Abstract
Brain metastases are the most common intracranial malignancy, and breast cancer is the second most common cancer to metastasize to the brain. Intracranial disease is a late manifestation of breast cancer with few effective treatment options, affecting 15-50% of breast cancer patients, depending upon molecular subtype. In this review article, we describe the genetic, molecular, and metabolic changes in breast cancer cells that facilitate breast to brain metastasis. We believe that advances in the understanding of breast to brain metastasis pathogenesis will lead to targeted molecular therapies and to improvements in the ability to prospectively identify patients at increased risk for developing intracranial disease.
Keywords: breast cancer, brain metastasis, neurosurgery, oncology, her2/neu
Introduction and background
A significant percentage of patients with breast cancer will acquire brain metastases at some point in their disease, with a significant impact on quality of life and life expectancy. The incidence of brain metastases is between 140,000 and 170,000 cases per year [1]. Breast carcinoma accounts for 12-20% of brain metastases, second only to lung cancer [2]. Autopsy studies have shown brain metastasis in up to 36% of breast cancer patients [3-5] and can involve up to half of patients with certain genetic markers. Breast cancer subtypes include luminal A, luminal B, HER2 positive/non-luminal, and triple negative [6-7]. In patients with breast-to-brain metastasis, HER2 positivity, and luminal-HER2 subtype were significant positive prognostic factors while cerebral progression was the most frequent cause of death [8-9]. Breast cancer brain metastasis is associated with young age, ER negativity [10], and HER-2 overexpression [11-14]. Brain metastasis is a significant cause of morbidity in breast cancer patients, with cognitive impairment detected on neuropsychological testing in up to 67% of patients [15-16]. Current treatment options, frequently used in combination, include surgery, whole-brain radiation therapy, chemotherapy, and stereotactic radiosurgery [17-18]. Without treatment or with corticosteroids alone, median survival of patients with brain metastasis is one and two months, respectively [19-20]. The one-year median survival of patients with brain metastases treated with surgical resection and adjuvant radiosurgery is approximately 50% [21]. As the treatment for systemic breast cancer improves, patients survive longer and the incidence of brain metastases increases.
The development of brain metastases is not random, but rather a coordinated accumulation of opportunistic mutations which enable the breast cancer cells to seed and flourish within the central nervous system (CNS). Successful colonization of distant tissue by tumor cells requires the establishment of a microenvironment in the host tissue that permits cell survival, growth, and invasion. Generally there is a latency of two to three years between surgical removal of primary breast cancer and the appearance of brain metastasis [4], suggesting that tumor cells undergo changes over time that bestow brain tropism. Like other carcinomas that metastasize to the brain, breast cancer has a predilection for brain regions with the highest perfusion, as 80% of metastases occur in the cerebral hemispheres, 15% are located in the cerebellum, and 5% occur in the brainstem [22]. We know breast cancer within the brain is distinct from the primary site: increased Ki67 indices, increased microvascular density, expression of a known pro-metastatic micro-RNAs and gene up-regulation [23-24]. Recently, efforts have been made to understand the genetic and molecular events that predispose cancer to metastasize [25-30], with the goal of prospectively identifying patients at highest risk of developing brain metastasis.
Consent was formally obtained or waived for all subjects present within this study.
Review
HER2-positive breast cancer predisposes to brain metastasis
HER2-positive tumors increase the likelihood of breast-to-brain metastasis or confer enhanced affinity for neural tissue. HER2 overexpression is found in approximately 20% of breast cancers [31-32] and is associated with breast-to-brain metastasis in nearly half of patients with this tumor subtype [5, 33]. Discordance in HER2 status, in which the primary tumor is negative for HER2 while the brain metastasis is HER2-positive, has been found in up to 24% of cases, and this is associated with decreased survival [34-35]. In addition, HER2-positive tumors that are also hormone-receptor-negative have increased risk of relapsing within the CNS [36]. Theories addressing the increased the rate of brain metastasis in HER2-positive breast cancers include homing and tropism of HER2-positive cells in brain parenchyma [37], general aggressiveness of HER2-positive breast cancer and tendency to metastasize to other tissues [38], and increased survival due to improvement in treatment options [39-41]. Molecular therapies that target HER2 include the monoclonal anti-HER2 antibody trastuzumab (Herceptin) and pertuzumab (Perjeta) (Genentech, South San Francisco, CA), and tyrosine kinase inhibitors, such as lapatinib (Tykerb) (GlaxoSmithKline, Middlesex, UK) [42]. As in primary breast cancer, it is hypothesized that trastuzumab functions by triggering the internalization and degradation of HER2 through the action of c-Cbl, a tyrosine kinase-ubiquitin ligase [43-44]. Pertuzumab is a monoclonal antibody that inhibits the dimerization of HER2 [45]. Lapatinib is a dual tyrosine kinase inhibitor that acts on both HER1 and HER2 by reversibly inhibiting the ATP-biding site of the tyrosine kinase domains of the HER receptors [46]. The proportion of patients with metastatic HER2-positive breast cancer who demonstrate a clinical response to trastuzumab, defined as at least a >50% decrease in tumor volume, is up to 34% [47].
