordoma, stable on molecular targeted therapy.
Case Reports
Case 1
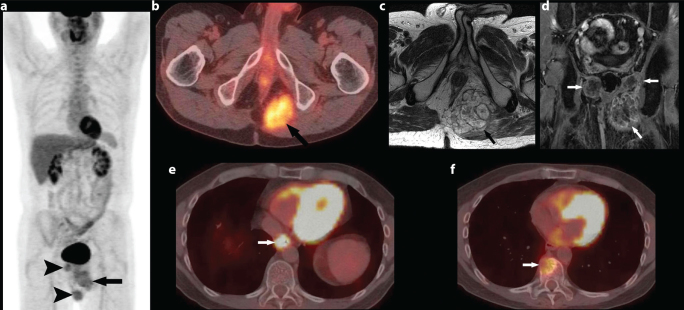
A 47 year old man presented with low back pain. He was diagnosed with a midline pericoccygeal mass for which he underwent en bloc excision. A diagnosis of chordoma was made on histopathology. He remained asymptomatic for 3 years, when he presented with left sciatic pain. Recurrent disease was diagnosed on MRI at an outside institution. F-18 FDG PET/CT study here, demonstrated mildly FDG avid bilateral ischiorectal fossa masses with extension into the left gluteus maximus muscle (SUV (Standardized uptake value) max 4.8) (Figure 1a, b). He received palliative radiotherapy for pain relief, with little response. MRI showed septated bilateral ischiorectal fossa masses that were iso- to hypointense to the muscle on T1 weighted images, hyperintense to muscle on T2 weighted images, with heterogeneous enhancement extending to the left gluteal musculature (Figure 1c, d). He received neoadjuvant chemotherapy with weekly low dose cisplatin along with 800 mg of imatinib mesylate (gleevec; Novartis) per day with partial pain relief and favourable response on imaging in the form of decrease in the size of the recurrent disease. He subsequently underwent a successful resection of the locally recurrent disease. He was started on adjuvant imatinib mesylate (gleevec; Novartis) 800 mg per day and remained disease free for 5 years. Routine restaging CT done earlier this year, revealed new lytic lesion of the right anterior aspect of T10 vertebral body, concerning the metastatic chordoma. In addition, there was focal wall thickening of the lower oesophagus and an enlarged paraoesophageal lymph node. F-18 FDG PET/CT showed intense uptake in the distal oesophagus (SUV max 25) (Figure 1e), intensely FDG avid paraoesophageal lymph node (SUV max 17.8) (not shown). The T10 lytic lesion was only mildly FDG- avid (SUV max 5.6) (Figure 1f).
Figure 1. a–f.
47 year-old male presented with low abdominal pain: Coronal image from F-18 FDG PET/CT shows three FDG avid masses, the largest one in the left ischiorectal fossa (arrow). Two smaller masses (arrowheads) are also seen adjacent to it and on the right suggesting recurrent disease (a). Axial image from F-18 FDG PET/CT shows FDG avid mass in the left ischiorectal fossa (arrow) (SUV (Standardized uptake value) Max 4.8) (b). Axial FSE T2 sequence shows large septated ill-defined T2 hyperintense mass in the ischiorectal fossa (arrow) (c). Coronal post contrast sequence shows heterogeneous enhancement in bilateral ischiorectal fossa (arrows) (d). Axial image from F-18 FDG PET/CT shows intense FDG uptake associated with a mass in the distal esophagus (SUV max 25) (arrow), consistent with biopsy proven oesophageal primary malignancy (e). Axial image from F-18 FDG PET/CT shows FDG-avid (SUV 5.6) lytic lesion involving T10 vertebral body (arrow), highly suspicious for metastatic disease (f).
The differential uptake in the T10 vertebral body lesion and the oesophageal lesion raised suspicion for dual pathology and prompted biopsy of both. A CT- guided biopsy of the T10 vertebral lesion was positive for metastatic chordoma. Endoscopy revealed fungating mass in the lower third of the esophagus, biopsy of which was consistent with primary oesophageal adenocarcinoma. He underwent oesophagogastrectomy with a complicated postoperative course and multiple organ failure, from which he eventually succumbed.
