Abstract
Purpose
Prostate-specific antigen (PSA) is a surrogate marker of disease progression; however, its predictive ability in the extreme ranges is unknown. We determined the predictors of survival in patients with bone metastatic prostate cancer (BMPCa) and with extremely high PSA levels.
Methods
Treatment-naïve patients (n = 248) diagnosed with BMPCa between December 2002 and June 2012 were retrospectively analyzed. Clinicopathological features at diagnosis, namely age, body mass index, serum alkaline phosphatase (ALP) and PSA levels, PSA nadir, time to PSA nadir and its maintenance period, PSA declining velocity, Gleason grade, clinical T stage, pain score, Eastern Cooperative Oncology Group performance score (ECOG PS), and the number of bone metastases were assessed. The patients were stratified according to PSA ranges of <20 ng/mL, 20–100 ng/mL, 100–1000 ng/mL, and 1000–10,000 ng/mL. Study endpoints were castration-resistant PCa (CRPC)-free survival and cancer-specific survival (CSS).
Results
Patients with higher PSA and ALP levels showed more bone lesions (P < 0.001). During the follow-up period (median, 39.9 months; interquartile range, 21.5–65.9 months), there were no differences between the groups in terms of the survival endpoints. High ALP levels, shorter time to PSA nadir, and pain were associated with an increased risk of progression to CRPC, and high ALP levels, ECOG PS ≥ 1, and higher PSA nadir independently predicted CSS.
Conclusions
PSA response to androgen deprivation therapy and serum ALP are reliable predictors of survival in patients with BMPCa presenting with extremely high PSA levels. These patients should not be deterred from active treatment based on baseline PSA values.
Keywords: Alkaline phosphatase, Bone, Metastasis, Prostate cancer, Prostate-specific antigen, Survival
1. Introduction
Over the course of the prostate-specific antigen (PSA) era, patients have presented with prostate cancer (PCa) at a younger age and with lower grade disease, and are now more likely to present with organ-confined cancers.1 However, bone metastasis still occurs in approximately 3% of all newly diagnosed patients. In a previous study, 12% of patients without initial evidence were found to develop bone metastases during a median follow-up of 2.2 years.2,3 In advanced stage PCa, bone is the most common site of metastasis, representing >80% of all cases, and this is associated with a high risk of morbidity.4 Given the heterogeneous natural course of bone metastatic PCa (BMPCa), determining the prognostic clinicopathological features for survival is critical for patient counseling and to ensure judicious application of multimodal treatments.
PSA is a surrogate marker of systemic progression and cancer-specific survival (CSS).1 Most prediction models define high risk PCa as a presenting PSA level of >20 ng/mL.5 However, the ability of an extremely high PSA level to reflect disease burden and its prognostic impact on survival has not been well described. Given the relationship between elevated PSA levels and inferior survival outcome, the concern about poorer outcome further intensifies when the PSA values are extremely elevated. Indeed, it is of substantial importance to identify patients at the highest risk of systemic dissemination, as aggressive definitive therapy targeting PCa may detrimentally impact the quality of life and performance status of these patients, without having any survival benefits.
To our knowledge, no prior studies have investigated the differential survival outcomes of patients with BMPCa and extremely high PSA levels. Hence, there is uncertainty regarding the clinical utility of PSA at this stage of the disease, and the optimal management and extent of treatment remain unclear. In this study, we aimed to investigate the predictors of castration-resistant PCa (CRPC)-free survival and CSS in patients initially diagnosed with BMPCa presenting with extremely high serum PSA levels. We believe that the relatively uncommon finding of a PSA level of such a great magnitude and the paucity of data pertaining to survival outcome in this population lend relevance to this study.
2. Methods
2.1. Study population
A retrospective analysis was performed using a prospectively collected database of 248 consecutive treatment-naïve patients diagnosed with BMPCa between December 2002 and June 2012. PCa staging was determined according to the seventh American Joint Committee on Cancer TNM system, with the definition of bone metastasis based on either demonstrable metastatic deposits upon imaging studies (bone scan, computed tomography, magnetic resonance imaging, or positron emission tomography) or by pathologic confirmation. Patients were excluded from the analysis if they met the following criteria: (1) incomplete clinical data, (2) presence of visceral metastasis, (3) presence of nonregional lymph node enlargement, (4) lost to follow-up, or (5) unknown cause of death. The initiation and regimen of intermittent or continuous androgen-deprivation therapy (ADT), secondary hormonal manipulations, and cytotoxic chemotherapy were decided at each treating physician's discretion.