HER2 expression has direct links to tumor biology that enhances growth in the brain. When HER2 is overexpressed in breast cancer cell lines, TGFβ production is increased, leading to activation of TGF/SMAD pathways and expression of transcriptional HER2 of E-cadherin, including SNAIL, SLUG, and ZEB-1. Inhibition of HER2 by cucurbitacin B leads to suppression of brain metastasis in vivo [48]. These findings support the notion that HER2 contributes to epithelial to mesenchymal transition (EMT) and breast-to-brain metastasis through the production of TGFβ, a known master regulator of EMT [49-51]. Given that HER2-positive tumors found to co-express SNAIL did not respond to trastuzumab therapy [52], expression of TGF dependent proteins may further contribute to trastuzumab resistance and EMT [53]. Breast cancer is the most common solid tumor to metastasize to the leptomeninges [54], and the incidence of leptomeningeal metastasis is higher in HER2-positive tumors compared to ER-positive lobular and triple negative breast cancer [5, 55-56]. HER2-positive tumors remain a difficult subtype to treat, given the marked predisposition for difficult to treat brain metastasis.
Brain endothelial cells interact with breast cancer metastases
The blood-brain barrier (BBB) poses a significant obstacle to infiltrating tumor cells via tight junctions, junctional adhesion molecules, and astrocyte foot processes [57-58]. Real-time imaging of metastasizing cancer cells in vivo has shown that brain metastasis is an inefficient process in which cells undergo high rates of attrition, and that early extravasation and persistent close contact with endothelial cells are critical features [59]. MRI studies of injected tumor cells show that approximately 1.5% of injected cells form metastases in the brain [60]. Examination of early micrometastases in the brain shows that 95% of metastatic cells grow along vessels as opposed to isolated colonies within the brain parenchyma [61], suggesting that vascular basement membrane may represent an important “soil” that facilitates brain metastasis [62].
Endothelial cells may help breast cancer enter the CNS. Breast cancer cell transmigration is augmented by human brain endothelial cells as endothelial cell expression of COX-2 induces expression of matrix metalloproteinases in cancer cells [63]. αB-crystallin, a molecular chaperone primarily expressed in triple negative breast cancer, is associated with poor prognosis. When overexpressed, metastatic breast cancer cells exhibit enhanced adhesion to human brain microvascular endothelial cells [64]. JAM-A, a component of the endothelial tight junction complex, is highly expressed in normal mammary epithelium, yet down-regulated in breast cancer cells in the brain [65]. JAM-A expression positivity is correlated with a poor prognosis [66]. Cathepsin S, a protein that mediates transmigration of tumor cells across the BBB via proteolytic processing of JAM, was independently associated with breast-to-brain metastasis [67]. A study involving the injection of human estrogen receptor (ER)-negative pleural malignant breast cancer cells intra-arterially in rats to select for brain tropism, revealed COX2, the EGFR ligand HBEGF, and the alpha2,6-sialyltransferase ST6GALNAC5 as important mediators of brain metastasis. In particular, ST6GALNAC5 was found to mediate adhesion of tumor cells to brain endothelial cells and subsequent entry through the blood brain barrier [68]. Breast cancer cells metastatic to the brain have refined a specific capacity to interact favorably with CNS endothelial cells.