Case 2
A 58 year old man presented with a well circumscribed, large sacral mass, for which he underwent coccygectomy. The tumour was pathologically proven to be a malignant chordoma, chondroid subtype. Postoperatively, the patient received a course of radiation therapy to the pelvis and tumour bed to a total of 6,400 cGy. Follow up CT five years after the initial treatment showed multiple enlarged mediastinal and hilar lymph nodes as well as pleural and lung parenchymal metastases. He subsequently, underwent left thoracotomy but the tumour was not fully resectable. Later that year, he was found to have new pulmonary metastases as well as local recurrence in the pelvis. Patient’s metastatic disease slowly progressed despite conventional chemotherapy for four years. F-18 FDG PET/CT done showed a large FDG-avid mass (SUV max 12.9) in the left obturator foramen, invading the pelvis with the destruction of the left inferior pubic ramus (Figure 2a). In addition, there were multiple FDG-avid pulmonary nodules/masses (SUV max 8.3) and FDG-avid nodule in the right lateral abdominal wall (SUV max 3.4) (Figure 2b, c). He was started on a combination of carboplatin and imatinib mesylate, and his disease has been stable for 5 years.
Figure 2. a–c.
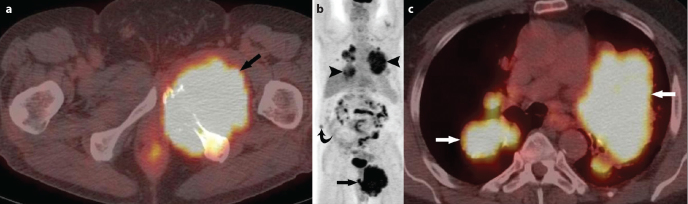
58 year-old man presented with pelvic pain: Axial image from F-18 FDG PET/CT shows a large FDG-avid mass in the left hemipelvis (SUV Standardized uptake value) 12.9) (arrow) with the destruction of the inferior pubic rami (a). Coronal image from F-18 FDG PET/CT shows large FDG-avid mass in the left hemipelvis with a SUV max of 12.9 (arrow), multiple FDG-avid pulmonary nodules/masses with a SUV max of 8.3 (arrowheads), mildly FDG avid soft tissue nodule in the right abdominal wall (SUV max 3.4) suspicious for metastatic implant (curved arrow) (b). Axial image from F-18 FDG PET/CT. Multiple FDG-avid pulmonary nodules/masses (arrows) are seen with a SUV max of 8.3 (c).
Discussion
Lushka first described chordomas in 1856 [5]. Chordomas have a predilection for the craniospinal axis and are thought to arise from notochordal rests which evolve into nucleus pulposus, explaining the predominant midline and paramedian locations [6]. Newer literature challenges this traditional concept suggesting that chordomas arise from pre-existing benign notochordal cell tumours [7–10]. Chordomas present between the fourth and seventh decade with a male to female ratio of 2:1 [6, 11]. On gross examination, chordoma has a lobular shape and internal fibrous septae. The lobule consists of tumour cells in a myxoid stroma. Classically, these tumours contain physaliferous cells which have small nuclei and large amounts of cytoplasm. Two variants of chordomas have been reported: aggressive sarcomatous or dedifferentiated high grade variety and chondroid variety, the latter having a better prognosis [12, 13].
Both CT and MR play an important role in delineating the primary tumour for surgical planning and in the assessment of treatment response. Contrast-enhanced studies are especially useful in differentiating the recurrent tumour versus postoperative seroma, as seromas do not enhance or only show rim enhancement [6, 14]. Whole body FDG PET/CT is useful at the time of diagnosis and when recurrent or metastatic disease is suspected. Since it provides both anatomical and metabolic detail, it can aid in biopsy planning where the most FDG-avid or metabolically active part of the tumour can be targeted. Its role in monitoring the response to the treatment is also well described [15–17].
The reported incidence of metastases in chordoma is between 3–48% [3]. Metastases are known to occur more commonly when the primary is located in the sacrococcygeal region, compared to the clivus [1]. Metastatic disease has been reported in almost every organ: lung, liver, bone, lymph node, brain, solid abdominal organs, peritoneum and even in the breast [1–4, 18]. Occurrence range of the metastases has been reported to be as early as 2–3 months [19, 20] to as late as 9–10 years after initial treatment [19].
Local recurrence is a significant predictor in the development of metastatic disease [2]. Prognosis of chordomas is variable. Tumour size, inadequate surgical margins, local aggressiveness, local recurrence, presence of tumour necrosis, Ki67 labelling index and invasive diagnostic procedure outside a tumour centre, are associated with a poorer prognosis [1, 21].
McMaster et al. [22] reported a survival rate of 68% at 5 years, falling to 40% at 10 years for all chordomas. A study by Bergh et al. [21] reported 10 year survival of 64%. After the development of metastatic disease, median survival has been less than 12 months [20]. In a more recent study 22% of chordoma patients developed metastatic disease, with a slightly improved median survival of roughly 3 years after the development of metastatic disease [23].