2.2. Prognostic factors and outcome variables
The clinical and pathological characteristics of the patients at diagnosis were retrieved from the institutional electronic medical record database. The data obtained consisted of age, baseline serum PSA and alkaline phosphatase (ALP) levels, PSA nadir, time to PSA nadir (TTN) and its maintenance period, PSA declining velocity, body mass index, Eastern Cooperative Oncology Group performance score (ECOG PS), Visual Analogue Scale pain score, Charlson Comorbidity Index, biopsy Gleason score, clinical T stage, progression to CRPC, and the number of metastatic bone lesions.
CRPC was defined as progression of disease or elevation of serum PSA using the Prostate Cancer Working Group 2 criteria,6 with the CRPC-free interval defined as the time from the date of the first radiographic bone metastasis to the date of CRPC diagnosis. The CSS interval was defined as the interval from the first date of diagnosis to the date of death from PCa. Patient data were considered missing if any of the above data were absent.
For the analyses, patients were stratified according to the following logarithmically scaled PSA ranges: <20 ng/mL, 20–100 ng/mL, 100–1000 ng/mL, and 1000–10,000 ng/mL. Patient survival and causes of death were investigated based on the National Cancer Registry Database or institutional electronic medical records. This study was approved by the institutional ethics committee after review of the protocol and procedures employed (3-2014-0112).
2.3. Statistical analysis
The clinicodemographic characteristics of the patients and tumors were compared using descriptive statistics. Appropriate comparative tests, such as analysis of variance and Fisher's exact test, were used to compare continuous and categorical variables. Kaplan–Meier curves were used to estimate the CRPC-free survival and CSS according to PSA, ALP, PSA nadir, and TTN, stratified by given cutoff values. Multivariate analysis was performed using Cox-proportional hazards regression models in order to adjust for potential confounders in predicting survival. Variables considered as potential predictors for multivariate modeling were selected by univariate analyses and subsequently tested in a stepwise forward conditional manner, with entry and retention in the model set at a significance level of 0.05. All statistical analyses were performed using SPSS version 18 (SPSS Inc., Chicago, IL, USA). All tests were two-sided, with statistical significance set at P < 0.05.
3. Results
3.1. Patient characteristics
The clinicopathologic features of each group are presented in Table 1. Patients with higher PSA levels were more likely to progress to CRPC, to harbor a greater number of metastatic bone lesions, and to have higher ALP levels than were patients with lower PSA levels. Of note, there were no differences in the distributions of biopsy Gleason grade, clinical T stage, and CSS rates between the PSA groups. Patients with ALP levels ≥200 IU/L harbored a greater number of metastatic bone lesions than those with ALP levels <200 IU/L (P < 0.001; data not shown).
Table 1.
Clinical and pathological features of 248 patients initially diagnosed with bone-metastatic prostate cancer.