Breast cancer adapts to the brain microenvironment
Astrocytes play an important role in the survival of breast cancer upon entering the brain. Once through the BBB, invading breast cancer cells are surrounded by reactive astrocytes, quickly localizing to tumor cells through the up-regulation of matrix metalloproteinase-9 [69]. Astrocytes have been shown to secrete matrix metalloproteinases (MMP), including MMP-2 and MMP-9, and that culturing metastatic breast cancer cells with astrocyte-conditioned media lead to increased invasive ability [70]. Reactive astrocytes defend against metastatic invasion by up-regulating plasmin, which leads to the secretion of the paracrine death signal FasL and inactivates L1CAM, a molecule used by metastatic tumor cells to disperse along capillaries. To combat this onslaught, metastatic breast cancer cells highly express serpins, anti-plasminogen activators, including neuroserpin and serpin B2 [59]. Reactive glia may also inadvertently support the growth of metastatic tumor cells. Co-culture experiments of tumor cells and glia resulted in a five-fold increase in tumor cell proliferation [59]. When co-cultured with murine astrocytes, breast cancer cells up-regulated survival genes, including GSTA5, BCL2L1, and TWIST1, and the degree of up-regulation correlated with resistance to chemotherapy [71]. In fact, astrocytes may protect cancer cells from chemotherapeutic agents [72]. Breast cancer stem cell expression of IL-1B leads to astrocyte activation and expression of JAG1 in astrocytes, which leads to increased Notch signaling in cancer stem cells and represents an important self-renewal pathway in metastatic tumor cells [73]. Adaptive strategies by breast cancer cells and reaction of astrocytes to breast cancer cells may facilitate breast tumor cell survival in the brain.
Gene expression profiling performed over time as breast cancer cells underwent progressive invasion of brain tissue reveals a transient activation of genes involved in homeostasis and stress, and then activation of genes involved in cell division and morphology as cells were faced with surviving in a new microenvironment [74]. These experiments showed that breast cancer cells co-cultured with brain tissue express many brain-specific genes, likely due to the effect of secreted signals [75].
Breast tumor metabolism within the brain niche enables cancer growth and survival. Despite the high energetic demand of neural tissue, glucose levels in the interstitial space of the brain are highly regulated by astrocytes and lower than blood glucose levels [76-77], suggesting that breast cancer cells must undergo metabolic reprogramming in order to thrive in the brain interstitial microenvironment. Breast-to-brain metastasis demonstrates enhanced ability to survive independently of glucose metabolism by undergoing high rates of gluconeogenesis and oxidation of glutamine and branched chain amino acids. Silencing of fructose-1,6-bisphosphatase, an essential component of gluconeogenesis, leads to improved survival in mice bearing orthotopic breast-to-brain metastasis [78].
Case presentation
A 59-year-old woman with a five-year history of invasive ductal ER, PR, and HER2/neu-positive breast cancer presented with a history of headache, nausea, and decreased oral intake. The patient had undergone a mastectomy, followed by neoadjuvant chemotherapy (doxorubicin, cyclophosphamide, paclitaxel, trastuzumab, paclitaxel), and radiation. On initial examination, she had decreased orientation and level of consciousness, hemineglect, and left-sided pronator drift. A head CT demonstrated a large right-sided temporal-parietal mass measuring 2.9 x 5.9 x 2.9 cm with surrounding edema, 10 mm of midline shift, and subfalcine herniation. A brain MRI re-demonstrated a large, right-sided, heterogeneously enhancing, dural-based, temporal-parietal mass (Figures 1A, 1B).
Figure 1. Resection of Dural-based Brain Metastasis.
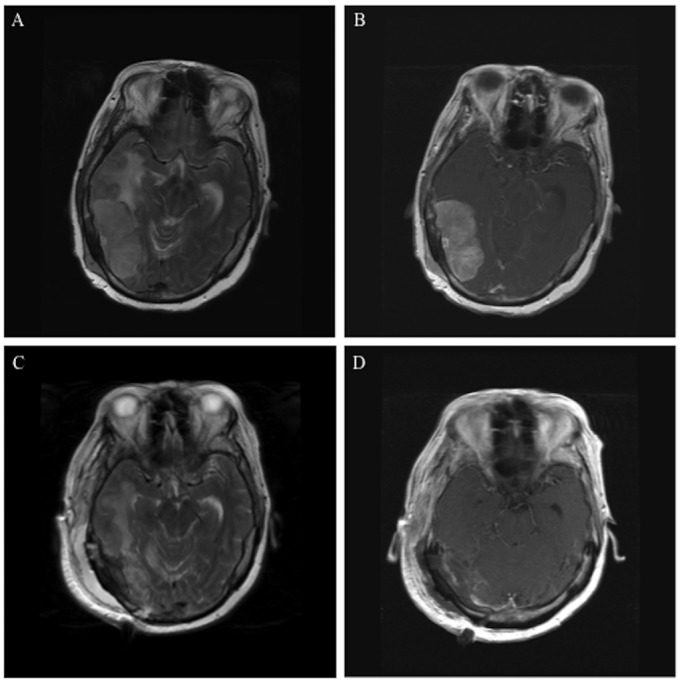
Pre- and postoperative MRI images demonstrate resection of a breast cancer dural-based metastasis. Preoperative axial T2 (A) and T1 with contrast images (B) show a heterogeneously enhancing 2.9 x 5.9 x 2.9 cm dural-based tumor in the right temporal-parietal region associated with significant peri-tumoral edema and midline shift. Postoperative T2 (C) and T1 with contrast images (D) demonstrate tumor resection.