Complete surgical excision is the best treatment option. En bloc excision provides a longer disease-free interval than incomplete resection, though quality of life and co-morbidity play an important role in decision making [2, 19, 24]. Radiation therapy may be used as an adjuvant or as definitive treatment when surgery is not possible [17]. Recent advances in radiation therapy such as intensity modulated radiation therapy offer better outcomes in unrespectable cases [25].
Chordomas are not particularly responsive to conventional chemotherapy. In the last decade, molecular targeted therapy has been encouraging in patients with metastatic disease or failure of surgery/radiotherapy. Imatinib is an inhibitor of multiple tyrosine kinases, mainly BCR-ABL, KIT, platelet-derived growth factor receptor-α (PDGFRA) and platelet-derived growth factor receptor-β (PDGFRB) [17]. Chordomas overexpress PDGFRB and its phosphorylated form, whereas PDGFRA and KIT are less expressed but phorsphorylated and thus activated. Treatment response observed in chordoma patients treated with imatinib is likely due to switching off of all three receptors [26], seen on imaging studies as marked decrease in the attenuation on CT, decreased contrast enhancement on CT and MRI and decrease in glucose uptake on PET.
Our two cases highlight stable disease in patients treated with imatinib (gleevec; Novartis) both in adjuvant and neoadjuvant settings, despite large tumour burden, local tumour recurrence and extensive metastatic disease as seen in our second patient. In both cases, PET/CT was helpful in evaluating the metastatic disease, biopsy planning and in differentiating metastatic disease due to chordoma from a second primary as in our first case based on SUV values.
In conclusion, no standard treatment regimen is available for widespread metastatic chordoma. Treatment varies from institution to institution. PET/CT in combination with other imaging modalities increases the sensitivity for the detection of recurrent as well as metastatic disease. Multimodality treatment including newer surgical and radiation techniques, in combination with molecular targeted therapy appears to benefit by improving the outcome in metastatic chordoma.
Footnotes
Informed Consent: Written informed consent was obtained from the patient/patients.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.R., N.H.R.; Design - J.P.J., S.A.H., A.B.S.; Supervision - N.H.R., K.M.K.; Materials - S.R., N.H.R.; Data Collection and/or Processing - S.R., N.H.R.; Analysis and/or Interpretation - S.R., N.H.R.; Literature Review - S.R., N.H.R.; Writing -S.R., N.H.R., J.P.J., S.A.H., A.B.S., K.M.K.; Critical Review - N.H.R., K.M.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Vergara G, Belinchón B, Valcárcel F, et al. Metastatic disease from chordoma. Clin Transl Oncol. 2008;10:517–21. doi: 10.1007/s12094-008-0243-4. [DOI] [PubMed] [Google Scholar]
- 2.McPherson CM, Suki D, McCutcheon IE, et al. Metastatic disease from spinal chordoma: a 10-year experience. J Neurosurg Spine. 2006;5:277–80. doi: 10.3171/spi.2006.5.4.277. [DOI] [PubMed] [Google Scholar]
- 3.Delank KS, Kriegsmann J, Drees P, Eckardt A, Eysel P. Metastasizing chordoma of the lumbar spine. Eur Spine J. 2002;11:167–71. doi: 10.1007/s00586-001-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sopta J, Tulic G, Mijucic V, et al. Solitary lymph node metastasis without local recurrence of primary chordoma. Eur Spine J. 2009;18(Supplement 2):191–5. doi: 10.1007/s00586-008-0800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez Carballal C, Gonzales Rodrigalvarez R, Lopez De La Riva M, et al. Dumbbell-shaped thoracic chondroid chordoma mimicking a neurinoma. Acta Neurochir (Wien) 2010;152:325–6. doi: 10.1007/s00701-009-0321-6. [DOI] [PubMed] [Google Scholar]
- 6.Farsad K, Kattapuram SV, Sacknoff R, Ono J, Nielsen GP. Sacral chordoma. Radiographics. 2009;29:1525–30. doi: 10.1148/rg.295085215. [DOI] [PubMed] [Google Scholar]