| PSA group (ng/mL) | <20 | 20–100 | 100–1000 | 1000–10,000 | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | 39 | (15.7) | 89 | (35.9) | 90 | (36.3) | 30 | (12.1) | NS |
| Age (y) | 73 | (67–79) | 65 | (58–72) | 71.5 | (66.5–76.5) | 71 | (65.5–76.5) | 0.803 |
| BMI (kg/m2) | 25.5 | (23–28.1) | 22.9 | (20.3–25.5) | 22.7 | (20.3–25.1) | 23.7 | (20.6–26.9) | 0.813 |
| ECOG PS (≥1) | 7 | (17.9) | 24 | (26.9) | 20 | (22.2) | 11 | (36.7) | 0.728 |
| CCI (≥4) | 32 | (82.1) | 67 | (75.3) | 68 | (75.6) | 24 | (80.0) | 0.744 |
| VAS pain score (≥1) | 9 | (23.1) | 26 | (29.2) | 42 | (46.7) | 14 | (46.7) | 0.086 |
| T stage | 0.409 | ||||||||
| T2 | 10 | (25.6) | 7 | (7.9) | 8 | (8.9) | 2 | (6.7) | |
| T3 | 23 | (59.1) | 49 | (55.1) | 40 | (44.5) | 18 | (60.0) | |
| T4 | 6 | (15.3) | 33 | (37.0) | 42 | (46.6) | 10 | (33.3) | |
| Gleason grade | 0.114 | ||||||||
| ≤7 | 11 | (28.2) | 23 | (25.8) | 15 | (16.8) | 1 | (3.3) | |
| 8 | 13 | (33.3) | 29 | (32.6) | 35 | (38.9) | 13 | (43.3) | |
| ≥9 | 15 | (38.5) | 37 | (41.6) | 40 | (44.3) | 16 | (53.4) | |
| ALP (IU/L) | 117 | (71.0–196.3) | 82.0 | (71.0–107) | 98.0 | (72.5–191) | 157 | (93.0–389) | 0.002 |
| Time to PSA nadir (mo) | 5.0 | (3.0–6.0) | 7.0 | (5.0–12.0) | 5.5 | (4.0–10.3) | 6.0 | (5.0–10.0) | 0.763 |
| PSA nadir (ng/mL) | 1.83 | (0.01–5.5) | 0.36 | (0.02–1.6) | 0.93 | (0.09–5.98) | 11.6 | (0.27–25.4) | 0.343 |
| PSA nadir maintenance period (mo) | 10.0 | (6.5–23.5) | 13.0 | (8.0–18.0) | 9.0 | (6.0–19.0) | 12.0 | (8.0–18.0) | 0.411 |
| PSA velocity (ng/mL/mo) | 10.0 | (2.7–39.2) | 11.3 | (3.9–18.1) | 45.3 | (26.2–101.1) | 472 | (176–817) | 0.051 |
| Progression to CRPC | 9 | (23.1) | 41 | (46.1) | 60 | (66.7) | 20 | (66.7) | 0.001 |
| No. of bone lesions | <0.001 | ||||||||
| ≤5 | 17 | (43.6) | 41 | (46.1) | 27 | (30.0) | 2 | (6.7) | |
| 6–20 | 11 | (28.2) | 41 | (46.1) | 30 | (33.3) | 6 | (20.0) | |
| >21 | 11 | (28.2) | 7 | (7.8) | 33 | (36.7) | 22 | (73.3) | |
| Follow-up period (mo) | 39.9 | (21.5–49.8) | 39.1 | (24.8–58.2) | 41.2 | (20.6–60.3) | 26.9 | (18.4–56.8) | 0.607 |
Data arepresented as n (%) and median (interquartile range).
ALP, alkaline phosphatase; BMI, body mass index; CCI, Charlson Comorbidity Index; CRPC, Castrate-resistance prostate cancer; ECOG PS, Eastern Cooperative Oncology Group performance score; LN, lymph node; PSA, prostate-specific antigen; VAS, Visual Analogue Scale.
3.2. Predictors of survival
The adjusted proportional hazard regression analyses for the potential prognostic factors of CRPC-free survival and CSS are summarized in Tables 2 and 3, respectively. High ALP level, shorter TTN, and the presence of pain were found to be associated with increased risks of progression to CRPC, whereas high ALP level, high PSA nadir, and ECOG PS ≥ 1 independently predicted cancer-specific mortality.
Table 2.
Predictors of progression to castration-resistant disease in patients with bone metastatic prostate cancer.