Given the progressive nature of the patient’s symptoms from the large dural-based mass causing midline shift, subfalcine herniation, and significant peritumoral edema, it was recommended that the patient undergo a craniotomy for tumor resection. The craniotomy was performed with care to maximize control of the transverse sinus. The tumor was devascularized from its base with meticulous hemostasis and microsurgical dissection between the edematous brain and the tumor capsule.
The patient tolerated the operation well. Postoperatively, the patient was immediately more responsive, pronator drift resolved, and she was referred for external beam radiation. Postoperative MRI confirmed excellent tumor resection (Figures 1C, 1D). Pathologic analysis showed metastatic carcinoma from a breast primary tumor. Abundant mitotic figures and foci of necrosis were evident within the tumor. Immunohistochemical analysis showed that the tumor was ER-positive, PR-negative, and HER2/neu-positive.
Conclusions
Brain metastasis is a common but late complication of breast cancer that contributes to significant morbidity and mortality. Patients with HER2-positive breast cancer are at increased risk of developing breast-to-brain metastasis, and inhibition of HER2 in experimental models has led to the suppression of brain metastasis. Recent work has demonstrated the genetic, molecular, and metabolic changes that occur in breast cancer cells as they gain the ability to survive in the brain microenvironment. We believe that further advances in the understanding of the pathogenesis of breast to brain metastasis will lead to improved targeting of brain lesions and the ability to prospectively identify breast cancer patients at highest risk of developing intracranial disease.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.SEER Cancer Statistics Review, 1975-2006. Horner MJ, Ries LAG, Krapcho M, et al. http://seer.cancer.gov/csr/1975_2006 National Cancer Institute. 2009
- 2.Brain metastases. Wen PY, Loeffler JS. Curr Treat Options Oncol. 2000;1:447–458. doi: 10.1007/s11864-000-0072-3. [DOI] [PubMed] [Google Scholar]
- 3.Brain metastases. Lassman AB, DeAngelis LM. Neurol Clin. 2003;21:1–23. doi: 10.1016/s0733-8619(02)00035-x. [DOI] [PubMed] [Google Scholar]
- 4.Breast cancer metastasis to the central nervous system. Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Am J Pathol. 2005;167:913–920. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, Tudor IC, Wang LI, Brammer MG, Shing M, Yood MU, Yardley DA. Clin Cancer Res. 2011;17:4834–4843. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- 6.Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ; Panel members. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molecular portraits of human breast tumours. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 8.Prognostic factors of brain metastases from breast cancer: Impact of targeted therapies. Braccini AL, Azria D, Thezenas S, Romieu G, Ferrero JM, Jacot W. Breast. 2013;22:993–998. doi: 10.1016/j.breast.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: A multicenter retrospective analysis. Niikura N, Hayashi N, Masuda N, Takashima S, Nakamura R, Watanabe K, Kanbayashi C, Ishida M, Hozumi Y, Tsuneizumi M, Kondo N, Naito Y, Honda Y, Matsui A, Fujisawa T, Oshitanai R, Yasojima H, Tokuda Y, Saji S, Iwata H. Breast Cancer Res Treat. 2014;147:103–112. doi: 10.1007/s10549-014-3090-8. [DOI] [PubMed] [Google Scholar]