- 7.McCormick M, Schroeder T, Benham S. Sacral chordoma: a case report with radiographic and histologic correlation and a review of the literature. WMJ. 2006;105:53–6. [PubMed] [Google Scholar]
- 8.Yamaguchi T, Suzuki S, Ishiiwa H, Ueda Y. Intraosseous benign notochordal cell tumours: overlooked precursors of classic chordomas? Histopathology. 2004;44:597–602. doi: 10.1111/j.1365-2559.2004.01877.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi T, Iwata J, Sugihara S, et al. Distinguishing benign notochordal cell tumours from vertebral chordoma. Skeletal Radiol. 2008;37:291–9. doi: 10.1007/s00256-007-0435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi T, Yamato M, Saotome K. First histologically confirmed case of a classic chordoma arising in a precursor benign notochordal lesion: differential diagnosis of benign and malignant notochordal lesions. Skeletal Radiol. 2002;31:413–8. doi: 10.1007/s00256-002-0514-z. [DOI] [PubMed] [Google Scholar]
- 11.Smith J, Ludwig RL, Marcove RC. Sacrococcygeal chordoma: a clinicoradiological study of 60 patients. Skeletal Radiol. 1987;16:37–44. doi: 10.1007/BF00349926. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher CDM. Diagnostic histopathology of tumours. 2nd edition. Churchill Livingstone; 2000. pp. 1584–5. [Google Scholar]
- 13.Casali PG, Stacchiotti S, Sangalli C, Olmi P, Gronchi A. Chordoma. Curr Opin Oncol. 2007;19:367–70. doi: 10.1097/CCO.0b013e3281214448. [DOI] [PubMed] [Google Scholar]
- 14.Sung MS, Lee GK, Kang HS, et al. Sacrococcygeal chordoma: MR imaging in 30 patients. Skeletal Radiol. 2005;34:87–94. doi: 10.1007/s00256-004-0840-4. [DOI] [PubMed] [Google Scholar]
- 15.Park SA, Kim HS. F-18 FDG PET/CT evaluation of sacrococcygeal chordoma. Clin Nucl Med. 2008;33:906–8. doi: 10.1097/RLU.0b013e31818c4e88. [DOI] [PubMed] [Google Scholar]
- 16.Miyazawa N, Ishigame K, Kato S, Satoh Y, Shinohara T. Thoracic chordoma: review and role of FDG-PET. J Neurosurg Sci. 2008;52:117–21. [PubMed] [Google Scholar]
- 17.Casali PG, Messina A, Stacchiotti S, et al. Imatinib mesylate in chordoma. Cancer. 2004;101:2086–97. doi: 10.1002/cncr.20618. [DOI] [PubMed] [Google Scholar]
- 18.Tot T. Metastatic chordoma of the breast: an extremely rare lesion mimicking mucinous cancer. APMIS. 2006;114:726–9. doi: 10.1111/j.1600-0463.2006.apm_437.x. [DOI] [PubMed] [Google Scholar]
- 19.Samson IR, Springfield DS, Suit HD, Mankin HJ. Operative treatment of sacrococcygeal chordoma. A review of twenty-one cases. J Bone Joint Surg Am. 1993;75:1476–84. doi: 10.2106/00004623-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Baratti D, Gronchi A, Pennacchioli E, et al. Chordoma: natural history and results in 28 patients treated at a single institution. Ann Surg Oncol. 2003;10:291–6. doi: 10.1245/aso.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Bergh P, Kindblom LG, Gunterberg B, et al. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88:2122–34. doi: 10.1002/(sici)1097-0142(20000501)88:9<2122::aid-cncr19>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 22.McMaster ML, Goldstein AM, Bromley CM, et al. Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control. 2001;12:1–11. doi: 10.1023/a:1008947301735. [DOI] [PubMed] [Google Scholar]
- 23.Stacchiotti S, Casali PG, Salvatore LV, et al. Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Ann Surg Oncol. 2010;17:211–9. doi: 10.1245/s10434-009-0740-x. [DOI] [PubMed] [Google Scholar]
- 24.Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine (Phila Pa 1976) 2006;31:493–503. doi: 10.1097/01.brs.0000200038.30869.27. [DOI] [PubMed] [Google Scholar]
- 25.Stacchiotti S, Casali PG. Systemic therapy options for unresectable and metastatic chordomas. Curr Oncol Rep. 2011;13:323–30. doi: 10.1007/s11912-011-0176-x. [DOI] [PubMed] [Google Scholar]
- 26.Tamborini E, Miselli F, Negri T, et al. Molecular and biochemical analyses of platelet-derived growth factor receptor (PDGFR) B, PDGFRA, and KIT receptors in chordomas. Clin Cancer Res. 2006;12:6920–8. doi: 10.1158/1078-0432.CCR-06-1584. [DOI] [PubMed] [Google Scholar]