| Univariate |
Multivariatea |
|||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P | HR | (95% CI) | P | |
| Age (y) | 0.991 | (0.967–1.016) | 0.471 | |||
| BMI | 1.027 | (0.963–1.095) | 0.422 | |||
| Gleason score (≥8) | 1.759 | (1.068–2.898) | 0.027 | |||
| T stage (≥T3) | 1.195 | (0.484–2.947) | 0.701 | |||
| Baseline PSA | 1.000 | (0.998–1.001) | 0.593 | |||
| Time to PSA nadir | 0.916 | (0.859–0.977) | 0.008 | 0.877 | (0.812–0.947) | 0.001 |
| PSA nadir | 1.000 | (0.993–1.006) | 0.931 | |||
| PSA nadir maintenance period | 0.968 | (0.939–0.998) | 0.041 | |||
| PSA velocity | 1.000 | (1.000–1.000) | 0.603 | |||
| Baseline ALP | 1.001 | (1.001–1.002) | <0.001 | 1.001 | (1.000–1.002) | 0.007 |
| VAS pain score (≥1) | 1.519 | (1.022–2.258) | 0.039 | 3.061 | (1.434–6.531) | 0.004 |
| ECOG PS (≥1) | 1.141 | (0.741–1.760) | 0.552 | |||
| Number of bone lesions | 1.016 | (0.997–1.036) | 0.095 | |||
ALP, alkaline phosphatase; BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; CRPC, Castrate-resistance prostate cancer; ECOG PS, Eastern Cooperative Oncology Group performance score; HR, hazards ratio; LN, lymph node; PSA, prostate-specific antigen; VAS, Visual Analogue Scale.
Forward step-wise conditional method.
Table 3.
Predictors of cancer-specific mortality in patients with bone metastatic prostate cancer.
| Univariate |
Multivariatea |
|||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P | HR | (95% CI) | P | |
| Age (y) | 1.009 | (0.985–1.034) | 0.447 | |||
| BMI | 1.044 | (0.961–1.135) | 0.315 | |||
| Gleason score (≥8) | 2.663 | (1.421–4.994) | 0.002 | |||
| T stage (≥T3) | 1.529 | (0.705–3.318) | 0.282 | |||
| PSA | 1.001 | (0.999–1.002) | 0.295 | |||
| Time to PSA nadir | 0.879 | (0.808–0.956) | 0.003 | |||
| PSA nadir | 1.001 | (1.001–1.002) | 0.002 | 1.006 | (0.001–1.011) | 0.012 |
| PSA nadir maintenance period | 0.924 | (0.868–0.962) | 0.003 | |||
| PSA velocity | 1.000 | (1.000–1.000) | 0.149 | |||
| ALP | 2.121 | (1.353–3.324) | 0.001 | 1.002 | (1.001–1.003) | 0.016 |
| VAS pain score (≥1) | 2.079 | (1.309–3.301) | 0.002 | |||
| ECOG PS (≥1) | 2.238 | (1.385–3.616) | 0.001 | 2.685 | (1.287–5.605) | 0.009 |
| Progression to CRPC | 2.255 | (1.442–3.526) | <0.001 | |||
| Number of bone lesions | 1.017 | (0.997–1.037) | 0.102 | |||
ALP, alkaline phosphatase; BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; CRPC, Castrate-resistance prostate cancer; ECOG PS, Eastern Cooperative Oncology Group performance score; HR, hazards ratio; LN, lymph node; PSA, prostate-specific antigen; VAS, Visual Analogue Scale.
Forward step-wise conditional method.
3.3. Survival outcome
Survival data as of March 2014 were used in this analysis. During the median follow-up period of 39.9 months (interquartile range, 21.5–65.9 months), there were no differences in the CRPC-free survival and CSS between the different PSA groups (Figs. 1A and 2A). Comparative survival outcomes of covariates considered potential predictors by the Cox-proportional hazards analysis were analyzed according to each cutoff value. Patients with high ALP levels (≥200 IU/L) were found to have significantly worse CRPC-free survival (P = 0.001) and CSS (P = 0.005) compared to patients with ALP levels < 200 IU/L (Figs. 1B and 2B). Moreover, patients who achieved a PSA nadir value of ≥0.2 ng/mL following ADT, revealed worse CSS compared to those with PSA nadir <0.2 ng/mL (P = 0.001; Fig. 2C). However, PSA nadir was not associated with an increased risk of CRPC progression (P = 0.614; Fig. 1C). Patients with a TTN of <9 months showed significantly worse CRPC-free survival (P = 0.024) and CSS (P = 0.022) compared to those with TTN of ≥9 months (Figs. 1D and 2D).
Fig. 1.