- 10.Brain metastases from breast cancer: Identification of a high-risk group. Evans AJ, James JJ, Cornford EJ, Chan SY, Burrell HC, Pinder SE, Gutteridge E, Robertson JF, Hornbuckle J, Cheung KL. Clin Oncol (R Coll Radiol) 2004;16:345–349. doi: 10.1016/j.clon.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Hicks DG, Short SM, Prescott NL, Tarr SM, Coleman KA, Yoder BJ, Crowe JP, Choueiri TK, Dawson AE, Budd GT, Tubbs RR, Casey G, Weil RJ. Am J Surg Pathol. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 12.Brain metastases: The HER2 paradigm. Lin NU, Winer EP. Clin Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 13.Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, Abdulkarim B. J Clin Oncol. 2006;24:5658–5663. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 14.Correlation between quantitative HER-2 protein expression and risk for brain metastases in HER-2+ advanced breast cancer patients receiving trastuzumab-containing therapy. Duchnowska R, Biernat W, Szostakiewicz B, Sperinde J, Piette F, Haddad M, Paquet A, Lie Y, Czartoryska-Arłukowicz B, Wysocki P, Jankowski T, Radecka B, Foszczynska-Kłoda M, Litwiniuk M, Debska S, Weidler J, Huang W, Buyse M, Bates M, Jassem J. Oncologist. 2012;17:26–35. doi: 10.1634/theoncologist.2011-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. Mehta MP, Rodrigus P, Terhaard CH, Rao A, Suh J, Roa W, Souhami L, Bezjak A, Leibenhaut M, Komaki R, Schultz C, Timmerman R, Curran W, Smith J, Phan SC, Miller RA, Renschler MF. J Clin Oncol. 2003;21:2529–2536. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 16.A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Chang EL, Wefel JS, Maor MH, Hassenbusch SJ 3rd, Mahajan A, Lang FF, Woo SY, Mathews LA, Allen PK, Shiu AS, Meyers CA. Neurosurg. 2007;60:277–283. doi: 10.1227/01.NEU.0000249272.64439.B1. [DOI] [PubMed] [Google Scholar]
- 17.Multidisciplinary management of brain metastases. Eichler AF, Loeffler JS. Oncologist. 2007;12:884–898. doi: 10.1634/theoncologist.12-7-884. [DOI] [PubMed] [Google Scholar]
- 18.The management of brain metastases. Patchell RA. Cancer Treat Rev. 2003;29:533–540. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 19.Sul J, Posner J. Brain Metastases. Vol. 136. Springer US; 2007. Brain Metastases: Epidemiology and Pathophysiology; pp. 1–21. [DOI] [PubMed] [Google Scholar]
- 20.Brain metastases: Epidemiology and pathophysiology. Gavrilovic IT, Posner JB. J Neurooncol. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 21.Survival after surgery and stereotactic radiosurgery for patients with multiple intracranial metastases: results of a single-center retrospective study. Smith TR, Lall RR, Lall RR, Abecassis IJ, Arnaout OM, Marymont MH, Swanson KR, Chandler JP. J Neurosurg. 2014;121:839–845. doi: 10.3171/2014.4.JNS13789. [DOI] [PubMed] [Google Scholar]
- 22.Distribution of brain metastases. Delattre JY, Krol G, Thaler HT, Posner JB. Arch Neurol. 1988;45:741–744. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- 23.Up-regulation of microRNA-10b is associated with the development of breast cancer brain metastasis. Ahmad A, Sethi S, Chen W, Ali-Fehmi R, Mittal S, Sarkar FH. Am J Transl Res. 2014;6:384–390. [PMC free article] [PubMed] [Google Scholar]
- 24.Differential role of angiogenesis and tumor cell proliferation in brain metastases according to primary tumor type: Analysis of 639 cases. Berghoff AS, Ilhan-Mutlu A, Dinhof C, Magerle M, Hackl M, Widhalm G, Hainfellner JA, Dieckmann K, Pichler J, Hutterer M, Melchardt T, Bartsch R, Zielinski CC, Birner P, Preusser M. Neuropathol Appl Neurobiol. 2014:0. doi: 10.1111/nan.12185. [DOI] [PubMed] [Google Scholar]
- 25.Current and emerging concepts in tumour metastasis. Coghlin C, Murray GI. J Pathol. 2010;222:1–15. doi: 10.1002/path.2727. [DOI] [PubMed] [Google Scholar]
- 26.Hallmarks of cancer: The next generation. Hanahan D, Weinberg RA. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 27.MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Olson P, Lu J, Zhang H, Shai A, Chun MG, Wang Y, Libutti SK, Nakakura EK, Golub TR, Hanahan D. Genes Dev. 2009;23:2152–2165. doi: 10.1101/gad.1820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Identifying site-specific metastasis genes and functions. Gupta GP, Minn AJ, Kang Y, Siegel PM, Serganova I, Cordón-Cardo C, Olshen AB, Gerald WL, Massagué J. Cold Spring Harb Symp Quant Biol. 2005;70:149–158. doi: 10.1101/sqb.2005.70.018. [DOI] [PubMed] [Google Scholar]
- 29.Metastasis: From dissemination to organ-specific colonization. Nguyen DX, Bos PD, Massagué J. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 30.Predictive molecular markers in metastases to the central nervous system: recent advances and future avenues. Berghoff AS, Bartsch R, Wöhrer A, Streubel B, Birner P, Kros JM, Brastianos PK, von Deimling A, Preusser M. Acta Neuropathol. 2014;128:879–891. doi: 10.1007/s00401-014-1350-7. [DOI] [PubMed] [Google Scholar]
- 31.Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF; American Society of Clinical Oncology; College of American Pathologists. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 32.ER and HER2 expression are positively correlated in HER2 non-overexpressing breast cancer. Pinhel I, Hills M, Drury S, Salter J, Sumo G, A'Hern R, Bliss JM, Sestak I, Cuzick J, Barrett-Lee P, Harris A, Dowsett M; NCRI Adjuvant Breast Cancer Trial Management Group. Breast Cancer Res. 2012;14:0. doi: 10.1186/bcr3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, Bunnell C, Rue M, Gelman R, Winer E. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 34.Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. Amir E, Miller N, Geddie W, Freedman O, Kassam F, Simmons C, Oldfield M, Dranitsaris G, Tomlinson G, Laupacis A, Tannock IF, Clemons M. J Clin Oncol. 2012;30:587–592. doi: 10.1200/JCO.2010.33.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. Niikura N, Liu J, Hayashi N, Mittendorf EA, Gong Y, Palla SL, Tokuda Y, Gonzalez-Angulo AM, Hortobagyi GN, Ueno NT. J Clin Oncol. 2012;30:593–599. doi: 10.1200/JCO.2010.33.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Impact of hormone receptor status on patterns of recurrence and clinical outcomes among patients with human epidermal growth factor-2-positive breast cancer in the National Comprehensive Cancer Network: A prospective cohort study. Vaz-Luis I, Ottesen RA, Hughes ME, Marcom PK, Moy B, Rugo HS, Theriault RL, Wilson J, Niland JC, Weeks JC, Lin NU. Breast Cancer Res. 2012;14:0. doi: 10.1186/bcr3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Central nervous system metastases in a cohort of metastatic breast cancer patients treated with trastuzumab. Montagna E, Cancello G, D'Agostino D, Lauria R, Forestieri V, Esposito A, Silvestro L, Accurso A, De Placido S, De Laurentiis M. Cancer Chemother Pharmacol. 2009;63:275–280. doi: 10.1007/s00280-008-0737-3. [DOI] [PubMed] [Google Scholar]
- 38.Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Kallioniemi OP, Holli K, Visakorpi T, Koivula T, Helin HH, Isola JJ. Int J Cancer. 1991;49:650–655. doi: 10.1002/ijc.2910490504. [DOI] [PubMed] [Google Scholar]
- 39.Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Musolino A, Ciccolallo L, Panebianco M, Fontana E, Zanoni D, Bozzetti C, Michiara M, Silini EM, Ardizzoni A. Cancer. 2011;117:1837–1846. doi: 10.1002/cncr.25771. [DOI] [PubMed] [Google Scholar]
- 40.Therapeutic approaches for HER2-positive brain metastases: Circumventing the blood-brain barrier. Mehta AI, Brufsky AM, Sampson JH. Cancer Treat Rev. 2013;39:261–269. doi: 10.1016/j.ctrv.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.A systematic review of trastuzumab and lapatinib in the treatment of women with brain metastases from HER2-positive breast cancer. Larsen PB, Kümler I, Nielsen DL. Cancer Treat Rev. 2013;39:720–727. doi: 10.1016/j.ctrv.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Monoclonal antibody-based targeted therapy in breast cancer: Current status and future directions. Bernard-Marty C, Lebrun F, Awada A, Piccart MJ. Drugs. 2006;66:1577–1591. doi: 10.2165/00003495-200666120-00004. [DOI] [PubMed] [Google Scholar]
- 43.Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Klapper LN, Waterman H, Sela M, Yarden Y. Cancer Res. 2000;60:3384–3388. [PubMed] [Google Scholar]