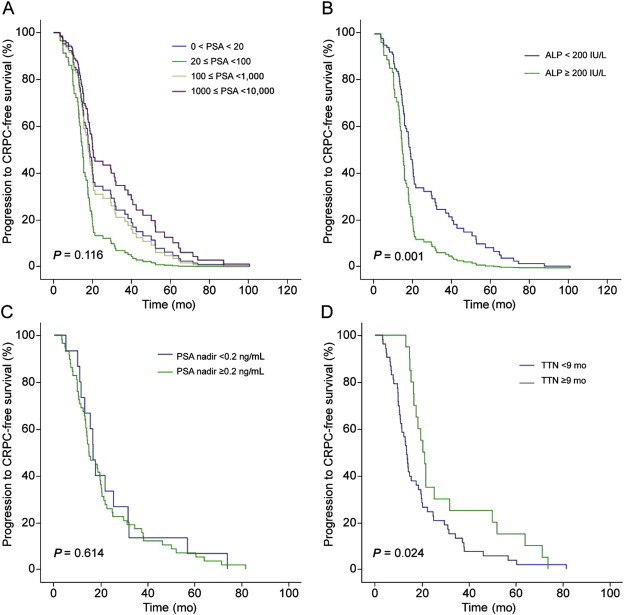
Comparative survival curves of patients with bone metastatic prostate cancer for progression to castration-resistant prostate cancer (CRPC)-free survival. (A) Survival curve stratified according to serum prostate-specific antigen (PSA) levels. (B) Survival curve stratified according to serum alkaline phosphatase (ALP) levels dichotomized at 200 IU/L. (C) Survival curve stratified according to PSA nadir levels dichotomized at 0.2 ng/mL. (D) Survival curve stratified according to time to PSA nadir (TTN) dichotomized at 9 months.
Fig. 2.
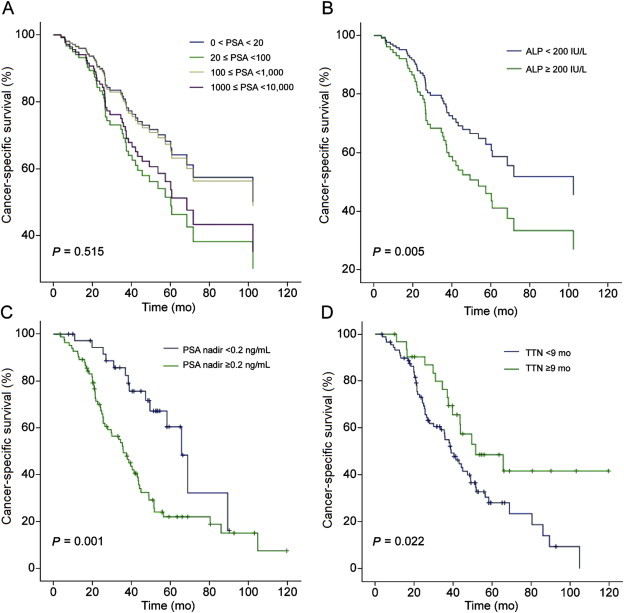
Comparative survival curves of patients with bone metastatic prostate cancer for cancer-specific survival. (A) Survival curve stratified according to serum prostate-specific antigen (PSA) levels. (B) Survival curve stratified according to. serum alkaline phosphatase (ALP) levels dichotomized at 200 IU/L. (C) Survival curve stratified according to. PSA nadir levels dichotomized at 0.2 ng/mL. (D) Survival curve stratified according to time to PSA nadir (TTN) dichotomized at 9 months.
4. Discussion
Most risk stratification schemes define high risk disease as a presenting PSA level of >20 ng/mL.1,5 In localized PCa, serum PSA has been demonstrated to correlate with the pathological stage, Gleason score, and the probability of organ-confined disease 7,8; whereas, in metastatic PCa, PSA has been shown to reflect the disease burden, and its post-hormone therapy response serves as a predictor of overall survival.9 However, in a number of previous population-based observational studies, a PSA level >50 ng/mL was observed to correlate with extreme-risk patients, who showed a worse response to treatment and survival outcome than other high risk patients.7,9,10 Therefore, it is conceivable that the high risk population includes a subset of patients who are at an extreme risk for disease progression and mortality.
The natural history of metastatic PCa is heterogeneous, and the diagnostic and treatment dilemmas intensify when a patient presents with extremely high PSA levels. Although an extremely high PSA level is commonly acknowledged to indicate extreme risk disease, no stratification model has yet demonstrated its prognostic value in this setting.11 If a patient diagnosed with BMPCa would be expected to have an unfavorable survival outcome based on an extremely high PSA level, this patient may not be regarded as a candidate for aggressive treatment, as this may have detrimental impacts on the quality of life and performance status of the patient without any survival benefits. For these reasons, in an initial attempt to investigate this issue, we limited the patient population in this study to men with BMPCa, which may limit the confounding variables and potentially provide a more accurate assessment of a specific population in which a prediction model would be clinically useful.
Bone metastasis in PCa is characterized by a distinct clinical pattern of bone-forming metastasis.12 Among the various bone-related biochemical markers that are known to be elevated in patients with BMPCa,13,14 serum ALP is a representative bone formation marker that has been demonstrated to be associated with both the extent of bone metastasis and survival.15,16 The importance of serum ALP as a prognostic factor for survival in metastatic PCa has been emphasized in previously developed survival models, in which the patient performance status as well as hemoglobin, albumin, lactate dehydrogenase, and ALP levels, was demonstrated to be significantly associated with overall survival.17–19 Our study confirmed patient performance status and serum ALP levels as independent predictors, even in patients with BMPCa presenting with extremely high PSA levels. Of note, our multivariate analysis revealed oncological features such as clinical T stage or Gleason grade to have only modest effects on survival outcome, which was also consistent with the results of certain previous studies.20,21 However, the inclusion of only patients with BMPCa who tended to have high risk disease may have limited the discriminatory value of these variables.
In clinical practice, PSA is the most widely utilized surrogate and prognostic marker used to evaluate disease burden and to predict survival prognosis.22 PSA has been shown to correlate with increased risks of bone-related clinical outcomes and overall survival in patients with metastatic CRPC23; and, moreover, in men with nonmetastatic CRPC, a higher PSA has been found to be associated with a shorter time to bone metastasis and reduced overall survival.14 However, it should be noted that the cohorts of these previous studies consisted of patients with relatively low PSA levels. Hence, our findings are of particular importance, given that extremely high PSA levels failed to show associations with all survival endpoints. Furthermore, the results of the current study are consistent with previous reports of patients on ADT, in which the association between baseline PSA and time to progression was confined only to nonmetastatic patients.24
PSA kinetics following ADT, namely, PSA nadir, TTN, and PSA velocity, have been shown to be useful predictors of disease progression and survival in various disease settings.25 Previous studies have reported a significant association between PSA nadir and progression to CRPC.20,26 Although there is currently no consensus on the optimal threshold of PSA nadir, we used the most widely reported cutoff value of 0.2 ng/mL in this study, and found that PSA nadir ≥0.2 ng/mL was an independent predictor of CSS.26,27 Likewise, there is also no consensus regarding the optimal threshold of TTN for predicting survival outcomes, and various studies have reported conflicting cutoff values, varying according to each study setting.27,28 Herein, we showed that a cutoff period of 9 months was capable of distinguishing patients with favorable CRPC-free survival and CSS rates; these findings are consistent with those of recent studies, which have reported that a longer TTN is associated with longer remission and survival.29,30 Although the exact mechanism underlying the association between PSA kinetics and survival prognosis is unclear, our results highlight the fact that the prognosis of patients diagnosed with BMPCa with extremely high PSA levels depends on multiple patient factors. Based on our results, we support the use of PSA kinetics following ADT and baseline serum ALP as alternative prognostic markers to PSA for risk stratification at this stage of the disease.
The main strength of the current study was the incorporation of detailed clinicopathological data, including information on treatment, comorbidities other than cancer, and performance status, available from each patient. Moreover, the use of a single institutional cohort may ensure uniformity of the data. However, at the same time, there were several potential limitations worth mentioning. The first is the retrospective nature of the study. This design is associated with a lack of a standard therapeutic approach, and strong patient and physician preferences existed regarding the implementation of specific treatments. As such, the indication to initiate therapy, choice of treatment modality, administration intervals, and type of agents lacked standardization. Nevertheless, we believe that this effect is inherent in any retrospective study and may reflect the real-world clinical practice in which the application of sequential therapies for metastatic PCa is not standardized. Secondly, our study cohort was limited to patients with BMPCa, and the prognostic factors identified were not determined until metastasis had developed. Thus, the results may not be generalizable to evaluating the risk for patients diagnosed with other metastases or after a certain treatment. However, despite these limitations, the current study provided information regarding the long-term survival outcomes in a unique cohort of potentially extreme-risk patients, for whom risk stratification is controversial.