- 44.Trastuzumab: Updated mechanisms of action and resistance in breast cancer. Vu T, Claret FX. Front Oncol. 2012;2:62. doi: 10.3389/fonc.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pertuzumab: Optimizing HER2 blockade. Metzger-Filho O, Winer EP, Krop I. Clin Cancer Res. 2013;19:5552–5556. doi: 10.1158/1078-0432.CCR-13-0518. [DOI] [PubMed] [Google Scholar]
- 46.Lapatinib - Member of a New Generation of ErbB-Targeting Drugs. Untch M, Lück HJ. Breast Care (Basel) 2010;5:8–12. doi: 10.1159/000285750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 48.HER2 mediated de novo production of TGFβ leads to SNAIL driven epithelial-to-mesenchymal transition and metastasis of breast cancer. Gupta P, Srivastava SK. Mol Oncol. 2014;8:1532–1547. doi: 10.1016/j.molonc.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The basics of epithelial-mesenchymal transition. Kalluri R, Weinberg RA. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Katsuno Y, Lamouille S, Derynck R. Curr Opin Oncol. 2013;25:76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- 51.The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. Taylor MA, Parvani JG, Schiemann WP. J Mammary Gland Biol Neoplasia. 2010;15:169–190. doi: 10.1007/s10911-010-9181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Epithelial-to-mesenchymal transition (EMT) confers primary resistance to trastuzumab (Herceptin) Oliveras-Ferraros C, Corominas-Faja B, Cufí S, Vazquez-Martin A, Martin-Castillo B, Iglesias JM, López-Bonet E, Martin ÁG, Menendez JA. Cell Cycle. 2012;11:4020–4032. doi: 10.4161/cc.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Epithelial-to-mesenchymal transition: What is the impact on breast cancer stem cells and drug resistance. Mallini P, Lennard T, Kirby J, Meeson A. Cancer Treat Rev. 2014;40:341–348. doi: 10.1016/j.ctrv.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Diagnosis and treatment of leptomeningeal metastases from solid tumors: Experience with 90 patients. Wasserstrom WR, Glass JP, Posner JB. Cancer. 1982;49:759–772. doi: 10.1002/1097-0142(19820215)49:4<759::aid-cncr2820490427>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 55.Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong YN, Blayney DW, Niland JC, Winer EP, Weeks JC. Cancer. 2012;118:5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Metastatic pattern of infiltrating lobular carcinoma of the breast: An autopsy study. Lamovec J, Bracko M. J Surg Oncol. 1991;48:28–33. doi: 10.1002/jso.2930480106. [DOI] [PubMed] [Google Scholar]
- 57.Brain metastases. El Kamar FG, Posner JB. Semin Neurol. 2004;24:347–362. doi: 10.1055/s-2004-861530. [DOI] [PubMed] [Google Scholar]
- 58.The role of claudin-5 in blood-brain barrier (BBB) and brain metastases (review) Jia W, Lu R, Martin TA, Jiang WG. Mol Med Rep. 2014;9:779–785. doi: 10.3892/mmr.2013.1875. [DOI] [PubMed] [Google Scholar]
- 59.Serpins promote cancer cell survival and vascular co-option in brain metastasis. Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, Massagué J. Cell. 2014;156:1002–1016. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Heyn C, Ronald JA, Ramadan SS, Snir JA, Barry AM, MacKenzie LT, Mikulis DJ, Palmieri D, Bronder JL, Steeg PS, Yoneda T, MacDonald IC, Chambers AF, Rutt BK, Foster PJ. Magn Reson Med. 2006;56:1001–1010. doi: 10.1002/mrm.21029. [DOI] [PubMed] [Google Scholar]
- 61.The vascular basement membrane as "soil" in brain metastasis. Carbonell WS, Ansorge O, Sibson N, Muschel R. PLoS One. 2009;4:0. doi: 10.1371/journal.pone.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.The seed and soil hypothesis: Vascularisation and brain metastases. Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. Lancet Oncol. 2002;3:53–57. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 63.Human brain endothelial cell-derived COX-2 facilitates extravasation of breast cancer cells across the blood-brain barrier. Lee KY, Kim YJ, Yoo H, Lee SH, Park JB, Kim HJ. Anticancer Res. 2011;31:4307–4313. [PubMed] [Google Scholar]