An extreme serum PSA level reflects the disease burden of BMPCa; however, it is not prognostic for CRPC-free survival or CSS. Patients with BMPCa initially presenting with extremely high PSA levels should not be deterred from active treatment and should be given a definitive therapeutic goal, as these patients may show comparable survival outcomes to that of men with relatively low PSA levels. Moreover, our observations support the use of the PSA response to ADT and serum ALP levels as alternative predictors of survival in these patients.
Conflict of interest
None of the contributing authors have any conflicts of interest, including specific financial interests, relationships, and affiliations relevant to the subject matter or materials discussed in the manuscript.
Acknowledgments
None.
References
- 1.D'Amico A.V., Cote K., Loffredo M., Renshaw A.A., Schultz D. Determinants of prostate cancer-specific survival after radiation therapy for patients with clinically localized prostate cancer. J Clin Oncol. 2002;20:4567–4573. doi: 10.1200/JCO.2002.03.061. [DOI] [PubMed] [Google Scholar]
- 2.Norgaard M., Jensen A.O., Jacobsen J.B., Cetin K., Fryzek J.P., Sorensen H.T. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007) J Urol. 2010;184:162–167. doi: 10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 4.Sturge J., Caley M.P., Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat Rev Clin Oncol. 2011;8:357–368. doi: 10.1038/nrclinonc.2011.67. [DOI] [PubMed] [Google Scholar]
- 5.Crawford E.D., Bennett C.L., Andriole G.L., Garnick M.B., Petrylak D.P. The utility of prostate-specific antigen in the management of advanced prostate cancer. BJU Int. 2013;112:548–560. doi: 10.1111/bju.12061. [DOI] [PubMed] [Google Scholar]
- 6.Scher H.I., Halabi S., Tannock I. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ou Y.C., Chen J.T., Cheng C.L., Ho H.C., Yang C.R. Radical prostatectomy for prostate cancer patients with prostate-specific antigen >20 ng/ml. Jpn J Clin Oncol. 2003;33:574–579. doi: 10.1093/jjco/hyg104. [DOI] [PubMed] [Google Scholar]
- 8.Feneley M.R., Partin A.W. Indicators of pathologic stage of prostate cancer and their use in clinical practice. Urol Clin North Am. 2001;28:443–458. doi: 10.1016/s0094-0143(05)70154-3. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues G., Bae K., Roach M. Impact of ultrahigh baseline PSA levels on biochemical and clinical outcomes in two radiation therapy oncology group prostate clinical trials. Int J Radiat Oncol Biol Phys. 2011;80:445–452. doi: 10.1016/j.ijrobp.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roach M., 3rd, Lu J., Pilepich M.V. Predicting long-term survival, and the need for hormonal therapy: a meta-analysis of RTOG prostate cancer trials. Int J Radiat Oncol Biol Phys. 2000;47:617–627. doi: 10.1016/s0360-3016(00)00577-0. [DOI] [PubMed] [Google Scholar]
- 11.Alexander A.S., Mydin A., Jones S.O. Extreme-risk prostate adenocarcinoma presenting with prostate-specific antigen (PSA) >40 ng/ml: prognostic significance of the preradiation PSA nadir. Int J Radiat Oncol Biol Phys. 2011;81:e713–e719. doi: 10.1016/j.ijrobp.2010.11.068. [DOI] [PubMed] [Google Scholar]
- 12.Logothetis C.J., Navone N.M., Lin S.H. Understanding the biology of bone metastases: key to the effective treatment of prostate cancer. Clin Cancer Res. 2008;14:1599–1602. doi: 10.1158/1078-0432.CCR-07-4603. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong A.J., Febbo P.G. Using surrogate biomarkers to predict clinical benefit in men with castration-resistant prostate cancer: an update and review of the literature. Oncologist. 2009;14:816–827. doi: 10.1634/theoncologist.2009-0043. [DOI] [PubMed] [Google Scholar]