- 64.αB-crystallin: A novel regulator of breast cancer metastasis to the brain. Malin D, Strekalova E, Petrovic V, Deal AM, Al Ahmad A, Adamo B, Miller CR, Ugolkov A, Livasy C, Fritchie K, Hamilton E, Blackwell K, Geradts J, Ewend M, Carey L, Shusta EV, Anders CK, Cryns VL. Clin Cancer Res. 2014;20:56–67. doi: 10.1158/1078-0432.CCR-13-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Attenuation of junctional adhesion molecule-A is a contributing factor for breast cancer cell invasion. Naik MU, Naik TU, Suckow AT, Duncan MK, Naik UP. Cancer Res. 2008;68:2194–2203. doi: 10.1158/0008-5472.CAN-07-3057. [DOI] [PubMed] [Google Scholar]
- 66.JAM-A expression positively correlates with poor prognosis in breast cancer patients. McSherry EA, McGee SF, Jirstrom K, Doyle EM, Brennan DJ, Landberg G, Dervan PA, Hopkins AM, Gallagher WM. Int J Cancer. 2009;125:1343–1351. doi: 10.1002/ijc.24498. [DOI] [PubMed] [Google Scholar]
- 67.Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, Brogi E, Brastianos PK, Hahn WC, Holsinger LJ, Massagué J, Leslie CS, Joyce JA. Nat Cell Biol. 2014;16:876–888. doi: 10.1038/ncb3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Genes that mediate breast cancer metastasis to the brain. Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massagué J. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Lorger M, Felding-Habermann B. Am J Pathol. 2010;176:2958–2971. doi: 10.2353/ajpath.2010.090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Astrocytes directly influence tumor cell invasion and metastasis in vivo. Wang L, Cossette SM, Rarick KR, Gershan J, Dwinell MB, Harder DR, Ramchandran R. PLoS One. 2013;8:0. doi: 10.1371/journal.pone.0080933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Kim SJ, Kim JS, Park ES, Lee JS, Lin Q, Langley RR, Maya M, He J, Kim SW, Weihua Z, Balasubramanian K, Fan D, Mills GB, Hung MC, Fidler IJ. Neoplasia. 2011;13:286–298. doi: 10.1593/neo.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.The brain microenvironment and cancer metastasis. Fidler IJ, Balasubramanian K, Lin Q, Kim SW, Kim SJ. Mol Cells. 2010;30:93–98. doi: 10.1007/s10059-010-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. Xing F, Kobayashi A, Okuda H, Watabe M, Pai SK, Pandey PR, Hirota S, Wilber A, Mo YY, Moore BE, Liu W, Fukuda K, Iiizumi M, Sharma S, Liu Y, Wu K, Peralta E, Watabe K. EMBO Mol Med. 2013;5:384–396. doi: 10.1002/emmm.201201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Effects of different tissue microenvironments on gene expression in breast cancer cells. Rondeau G, Abedinpour P, Desai P, Baron VT, Borgstrom P, Welsh J. PLoS One. 2014;9:0. doi: 10.1371/journal.pone.0101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adaptation versus selection: The origins of metastatic behavior. Scheel C, Onder T, Karnoub A, Weinberg RA. Cancer Res. 2007;67:11476–11479. doi: 10.1158/0008-5472.CAN-07-1653. [DOI] [PubMed] [Google Scholar]
- 76.Direct measurement of brain glucose concentrations in humans by 13C NMR spectroscopy. Gruetter R, Novotny EJ, Boulware SD, Rothman DL, Mason GF, Shulman GI, Shulman RG, Tamborlane WV. Proc Natl Acad Sci U S A. 1992;89:1109–1112. doi: 10.1073/pnas.89.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simultaneous measurement of glucose transport and utilization in the human brain. Shestov AA, Emir UE, Kumar A, Henry PG, Seaquist ER, Öz G. Am J Physiol Endocrinol Metab. 2011;301:0–9. doi: 10.1152/ajpendo.00110.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gain of glucose-independent growth upon metastasis of breast cancer cells to the brain. Chen J, Lee HJ, Wu X, Huo L, Kim SJ, Xu L, Wang Y, He J, Bollu LR, Gao G, Su F, Briggs J, Liu X, Melman T, Asara JM, Fidler IJ, Cantley LC, Locasale JW, Weihua Z. Cancer Res. 2014;Dec 15:0. doi: 10.1158/0008-5472.CAN-14-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]