- 14.Smith M.R., Cook R., Lee K.A., Nelson J.B. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer. 2011;117:2077–2085. doi: 10.1002/cncr.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demers L.M., Costa L., Lipton A. Biochemical markers and skeletal metastases. Cancer. 2000;88:2919–2926. doi: 10.1002/1097-0142(20000615)88:12+<2919::aid-cncr7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Coleman R.E., Major P., Lipton A. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23:4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 17.Smaletz O., Scher H.I., Small E.J. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20:3972–3982. doi: 10.1200/JCO.2002.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Chodak G.W., Vogelzang N.J., Caplan R.J., Soloway M., Smith J.A. Independent prognostic factors in patients with metastatic (stage D2) prostate cancer. The Zoladex study group. JAMA. 1991;265:618–621. [PubMed] [Google Scholar]
- 19.Halabi S., Small E.J., Kantoff P.W. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 20.Kwak C., Jeong S.J., Park M.S., Lee E., Lee S.E. Prognostic significance of the nadir prostate specific antigen level after hormone therapy for prostate cancer. J Urol. 2002;168:995–1000. doi: 10.1016/S0022-5347(05)64559-4. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki T., Onishi T., Hoshina A. Nadir PSA level and time to PSA nadir following primary androgen deprivation therapy are the early survival predictors for prostate cancer patients with bone metastasis. Prostate Cancer Prostatic Dis. 2011;14:248–252. doi: 10.1038/pcan.2011.14. [DOI] [PubMed] [Google Scholar]
- 22.Scher H.I., Morris M.J., Basch E., Heller G. End points and outcomes in castration-resistant prostate cancer: from clinical trials to clinical practice. J Clin Oncol. 2011;29:3695–3704. doi: 10.1200/JCO.2011.35.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saad F., Segal S., Eastham J. Prostate-specific antigen kinetics and outcomes in patients with bone metastases from castration-resistant prostate cancer treated with or without zoledronic acid. Eur Urol. 2014;65:146–153. doi: 10.1016/j.eururo.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Ross R.W., Xie W., Regan M.M. Efficacy of androgen deprivation therapy (ADT) in patients with advanced prostate cancer: association between gleason score, prostate-specific antigen level, and prior ADT exposure with duration of ADT effect. Cancer. 2008;112:1247–1253. doi: 10.1002/cncr.23304. [DOI] [PubMed] [Google Scholar]
- 25.Hong S.Y., Cho D.S., Kim S.I., Ahn H.S., Kim S.J. Prostate-specific antigen nadir and time to prostate-specific antigen nadir following maximal androgen blockade independently predict prognosis in patients with metastatic prostate cancer. Korean J Urol. 2012;53:607–613. doi: 10.4111/kju.2012.53.9.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morote J., Esquena S., Abascal J.M. Usefulness of prostate-specific antigen nadir as predictor of androgen-independent progression of metastatic prostate cancer. Int J Biol Markers. 2005;20:209–216. doi: 10.1177/172460080502000403. [DOI] [PubMed] [Google Scholar]
- 27.Choueiri T.K., Xie W., D'Amico A.V. Time to prostate-specific antigen nadir independently predicts overall survival in patients who have metastatic hormone-sensitive prostate cancer treated with androgen-deprivation therapy. Cancer. 2009;115:981–987. doi: 10.1002/cncr.24064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamey T.A., Kabalin J.N., Ferrari M., Yang N. Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. IV. Anti-androgen treated patients. J Urol. 1989;141:1088–1090. doi: 10.1016/s0022-5347(17)41177-3. [DOI] [PubMed] [Google Scholar]
- 29.Furuya Y., Akimoto S., Akakura K. Response of prostate-specific antigen after androgen withdrawal and prognosis in men with metastatic prostate cancer. Urol Int. 1998;60:28–32. doi: 10.1159/000030199. [DOI] [PubMed] [Google Scholar]
- 30.Park Y.H., Hwang I.S., Jeong C.W., Kim H.H., Lee S.E., Kwak C. Prostate specific antigen half-time and prostate specific antigen doubling time as predictors of response to androgen deprivation therapy for metastatic prostate cancer. J Urol. 2009;181:2520–2524. doi: 10.1016/j.juro.2009.01.104. [DOI] [PubMed] [Google Scholar]


